Introduction
Osteosarcoma is frequently diagnosed in children and
adolescents (1). Patients <25
years of age exhibit a higher incidence and constituent ratio
compared with any other age group (2). Studies suggested patients <25 years
of age with osteosarcoma constitute as a separate specific subgroup
of the population (3,4). Mirabello et al (2) reported that the epidemiologic features
of osteosarcoma were unique among the 0–24; 25–59 and ≥60 years age
groups, therefore emphasizing the need to study the aforementioned
age groups separately. In the present study, osteosarcoma was
examined in the younger age groups by conducting a systematic study
of patients <25 years of age.
Single center and nationwide studies on osteosarcoma
have been indicated to provide limited sample size (5,6). The
Surveillance, Epidemiology and End Results (SEER) program, which
currently consists of 17 geographically defined registries and
covers ~26% of the U.S. population, is able to provide a large
sample size. For osteosarcoma, the SEER program provides
information regarding tumor site, histologic type, surgical type
and incidence, which are useful parameters for clinical
researchers. Therefore, the SEER program may assist clinicians with
regard to early diagnosis and optimal treatment of the
aforementioned tumor type.
Previous studies have primarily focused on all types
of tumors, particularly bone tumors in <25 years of age
(7–10)
or osteosarcoma cases of all ages (3,4,11). However, further systematic and
comprehensive studies focusing on osteosarcoma in children and
adolescents are required.
In the present study, the incidence based on year of
diagnosis, age, sex, race, region, and metastasis was examined from
different sites and histologic types. In addition, the study
assessed the risk factors for survival outcomes from 15 factors,
performed a pairwise comparison of these factors and elucidated
optimal surgical options to provide additional knowledge regarding
the characteristics of osteosarcoma in patients <25 years of
age. The aim of the present cohort study was to identify useful
factors for the prevention and treatment of osteosarcoma.
Materials and methods
Data source
All data were obtained from the SEER program
(https://seer.cancer.gov/) and the SEER*Stat
application 8.3.4 software (Surveillance Research Program, National
Cancer Institute, Bethesda, MD, USA) was used for analysis.
Patients between 0 and 24 years of age, who were diagnosed between
1973 and 2012 were selected for the present study. Histologic Type
International Classification of Disease (ICD)-O-3 was input as
9180–9187 and 9192–9195, and Primary Site-Labeled was input as
C40.0-C41.9 in the software to represent osteosarcoma. A total of
3,085 cases were available. Incidence, frequency and survival
outcomes were analyzed according to the following 15 factors.
Study design
A total of 15 factors, including patient- associated
factors, tumor-associated factors and treatment-associated factors,
were included in the present study. Patient-associated factors
consisted of year of diagnosis, sex, age at diagnosis, race,
Contract Health Service Delivery Areas (CHSDA) region, and rural or
urban. Tumor-associated factors included stage, grade, tumor size,
laterality, Histologic Type ICD-O-3 and Primary Site-Labeled.
Finally, treatment-associated factors consisted of surgery, surgery
type and radiation.
The year of diagnosis was divided into the following
4 groups: 1973–1982; 1983–1992; 1993–2002; and 2003–2012. Age at
diagnosis was divided as follows: 0–4 years; 5–9 years; 10–14
years; 15–19 years; and 20–24 years. Individuals were also
categorized as Caucasian, African descent or other, which included
American Indian/Alaska (AK) Native and Asian/Pacific Islander.
CHSDA region was categorized as East, Northern Plains, Pacific
Coast and Southwest. Rural or urban: Urban for patients in a
metropolitan area and rural for patients not in a metropolitan
area. Stage was divided into localized, regional and distant. Grade
was divided into well-differentiated, moderately differentiated,
poorly differentiated and undifferentiated. Tumor size was divided
into the following groups: <50 mm; 50–99 mm; 100–119 mm; and
≥120 mm. Laterality was divided into right and left, and surgery
type was divided into no surgery, local excision, radical excision
and amputation. Various histologic types, which had small samples
in the univariate analysis, were excluded, while osteosarcoma, not
otherwise specified, chondroblastic, fibroblastic, telangiectatic
and parosteal were included. For Primary Site-Labeled the following
were combined: C40.0 and C40.1, upper limbs; C40.2 and C40.3, lower
limbs; C41.0 and C41.1, skull and mandible; C41.2 and C41.3,
vertebral and chest bones; C41.4, pelvic bones.
Variables that had incomplete data among the 3,085
patients included surgery type, tumor size and grade. Cases for the
present study were available through the SEER program, including
1,976 cases for surgery type recorded since 1998, and 1,074 cases
for tumor size recorded since 2004. Therefore, in the survival
curve, the x-axis for surgery type and tumor size did not
correspond to 40 years. For tumor grade data, 1,834 cases were
available in total, distributed throughout 1973–2012; however,
there were numerous missing data.
Statistical analysis
The SEER*Stat application 8.3.4 software was used
for statistical analysis of the data. Rate session was used to
calculate incidence, and frequency session was used to calculate
frequency. Incidence is indicated as the number per 1,000,000. Case
listing session was used to collect the data of each patient and
for further survival analysis. The SPSS software 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used to perform the survival analysis,
log-rank testing, pairwise comparisons, five-year survival rate
analysis, univariate analysis and multivariate Cox regression
analysis. Associations among histological type, tumor site and
stage were analyzed using χ2 tests. P<0.05 was
considered to indicate a statistically significant difference.
The aforementioned 15 factors were used to plot
survival curves, and in log-rank testing, pairwise comparisons,
five-year survival rate analysis and univariate analysis. A total
of 3 factors, including grade, tumor size and surgery type, had
incomplete data, and therefore, only 12 factors were included in
the multivariate Cox regression analysis. Model 1 included all 12
factors, whereas Model 2 included 9 factors subsequent to excluding
3 factors, which exhibited no significant difference in the
univariate analysis. The association between surgery type and
survival was analyzed as a whole, but also for each stage and
grade. The numbers of each case, the sequence of survival outcomes
ranked from best to worst, and pairwise comparisons were
calculated.
Results
Osteosarcoma incidence, age at
diagnosis and survival for all age groups
As indicated in Fig.
1, the present study of osteosarcoma was performed in all age
groups between 1973 and 2012. In the line chart two peaks for
osteosarcoma incidence were indicated. The highest peak
corresponded to the 0–24 age group, and the other peak corresponded
to the ≥60 age group (Fig. 1A). The
majority of osteosarcoma cases were exhibited among patients
between 10 and 14 years of age (7.6 per million) and between 15 and
19 years of age (8.2 per million) (Fig.
1A). As indicated in Fig. 1B the
ratios of osteosarcoma were 56.8, 27.6 and 15.6% for 0–24, 25–59
and ≥60 years of age, respectively. A survival curve indicated that
for the three age groups, patients between 0 and 24 years of age
had the best prognosis, while patients ≥60 years of age had the
worst prognosis (P<0.001; Fig.
1C).
Incidence of osteosarcoma in patients
<25 years of age
The incidence of osteosarcoma according to
generation, sex, race, age group and CHSDA region are demonstrated
in Table I. The overall incidence
rate of osteosarcoma was 4.5 per million. The time span 1973–1982
had the lowest incidence rate, while the following 3 decades
exhibited an increase in incidence rates compared with previous
decades. However, differences among the 3 decades were not
significant (P>0.05). Male patients had a higher incidence of
osteosarcoma compared with female patients within each decade. The
incidence rate in male patients with osteosarcoma increased between
1973 and 2003, and decreased between 2003 and 2012. Changes in
female patients, according to generation, were not clear. Within
each decade, races such as American Indian/Alaska Native and
Asian/Pacific Islander, had the highest incidence rate, followed by
patients of African descent and Caucasian. As time progressed, the
incidence rate of osteosarcoma among Caucasian patients increased.
However, the incidence rate of osteosarcoma decreased among other
races, and remained unchanged among patients of African descent. No
visible trend over time was observed for the 0–24-year-old group,
but there were significant differences (P<0.05) within the age
group, with the highest incidence rate indicated in patients
between 10 and 19 years of age, followed by those of 20 and 24
years of age. No obvious findings for CHSDA regions were
identified, except for the East region, which had the lowest
incidence of osteosarcoma.
 | Table I.Incidence of osteosarcoma in patients
<25 years of age between 1973 and 2012 over 10-year intervals,
according to sex, age at diagnosis, race and CHSDA region. |
Table I.
Incidence of osteosarcoma in patients
<25 years of age between 1973 and 2012 over 10-year intervals,
according to sex, age at diagnosis, race and CHSDA region.
| Variables | 1973–1982 | 1983–1992 | 1993–2002 | 2003–2012 | All |
|---|
| Sex |
|
Male | 4.2 | 5.2 | 5.6 | 5.2 | 5.1 |
|
Female | 3.8 | 4.0 | 3.9 | 4.0 | 3.9 |
| Age at diagnosis
(years) |
|
|
|
|
|
|
0–4 | 0.5 | 0.5 | 0.3 | 0.4 | 0.4 |
|
5–9 | 1.5 | 2.9 | 2.7 | 2.7 | 2.5 |
|
10–14 | 7.3 | 7.3 | 7.9 | 7.7 | 7.6 |
|
15–19 | 7.4 | 8.6 | 8.8 | 7.9 | 8.2 |
|
20–24 | 3.0 | 3.6 | 4.0 | 4.1 | 3.7 |
| Race |
|
|
|
|
|
|
Caucasian | 3.7 | 4.4 | 4.6 | 4.5 | 4.3 |
| African
descent | 4.8 | 5.0 | 5.1 | 5.3 | 5.1 |
|
Other | 5.6 | 5.6 | 5.3 | 4.0 | 4.9 |
| CHSDA region |
|
|
|
|
|
|
East | 3.8 | 4.1 | 4.1 | 4.1 | 4.0 |
|
Northern plains | 3.7 | 4.5 | 5.1 | 5.4 | 4.6 |
| Pacific
coast | 4.3 | 5.0 | 4.5 | 4.4 | 4.6 |
|
Southwest | 4.1 | 4.9 | 5.6 | 4.6 | 4.8 |
|
All | 4.0 | 4.6 | 4.8 | 4.6 | 4.5 |
Incidence of osteosarcoma according to
sex and age
Table II indicates
the incidence rate of osteosarcoma in male and female patients in
different age groups. It was demonstrated that female patients had
a higher incidence rate of osteosarcoma compared with male
patients, between 0–14 years of age, particularly between the ages
of 0–4, 5–9 and 10–14 diagnosed between 1983 and 2002, 1993 and
2012, and 1973 and 1992, respectively. When combining the 4
decades, 1973–1982, 1983–1992, 1993–2002 and 2003–2012, female
patients had a higher incidence at 5–9 years of age (Table II).
 | Table II.Incidence of osteosarcoma in patients
<25 years of age between 1973 and 2012, according to age group
and sex. |
Table II.
Incidence of osteosarcoma in patients
<25 years of age between 1973 and 2012, according to age group
and sex.
|
| 1973–1982 | 1983–1992 | 1993–2002 | 2003–2012 | All |
|---|
|
|
|
|
|
|
|
|---|
| Age at diagnosis
(years) | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
|---|
| 0–4 | 0.6 | 0.3 | 0.3 | 0.6 | 0.1 | 0.4 | 0.5 | 0.3 | 0.4 | 0.4 |
| 5–9 | 1.6 | 1.4 | 2.9 | 2.8 | 2.6 | 2.8 | 2.4 | 3.0 | 2.4 | 2.6 |
| 10–14 | 6.6 | 8.0 | 7.3 | 7.4 | 8.5 | 7.2 | 8.2 | 7.1 | 7.7 | 7.4 |
| 15–19 | 8.6 | 6.2 | 10.9 | 6.2 | 12.5 | 4.8 | 10.1 | 5.7 | 10.5 | 5.7 |
| 20–24 | 3.2 | 2.8 | 4.4 | 2.7 | 3.9 | 4.1 | 4.7 | 3.5 | 4.1 | 3.2 |
Association among histologic type,
tumor site and metastasis
Table III indicated
the association between histologic type and site with risk of
metastatic disease. The ‘distant’ stage was defined as metastasis.
The results of the present study indicated that the chest and
pelvic bones had a higher prevalence rate of metastatic disease,
while the long bone of the upper limbs had a higher prevalence rate
of metastatic disease compared with the lower limbs. Parosteal and
periosteal osteosarcoma were two histologic types with a low risk
of metastasis.
 | Table III.Association among histologic type,
tumor site and stage in patients <25 years of age with
osteosarcoma between 1973 and 2012. |
Table III.
Association among histologic type,
tumor site and stage in patients <25 years of age with
osteosarcoma between 1973 and 2012.
| Variables | Localized, n
(%) | Regional, n
(%) | Distant, n (%) | P-value |
|---|
| Histologic type
ICD-O-3 |
|
|
| aP<0.001 |
|
Osteosarcoma, NOS | 724 (35.0) | 905 (43.8) | 438 (21.2) |
|
|
Chondroblastic
osteosarcoma | 117 (29.7) | 208 (52.8) | 69 (17.5) |
|
|
Fibroblastic osteosarcoma | 42 (35.9) | 56 (47.9) | 19 (16.2) |
|
|
Telangiectatic
osteosarcoma | 39 (36.4) | 52 (48.6) | 16 (15.0) |
|
|
Osteosarcoma in Paget disease
of bone | 1 (100.0) | 0 (0.0) | 0 (0.0) |
|
| Small
cell osteosarcoma | 9 (39.1) | 10 (43.5) | 4 (17.4) |
|
| Central
osteosarcoma | 16 (32.0) | 28 (56.0) | 6 (12.0) |
|
|
Intraosseous
well-differentiated osteosarcoma | 1 (50.0) | 1 (50.0) | 0 (0.0) |
|
|
Parosteal osteosarcoma | 69 (65.7) | 31 (29.5) | 5 (4.8) |
|
|
Periosteal osteosarcoma | 16 (57.1) | 10 (35.7) | 2 (7.1) |
|
|
High-grade surface
osteosarcoma | 1 (12.5) | 4 (50.0) | 3 (37.5) |
|
| Primary
site-labeled |
|
|
| aP<0.001 |
|
C40.0-Long bones: Upper limb,
scapula, and associated joints | 118 (34.3) | 149 (43.3) | 77 (22.4) |
|
|
C40.1-Short bones of upper
limb and associated joints | 5 (50.0) | 5 (50.0) | 0 (0.0) |
|
|
C40.2-Long bones of lower limb
and associated joints | 796 (36.8) | 973 (45.0) | 394 (18.2) |
|
|
C40.3-Short bones of lower
limb and associated joints | 13 (36.1) | 17 (47.2) | 6 (16.7) |
|
|
C41.0-Bones of skull and face
and associated joints | 32 (36.8) | 39 (44.8) | 16 (18.4) |
|
| C41.1
Mandible | 23 (39.7) | 30 (51.7) | 5 (8.6) |
|
| C41.2
Vertebral column | 13 (41.9) | 13 (41.9) | 5 (16.1) |
|
| C41.3
Rib, Sternum, Clavicle and associated joints | 12 (27.3) | 18 (40.9) | 14 (31.8) |
|
|
C41.4-Pelvic bones, sacrum,
coccyx and associated joints | 22 (17.9) | 59 (48.0) | 42 (34.1) |
|
|
C41.9-Bone, NOS | 1 (16.7) | 2 (33.3) | 3 (50.0) |
|
The five-year survival rate,
univariate analysis and pairwise comparisons
Table IV summarized
the five-year survival rates and univariate analyses for 15
factors. Survival curves and the results of pairwise comparisons
are presented in Fig. 2 for
patient-associated factors and Fig. 3
for tumor-associated factors and treatment-associated factors.
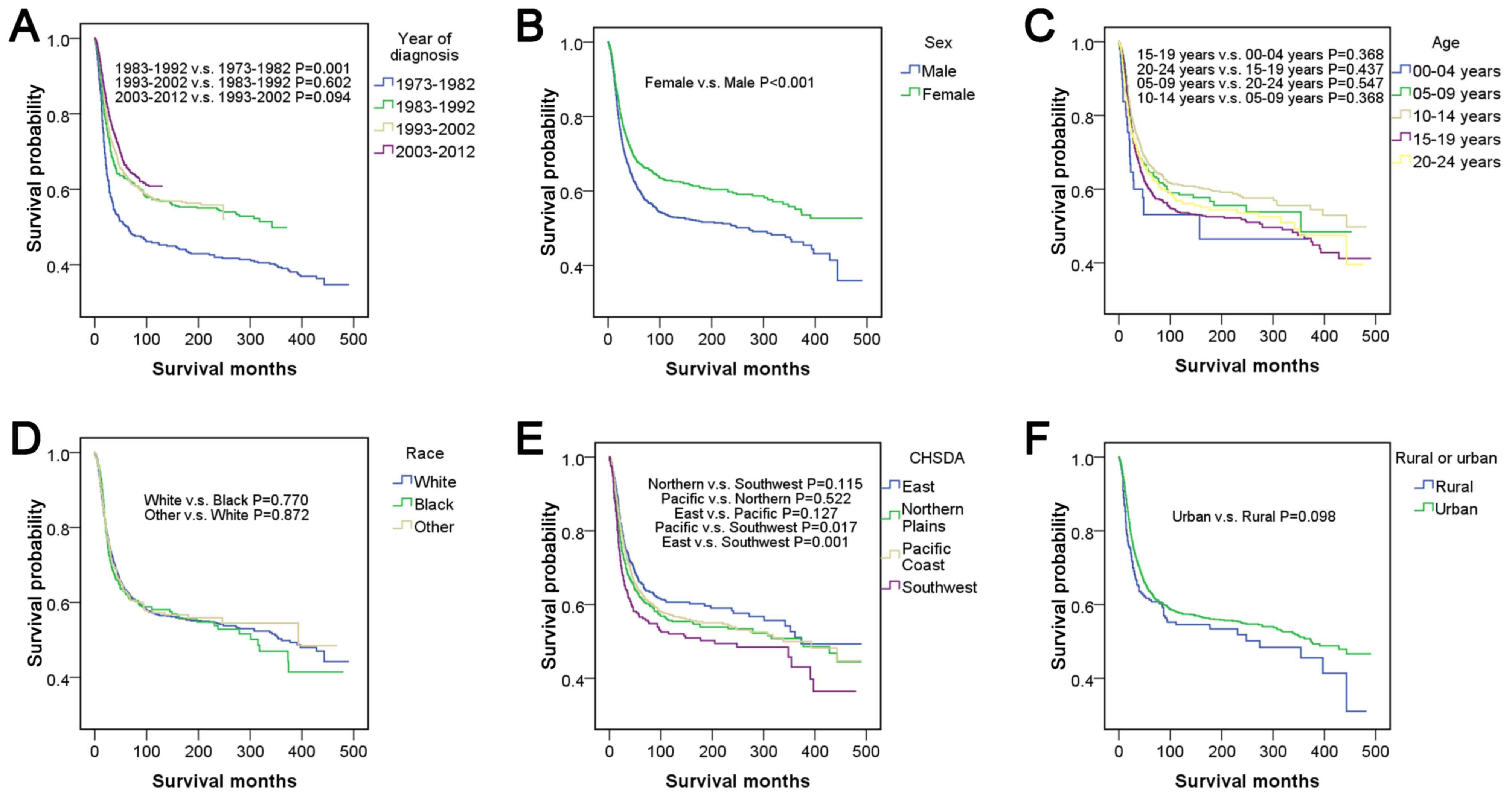
Survival outcome was worst between 1973–1982, and the following 3
decades exhibited an improved survival outcome. When each of the 3
decades was compared with 1973–1982, all results exhibited
significant differences (P<0.05), but comparisons within the 3
decades indicated no differences (P>0.05). Female patients had
relatively good survival outcomes compared with male patients
(P<0.001). The survival outcome from best to worst among the
different age groups was as follows: 10–14; >5-9; >20-24;
>15–19 and >0–4 years of age, but there were no significant
differences in pairwise comparisons among the groups
(P>0.05).
 | Table IV.Five-year survival rate and
univariate analysis in patients with osteosarcoma <25 years of
age between 1973 and 2012. |
Table IV.
Five-year survival rate and
univariate analysis in patients with osteosarcoma <25 years of
age between 1973 and 2012.
|
|
|
| Univariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n (%) | Survival (95%
CI) | HR (95% CI) | P-value |
|---|
| Year of
diagnosis |
|
|
| aP<0.001 |
|
1973–1982 | 357 (11.6) | 50.1
(45.0–55.2) | Reference |
|
|
1983–1992 | 404 (13.1) | 62.7
(58.0–67.4) | 0.701
(0.576–0.852) | aP<0.001 |
|
1993–2002 | 851 (27.6) | 63.5
(60.2–66.8) | 0.661
(0.557–0.784) | aP<0.001 |
|
2003–2012 | 1,473 (47.7) | 66.3
(63.6–69.0) | 0.582
(0.493–0.687) | aP<0.001 |
| Sex |
|
|
| aP<0.001 |
|
Male | 1,757 (57.0) | 60.1
(57.7–62.5) | Reference |
|
|
Female | 1,328 (43.0) | 67.3
(64.8–69.8) | 0.757
(0.675–0.849) | aP<0.001 |
| Age at diagnosis
(years) |
|
|
| a0.014 |
|
0–4 | 49 (1.6) | 54.1
(39.2–69.0) | Reference |
|
|
5–9 | 368 (11.9) | 64.2
(59.1–69.3) | 0.728
(0.468–1.131) | 0.158 |
|
10–14 | 1,064 (34.5) | 66.4
(63.5–69.3) | 0.666
(0.437–1.014) | 0.058 |
|
15–19 | 1,102 (35.7) | 60.0
(57.1–62.9) | 0.828
(0.545–1.258) | 0.376 |
|
20–24 | 502 (16.3) | 63.8
(59.5–68.1) | 0.776
(0.504–1.194) | 0.249 |
| Race |
|
|
| 0.939 |
|
Caucasian | 2,289 (74.2) | 63.2
(61.0–65.4) | Reference |
|
| African
descent | 476 (15.4) | 62.1
(57.6–66.6) | 1.023
(0.877–1.194) | 0.771 |
|
Other | 296 (9.6) | 62.6
(56.9–68.3) | 0.985
(0.814–1.191) | 0.872 |
| CHSDA region |
|
|
| a0.011 |
|
East | 843 (27.3) | 65.8
(62.5–69.1) | Reference |
|
|
Northern Plains | 479 (15.5) | 61.8
(57.3–66.3) | 1.169
(0.981–1.394) | 0.081 |
| Pacific
Coast | 1,465 (47.5) | 63.4
(60.9–65.9) | 1.114
(0.970–1.280) | 0.127 |
|
Southwest | 295 (9.6) | 56.5
(50.6–62.4) | 1.394
(1.142–1.701) | a0.001 |
| Rural or urban |
|
|
| 0.098 |
|
Rural | 264 (8.6) | 60.8
(54.7–66.9) | Reference |
|
|
Urban | 2,758 (89.4) | 63.5
(61.5–65.5) | 0.852
(0.704–1.031) | 0.098 |
| Stage |
|
|
| aP<0.001 |
|
Localized | 1,034 (33.5) | 77.5
(74.8–80.2) | Reference |
|
|
Regional | 1,305 (42.3) | 64.7
(62.0–67.4) | 1.640
(1.416–1.900) | aP<0.001 |
|
Distant | 562 (18.2) | 31.1
(27.0–35.2) | 4.442
(3.798–5.196) | aP<0.001 |
| Grade |
|
|
| aP<0.001 |
|
Well | 86 (2.8) | 81.1
(72.5–89.7) | Reference |
|
|
Moderately | 125 (4.1) | 78.0
(70.4–85.6) | 1.142
(0.653–1.997) | 0.641 |
|
Poorly | 524 (17.0) | 61.4
(56.9–65.9) | 2.073
(1.307–3.287) | a0.002 |
|
Undifferentiated | 1,100 (35.7) | 64.5
(61.6–67.4) | 2.015
(1.286–3.163) | a0.002 |
| Tumor size
(mm) |
|
|
| aP<0.001 |
|
<50 | 127 (4.1) | 79.5
(71.7–87.3) | Reference |
|
|
50–99 | 458 (14.8) | 73.2
(68.7–77.7) | 1.250
(0.809–1.930) | 0.315 |
|
100–119 | 149 (4.8) | 64.3
(55.9–72.7) | 1.751
(1.082–2.836) | a0.023 |
|
≥120 | 340 (11.0) | 56.4
(49.9–62.9) | 2.119
(1.376–3.263) | a0.001 |
| Laterality |
|
|
| 0.371 |
|
Right | 1,364 (44.2) | 50.0
(47.5–52.5) | Reference |
|
|
Left | 1,367 (44.3) | 63.3
(60.6–66.0) | 1.056
(0.937–1.191) | 0.371 |
| Histologic Type
ICD-O-3 |
|
|
| aP<0.001 |
|
Osteosarcoma, NOS | 2,223 (72.1) | 60.8
(58.6–63.0) | Reference |
|
|
Chondroblastic | 407 (13.2) | 62.4
(57.5–67.3) | 0.912
(0.770–1.080) | 0.286 |
|
Fibroblastic | 122 (4.0) | 69.9
(61.7–78.1) | 0.732
(0.539–0.994) | a0.045 |
|
Telangiectatic | 109 (3.5) | 70.2
(61.0–79.4) | 0.774
(0.557–1.075) | 0.127 |
|
Parosteal | 111 (3.6) | 89.0
(82.9–95.1) | 0.287
(0.180–0.457) | aP<0.001 |
| Primary
site-labeled |
|
|
| aP<0.001 |
| Upper
limb | 378 (12.3) | 56.8
(51.5–62.1) | Reference |
|
| Lower
limb | 2,321 (75.2) | 66.4
(64.4–68.3) | 0.737
(0.627–0.867) | aP<0.001 |
| Skull
and mandible | 155 (5.0) | 67.6
(60.0–75.2) | 0.694
(0.511–0.944) | a0.02 |
|
Vertebral and chest bones | 79 (2.6) | 48.2
(37.0–59.4) | 1.301
(0.943–1.795) | 0.109 |
| Pelvic
bones | 135 (4.4) | 31.1
(22.9–39.3) | 2.339
(1.827–2.993) | aP<0.001 |
| Surgery |
|
|
| aP<0.001 |
|
Yes | 2,613 (84.7) | 66.3
(64.3–68.3) | Reference |
|
| No | 399 (12.9) | 44.6
(39.5–49.7) | 2.113
(1.831–2.438) | aP<0.001 |
| Surgery type |
|
|
| aP<0.001 |
| No
surgery | 244 (7.9) | 42.4
(35.9–48.9) | Reference |
|
| Local
excision | 208 (6.7) | 75.3
(69.2–81.4) | 0.310
(0.226–0.426) | aP<0.001 |
| Radical
excision | 1,076 (34.9) | 71.1
(68.2–74.0) | 0.344
(0.281–0.422) | aP<0.001 |
|
Amputation | 389 (12.6) | 61.7
(56.6–66.8) | 0.494
(0.391–0.624) | aP<0.001 |
| Radiation |
|
|
| aP<0.001 |
|
Yes | 163 (5.3) | 35.3
(27.7–42.9) | Reference |
|
| No | 2,881 (93.4) | 64.8
(63.0–66.6) | 0.373
(0.308–0.450) | aP<0.001 |
There were no significant differences among races
(P>0.05). In addition, no significant differences were observed
among CHSDA regions (P>0.05), except that the region with the
best outcome, East, was significantly different compared with the
region with the worst outcome, Southwest (P<0.05). Patients from
rural and urban areas had no significant difference in survival
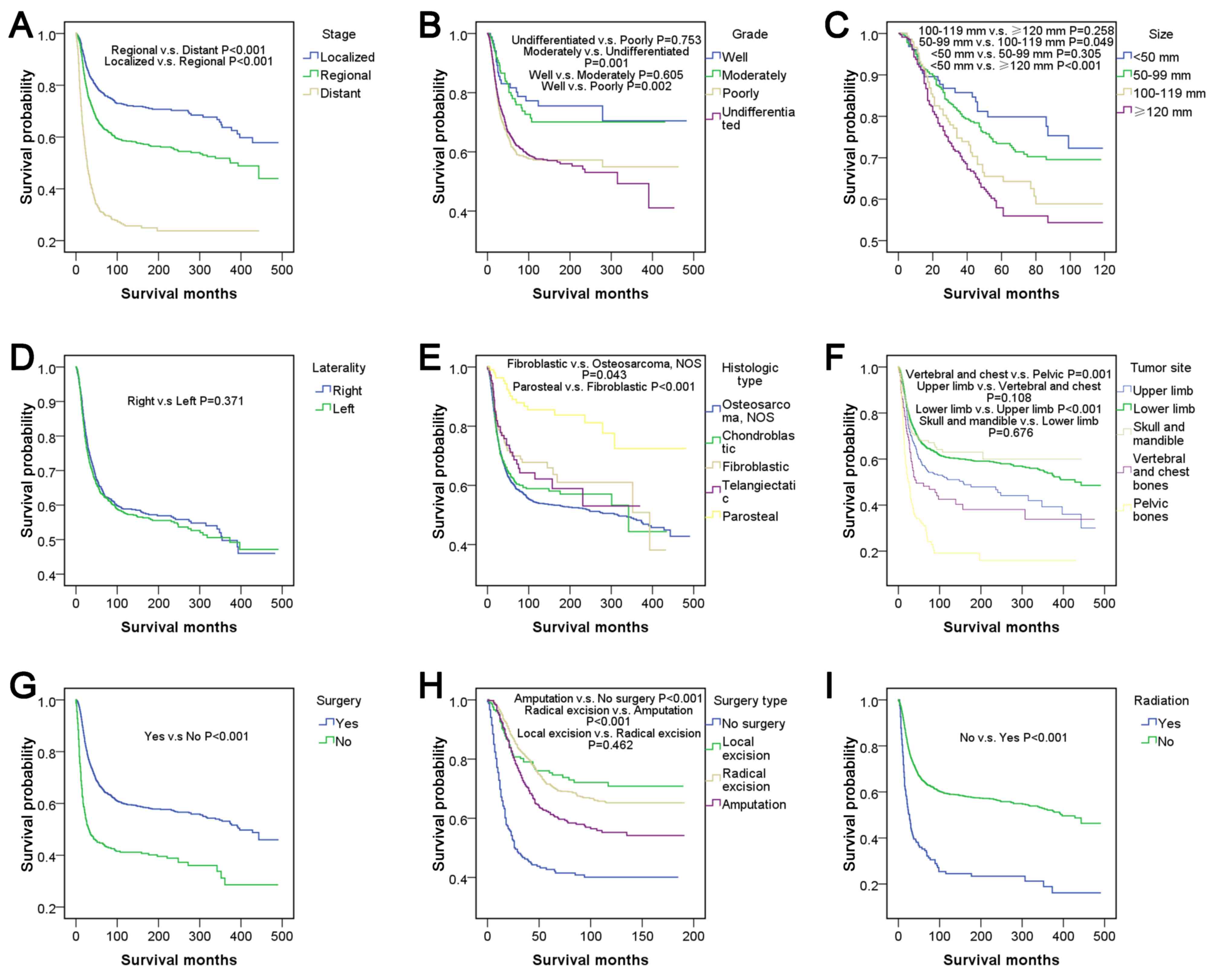
outcome (P>0.05). There were significant differences in stage
according to pairwise comparisons and the entire comparison
(P<0.001). Well- and moderately differentiated subtypes of grade
corresponded to relatively good survival outcomes compared with the
poorly differentiated and undifferentiated subtypes (P<0.05),
but no significant differences were demonstrated within well- and
moderately differentiated or within poorly differentiated and
undifferentiated subtypes (P>0.05). The survival curve indicated
that a large tumor size was associated with relatively poor
survival outcomes, and there was a significant difference in
survival outcome between patients with a tumor size <50 mm and
patients with a tumor size >100 mm (P<0.05). There was no
significant difference between tumors located on the left and right
lateral (P>0.05). Parosteal osteosarcoma had the best survival
outcome with a five-year survival rate of 89.0% and was
significantly different from all other histological types of
osteosarcoma (P<0.001). The tumor sites ranked from best to
worst survival outcome were as follows: Skull and mandible; lower
limb; upper limb; vertebral and chest bones, and pelvic bones. In
pairwise comparisons, there were significant differences between
chest bones and pelvic bones (P=0.001) and between lower limbs and
upper limbs (P<0.001). Patients who underwent surgery had
relatively good survival outcomes compared with those who did not
(P<0.001). The surgery types, which were ranked from best to
worst for survival outcome were local excision, radical excision,
amputation and no surgery. There was no significant difference
between local excision and radical excision, according to pairwise
comparisons (P>0.05), however, all other types of surgery were
associated with significant differences (P<0.001). Patients who
underwent radiation had relatively poor survival outcomes compared
with patients who did not receive radiation (P<0.001).
Association between surgery type and
survival outcome
As the association between surgery type and survival
outcome may be confounded by other factors, including stage and
grade of osteosarcoma, survival curves were plotted and log-rank
tests were performed for the same stage or grade (Table V). The frequency of amputation as a
treatment for osteosarcoma was higher among patients with
‘localized’ stage of osteosarcoma, while patients with ‘distant’
stage of osteosarcoma were not surgically treated. In addition,
amputation was indicated to be higher among patients with
‘undifferentiated’ grade of osteosarcoma. In the comparison of
surgery types among patients with the same stage or grade of
osteosarcoma, results of survival outcome based on surgery type
were almost identical. The results of the present study indicated
that for types of surgery the best to worst survival outcomes were
as follows: Local excision, radical excision, amputation and no
surgery. The aforementioned result was also indicated for the total
number of patients. Therefore, local excision may be the optimal
choice for patients with any type of osteosarcoma, conflicting with
the previous notion that amputation is the optimal choice of
treatment. In addition, as indicated in the results of Table V, radical excision may be an optimal
choice for patients with metastatic disease.
 | Table V.Association between surgery type and
survival outcome in patients <25 years of age with osteosarcoma
between 1973 and 2012, according to stage and grade. |
Table V.
Association between surgery type and
survival outcome in patients <25 years of age with osteosarcoma
between 1973 and 2012, according to stage and grade.
|
| Stage
(n=1,915) | Grade
(n=1,439) |
|
|---|
|
|
|
|
|
|---|
| Variables | Localized | Regional | Distant | Well | Moderately | Poorly |
Undifferentiated | All (n=1977) |
|---|
| Number |
| No
surgery | 53 | 53 | 107 | 2 | 4 | 42 | 88 | 244 |
| Local
excision | 98 | 75 | 29 | 16 | 13 | 48 | 78 | 208 |
| Radical
excision | 396 | 504 | 163 | 25 | 53 | 229 | 492 | 1076 |
|
Amputation | 101 | 235 | 101 | 8 | 12 | 91 | 238 | 449 |
| Order of
outcome |
|
Best | 2 | 2 | 3 | 1 | 3 | 2 | 2 | 2 |
|
Relatively good | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 3 |
|
Relatively poor | 4 | 4 | 4 | 2 | 1 | 4 | 4 | 4 |
|
Worst | 1 | 1 | 1 | 4 | 4 | 1 | 1 | 1 |
| Significance |
| No
surgery vs. local excision | a0.031 | aP<0.001 | a0.011 | 0.622 | 0.523 | a0.005 | aP<0.001 | aP<0.001 |
| No
surgery vs. radical excision | a0.046 | aP<0.001 | aP<0.001 | 0.724 | 0.113 | aP<0.001 | aP<0.001 | aP<0.001 |
| No
surgery vs. amputation | 0.478 | aP<0.001 | a0.001 | 0.385 | 0.994 | a0.033 | aP<0.001 | aP<0.001 |
| Local
excision vs. radical excision | 0.469 | 0.557 | 0.202 | 0.505 | 0.393 | 0.682 | 0.576 | 0.462 |
| Local
excision vs. amputation | 0.092 | 0.243 | 0.589 | a0.037 | 0.358 | 0.165 | 0.087 | a0.001 |
| Radical
excision vs. amputation | 0.139 | 0.263 | a0.002 | a0.006 | a0.014 | 0.081 | a0.042 | aP<0.001 |
Multivariate Cox regression
analysis
In the univariate analysis, factors with significant
differences included year of diagnosis, sex, age at diagnosis,
CHSDA region, stage, grade, tumor size, histologic type, tumor
site, surgery, surgery type and radiation (P<0.05). The results
of the multivariate Cox regression analysis are indicated in
Table VI. Year of diagnosis, sex,
age at diagnosis, CHSDA region, stage, histologic type, tumor site,
surgery and radiation were independent risk factors in Model 1 of
the multivariate Cox regression analysis (P<0.05). In Model 2 of
the multivariate Cox regression analysis, independent risk factors
included year of diagnosis, sex, CHSDA region, stage, histologic
type, tumor site, surgery and radiation (P<0.05).
 | Table VI.Multivariate Cox regression analysis
in patients <25 years of age with osteosarcoma between 1973 and
2012. |
Table VI.
Multivariate Cox regression analysis
in patients <25 years of age with osteosarcoma between 1973 and
2012.
|
| Model 1 | Model 2 |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95%CI) | P-value |
|---|
| Year of
diagnosis |
| P<0.001 |
| aP<0.001 |
|
1973–1982 | Reference |
| Reference |
|
|
1983–1992 | 0.649
(0.500–0.841) | 0.001 | 0.657
(0.529–0.816) | aP<0.001 |
|
1993–2002 | 0.611
(0.484–0.771) | P<0.001 | 0.594
(0.491–0.719) | aP<0.001 |
|
2003–2012 | 0.528
(0.418–0.666) | P<0.001 | 0.507
(0.419–0.613) | aP<0.001 |
| Sex |
| 0.042 |
| aP<0.001 |
|
Male | Reference |
| Reference |
|
|
Female | 0.866
(0.754–0.995) | 0.042 | 0.805
(0.710–0.912) | aP<0.001 |
| Age at diagnosis
(years) |
| 0.042 |
| 0.081 |
|
0–4 | Reference |
| Reference |
|
|
5–9 | 0.859
(0.518–1.423) | 0.554 | 0.837
(0.52–1.349) | 0.466 |
|
10–14 | 0.726
(0.448–1.176) | 0.193 | 0.716
(0.454–1.129) | 0.151 |
|
15–19 | 0.909
(0.561–1.471) | 0.697 | 0.832
(0.528–1.311) | 0.428 |
|
20–24 | 0.920
(0.558–1.516) | 0.743 | 0.902
(0.566–1.440) | 0.667 |
| Race |
| 0.452 |
|
|
|
Caucasian | Reference |
|
|
|
| African
descent | 1.123
(0.935–1.348) | 0.214 |
|
|
|
Other | 1.043
(0.818–1.332) | 0.733 |
|
|
| CHSDA region |
| 0.003 |
| a0.035 |
|
East | Reference |
| Reference |
|
|
Northern Plains | 1.372
(1.101–1.711) | 0.005 | 1.190
(0.978–1.448) | 0.082 |
| Pacific
Coast | 1.164
(0.978–1.385) | 0.088 | 1.133
(0.971–1.323) | 0.114 |
|
Southwest | 1.515
(1.187–1.934) | 0.001 | 1.378
(1.108–1.715) | a0.004 |
| Rural or urban |
| 0.71 |
|
|
|
Rural | Reference |
|
|
|
|
Urban | 1.046
(0.824–1.328) | 0.71 |
|
|
| Stage |
| P<0.001 |
| aP<0.001 |
|
Localized | Reference |
| Reference |
|
|
Regional | 1.612
(1.360–1.911) | P<0.001 | 1.585
(1.360–1.848) | aP<0.001 |
|
Distant | 4.036
(3.357–4.853) | P<0.001 | 3.899
(3.292–4.616) | aP<0.001 |
| Laterality |
| 0.944 |
|
|
|
Right | Reference |
|
|
|
|
Left | 0.995
(0.873–1.135) | 0.944 |
|
|
| Histologic type
ICD-O-3 |
| P<0.001 |
| aP<0.001 |
|
Osteosarcoma, NOS | Reference |
| Reference |
|
|
Chondroblastic | 0.821
(0.669–1.008) | 0.060 | 0.869
(0.721–1.046) | 0.137 |
|
Fibroblastic | 0.669
(0.464–0.965) | 0.032 | 0.779
(0.567–1.070) | 0.124 |
|
Telangiectatic | 0.770
(0.538–1.102) | 0.154 | 0.782
(0.553–1.107) | 0.166 |
|
Parosteal | 0.365
(0.218–0.614) | P<0.001 | 0.354
(0.218–0.576) | aP<0.001 |
| Primary
site-labeled |
| P<0.001 |
| aP<0.001 |
| Upper
limb | Reference |
| Reference |
|
| Lower
limb | 0.737
(0.617–0.881) | 0.001 | 0.777
(0.654–0.923) | a0.004 |
| Skull
and mandible | 0.668
(0.316–1.495) | 0.344 | 0.689
(0.494–0.960) | a0.028 |
|
Vertebral and chest bones | 0.834
(0.487–1.429) | 0.509 | 0.982
(0.691–1.395) | 0.917 |
| Pelvic
bones | 1.625
(1.157–2.281) | 0.005 | 1.612
(1.210–2.147) | a0.001 |
| Surgery |
| P<0.001 |
| aP<0.001 |
|
Yes | Reference |
| Reference |
|
| No | 1.726
(1.434–2.077) | P<0.001 | 1.645
(1.391–1.946) | aP<0.001 |
| Radiation |
| P<0.001 |
| aP<0.001 |
|
Yes | Reference |
| Reference |
|
| No | 0.600
(0.461–0.781) | P<0.001 | 0.548
(0.442–0.679) | aP<0.001 |
Discussion
At the start of the present study, the distribution
characteristics for the incidence of osteosarcoma was indicated
according to age. The results demonstrated that patients <25
years of age with osteosarcoma had relatively good survival
outcomes, but also had the highest ratio (56.8%) and incidence (8.2
per million) among all age groups. The aforementioned result may be
due to certain characteristics of this age group, which remain
unknown. Therefore, this specific age group requires further study
in terms of incidence, metastasis, survival prognosis and treatment
options for osteosarcoma, according to the aforementioned 15
factors.
The lowest incidence rate of osteosarcoma and worst
survival outcomes were observed between 1973 and 1982, while the
subsequent 3 decades had the highest incidence rate and best
outcomes. Within these 3 decades, incidences and survival outcomes
minimally changed. The five-year survival rate was 50.1% prior to
1982 and >60% subsequent to this year. The increased incidence
of osteosarcoma, following 1982, may be due to the diagnostic
improvements for osteosarcoma. Numerous studies have also observed
that there was improvement in survival for patients diagnosed with
osteosarcoma subsequent to 1982, which according to the studies may
be due to the introduction of chemotherapeutic regimens (12,13).
Duffaud et al (14) reported
that localized high-grade osteosarcoma had a long-term disease-free
survival rate of <20% prior to the administration of intensive
chemotherapy and 55–75% subsequent to the introduction of the
aforementioned treatment. The patient-derived orthotopic xenograft
model, developed over the past 30 years, is a promising research
method for effective individualized therapy, which has been applied
to various types of cancer, including breast, ovarian, lung,
cervical, colon, stomach, pancreatic, melanoma, sarcoma, and
osteosarcoma (15). Using the
aforementioned model, Murakami et al (16) and Igarashi et al (17) indicated that the tumor-targeting
Salmonella typhimurium A1-R is a powerful treatment option and they
reported that it was able to regress osteosarcoma. The authors of
the aforementioned studies also used this model to screen drugs and
identify effective treatment drugs or drug combinations for
osteosarcoma (18,19).
Sex-associated differences revealed that male
patients had a higher incidence rate of osteosarcoma compared with
female patients, which was consistent even within the same race and
region (data not shown). The only exception was that female
patients 5–9 years of age had a higher incidence rate of
osteosarcoma compared with male patients. The aforementioned
results may be due to the active bone growth reported in males and
females (20). It has been reported
that males undergo more rapid bone growth compared with females
(21). However, females between the
ages of 11 and 13 have been reported to be taller and undergo rapid
bone growth compared with age-matched males (22). Numerous studies have reported that as
the height of an individual increases so does the risk of
osteosarcoma (20,23–25). A
higher incidence rate of osteosarcoma was additionally observed in
female patients between 0 and 14 years of age in a study by
Mirabello et al (2) and in
female patients between 10 and 14 years of age in a study by Homa
et al (26). Regarding
sex-associated differences in survival, numerous studies (27,28) have
reported that females have a longer life span compared with males;
results which confirm the present study's findings. Researchers
have attributed the aforementioned survival difference to the
relatively poor response reported in male patients to chemotherapy,
and their high recurrence rate (29,30). In
the present study it was additionally proposed that males may
exhibit symptoms in the long-term and therefore, do not participate
actively in treatment.
Regarding age, the highest incidence of osteosarcoma
was among those 10–19 years of age, followed by those between 20
and 24 years of age, which may be due to the rapid bone growth of
the aforementioned age groups. The optimal survival rate was
observed in patients between 10 and 14 years of age and between 5
and 9 years of age, whereas the worst survival rate was observed in
patients between 1 and 4 years of age. The survival results of the
present study were verified by other single-center studies
(31–33). Guillon et al (31) analyzed 15 patients <5 years of age
with osteosarcoma and reported a mortality rate of 45% (7 patients)
within 5 years of follow-up, suggesting that osteosarcoma is highly
invasive in patients <5 years of age. Worch et al
(32) reported that the five-year
survival rate of children ≤5 years of age and >5 years of age
was 51.9 and 67.3%, respectively. Sugalski et al (33) reported that the five-year survival
rate of children <12 years of age and >12 years of age was 11
and 57%, respectively. However, Hagleitner et al (34) reported an opposite trend, where the
5-year overall survival rate was 70.6±0.8, 52.5±1.1, 33.3±0.9% in
patients ≤14, 15–19 and 20–40 years of age, respectively. The
reason for the decrease in survival rate in young patients remains
unclear, however, the aforementioned findings suggest that tumors
in patients with different ages have different biological
characteristics. Furthermore, limb salvage surgery poses surgical
challenges for skeletally immature patients, as it may cause
leg-length inequality in the long-term (35). It has been reported that patients
<5 years of age undergo amputation at a higher rate, whereas
only a number of patients receive chemotherapy (32).
Miller et al (36) defined patients at the stage of
‘distant’ in the SEER database as metastatic, whereas patients at
the stage of ‘localized’ or ‘regional’ were defined as
non-metastatic. Miller et al (36), including other researchers, have
reported that patients with metastatic osteosarcoma had a
relatively poor prognosis compared with patients with localized
osteosarcoma (37,38). The present study compared the
aforementioned three subtypes of osteosarcoma and the differences
were reported as significant for all 15 factors in pairwise
comparisons and the overall comparison. This may be due to the fact
that the predominant factor associated to survival was metastasis
vs. non-metastasis. Lee (39)
reported that the event-free survival (EFS) rate of Korean children
and adolescents with osteosarcoma at 5 years following diagnosis
was 27.0 and 65.3% with and without metastasis, respectively.
Kantar et al (40) reported
that the 5-year EFS rates were 67 and 25% in patients with
non-metastatic and metastatic disease, respectively. The present
study demonstrated that the five-year survival rates in localized,
regional and distant stages were 77.5, 64.7 and 31.1%,
respectively.
Two factors, grade and tumor size, had incomplete
data and have been rarely analyzed in other studies. In the present
study, tumors with grades of well and moderate differentiation had
relatively good outcomes compared with those that were poorly
differentiated and undifferentiated in the univariate analysis,
possibly due to the fact that tumor differentiation reflects tumor
malignancy. Larger tumors were reported to have relatively poor
prognoses (41), which was confirmed
in the present study, where tumors >100 mm in size had
relatively poor outcomes compared with tumors <50 mm. Therefore,
larger tumor sizes may reflect a more advanced stage of tumor
development.
Miller et al (36) performed a histological analysis for
patients with osteosarcoma of all ages, among which Paget diseases
were reported to be more common in the elderly group (≥60 years of
age). However, in the present study only one case of Paget disease
presented in the younger group (<25 years of age). In addition,
small cell osteosarcoma was commonly associated with metastatic
disease in the aforementioned study, in contrast to the present
study. Nakajima et al (41)
reviewed 72 cases with small cell osteosarcoma, concluding that
this subtype of osteosarcoma is highly aggressive and less
responsive to chemotherapy. The present study revealed two
histologic types, parosteal and periosteal osteosarcoma, which were
not associated with metastatic diseases. Parosteal osteosarcoma had
relatively good survival outcomes compared with any other type of
tumor, in accordance with the study of Mankin et al
(42). Bacci et al (43) reported that fibroblastic and
telangiectatic tumors had significantly higher 5-year overall
survival rates, whereas chondroblastic and osteoblastic tumors had
significantly lower 5-year overall survival rates. The effect of
histologic type on metastasis and survival may be determined by
biological characteristics of the tumors, however, further
investigation is required.
The results of the present study indicated that the
long bones of the four limbs were predilection sites for
osteosarcoma. Lee (39) further
reported that the most frequently affected site in children and
adolescents was the distal femur (52.3%). In the present study,
extremity osteosarcoma had low metastasis and relatively good
outcome, while axial skeletal osteosarcoma had the highest
metastasis and worst outcome, confirming previous observations by
Janinis et al (44), who
reported that extremity tumors had a 2- and 3-year survival rate of
50 and 21%, respectively, and axial skeletal tumors had a 2- and
3-year survival rate of 19 and 13%, respectively. Meazza et
al (45) studied 20 patients
between the ages of 3 and 19 with axial skeletal osteosarcoma and
reported a 5-year overall survival rate as low as 40%. Among 129
cases of osteosarcomas, Akyuz et al (46) observed 6 cases of axial skeletal
osteosarcomas, in which mortality occurred in 5 cases between three
and sixteen months subsequent to diagnosis, indicating a poor
prognosis. In the present study, the recorded sites were divided
into 5 types to provide detailed information of tumor site and
prognosis. According to the analysis, the tumor types ranked from
best to worst survival outcome followed skull and mandible, lower
limb, upper limb, vertebral and chest bones, and pelvic bones. An
explanation for the aforementioned results is that axial skeletal
osteosarcomas in vertebral, chest and pelvic bones may in close
proximity to important organs, vessels or nerves, therefore, making
complete resection difficult and possibly contributing to poor
survival.
The type of surgery was analyzed in detail in the
present study. Previous meta-analyses (47,48) have
reported that limb-salvage surgery confers better survival compared
with amputation. Schrager et al (7) additionally reported the same trend in
children and adolescents. Once modern prosthetics became available
in the 1970s, there was an increase in limb-salvage surgeries
performed, which corresponded to survival outcomes in comparison to
amputation (7). It has been reported
that limb-salvage surgery is the optimal choice in 85% of children
patients with osteosarcoma (49,50).
Limb-salvage surgery may provide better outcomes compared with
amputation, as amputation can cause psychological and functional
impairment in young patients (51,52).
Furthermore, the present study compared survival outcomes among
different types of surgery of the same stage or grade to exclude
confounding factors and provide reliable results, as favorable
outcomes may be due to mild disease rather than type of surgery.
According to the order of best to worst survival outcome reported
in the present study, surgery provided better outcomes compared
with non-surgical treatment approaches, while excision provided
better outcomes compared with amputation. Therefore, although
removing the entire tumor may be the ideal choice for the treatment
of osteosarcoma, excessive excision negatively affects body
recovery. An interesting finding in the present study was that
patients with metastatic diseases had a better survival rate when
radical excision was chosen as a treatment option for osteosarcoma,
possibly due to the fact that metastatic tumors tend to be larger
and require to be thoroughly removed. Therefore, radical excision
should be recommended for patients with metastatic diseases. Picci
et al (53) identified
inadequate margins in surgery as a risk factor for poor prognosis,
confirming to an extent the results of the present study.
The results of the present study initially indicated
poor outcomes in patients who underwent radiation, however, further
analysis indicated that the aforementioned result was not entirely
valid. A total of 11.2% patients with metastatic diseases had
undergone radiotherapy, whereas 3.9% of patients with
non-metastatic diseases had undergone radiotherapy (data not
shown). Therefore, a higher number of patients with metastatic
diseases had received radiotherapy, indicating that the observed
negative effect of radiotherapy was because patients with
metastatic diseases had relatively poor overall prognosis.
The systematic analysis of the present study may
provide useful information for guiding clinical work. Males
patients between 10 and 19 years of age had a high incidence rate
of osteosarcoma, suggesting the requirement for an early screening
of this aforementioned high-risk population for osteosarcoma. Chest
and pelvic bones were at high risk of metastasis, therefore,
metastatic lesions should be checked among high-risk patients. Year
of diagnosis, sex, CHSDA region, stage, histologic type, tumor
site, surgery and radiation were demonstrated in the present study
to be independent risk factors in the multivariate Cox regression
analysis. In order to improve survival rate, local excision can be
used in the majority of patients with osteosarcoma, and radical
excision is suggested for patients with metastatic diseases.
However, the present study presents certain
limitations. Firstly, information regarding chemotherapy treatment
is not available in the SEER database, therefore extracting
information associated with the type of drugs used for chemotherapy
treatments was not possible. Secondly, no detailed record of the
type of surgery was available, therefore, determining for example
if patients had undergone joint replacement was not possible.
Thirdly, the present study was retrospective rather than a
randomized controlled trial, therefore, whether to perform a
limb-salvage surgery or an amputation depended on the doctors'
suggestion and the patient's requirement rather than random
assignment. Finally, there were no therapy records of biologic
markers and no records of tumor recurrence. Despite the
aforementioned limitations, the present study incorporated a
relatively large number of osteosarcoma cases from the SEER
database, increasing the accuracy of present study's results.
Acknowledgements
The authors thank the National Natural Science
Foundation of China (Beijing, China). The authors also thank the
National Cancer Institute (Bethesda, USA), who provided access to
the public SEER database.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant, no., 81672154; Beijing,
China).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Surveillance, Epidemiology, and
End Results (SEER) repository, https://seer.cancer.gov/.
Authors' contributions
ZN acquired data, analyzed data and wrote the
manuscript. HP acquired data, designed the study, revised the
manuscript and gave final approval of the version to be published.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethics approval and consent to participate are not
needed as this study is based on already existing data from the
National Cancer Institute. The present study was approved by the
National Cancer Institute.
Patient's consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma, and ewing's sarcoma: National cancer
data base report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anfinsen KP, Devesa SS, Bray F, Troisi R,
Jonasdottir TJ, Bruland OS and Grotmol T: Age-period-cohort
analysis of primary bone cancer incidence rates in the United
States (1976–2005). Cancer Epidemiol Biomarkers Prev. 20:1770–1777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duong LM and Richardson LC: Descriptive
epidemiology of malignant primary osteosarcoma using
population-based registries, United States, 1999–2008. J Registry
Manag. 40:59–64. 2013.PubMed/NCBI
|
|
5
|
Tsuda Y, Ogura K, Shinoda Y, Kobayashi H,
Tanaka S and Kawai A: The outcomes and prognostic factors in
patients with osteosarcoma according to age: A Japanese nationwide
study with focusing on the age differences. BMC Cancer. 18:6142018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Yang J, Wang Y, Wang D, Han G, Jia
J, Xu M and Bi W: Survival and prognostic factors in Chinese
patients with osteosarcoma: 13-year experience in 365 patients
treated at a single institution. Pathol Res Pract. 213:119–125.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schrager J, Patzer RE, Mink PJ, Ward KC
and Goodman M: Survival outcomes of pediatric osteosarcoma and
Ewing's sarcoma: A comparison of surgery type within the SEER
database, 1988–2007. J Registry Manag. 38:153–161. 2011.PubMed/NCBI
|
|
8
|
Perkins SM, Shinohara ET, DeWees T and
Frangoul H: Outcome for children with metastatic solid tumors over
the last four decades. PLoS One. 9:e1003962014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gatta G, Capocaccia R, Coleman MP, Ries LA
and Berrino F: Childhood cancer survival in europe and the united
states. Cancer. 95:1767–1772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novakovic B: U.S. childhood cancer
survival, 1973–1987. Med Pediatr Oncol. 23:480–486. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duchman KR, Gao Y and Miller BJ:
Prognostic factors for survival in patients with high-grade
osteosarcoma using the surveillance, epidemiology, and end results
(SEER) program database. Cancer Epidemiol. 39:593–599. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster L, Dall GF, Reid R, Wallace WH and
Porter DE: Twentieth-century survival from osteosarcoma in
childhood. Trends from 1933 to 2004. J Bone Joint Surg Br.
89:1234–1238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duffaud F, Digue L, Mercier C, Dales JP,
Baciuchka-Palmaro M, Volot F, Thomas P and Favre R: Recurrences
following primary osteosarcoma in adolescents and adults previously
treated with chemotherapy. Eur J Cancer. 39:2050–2057. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaguchi K, Igarashi K, Li S, Han Q, Tan
Y, Miyake K, Kiyuna T, Miyake M, Murakami T, Chmielowski B, et al:
Recombinant methioninase (rMETase) is an effective therapeutic for
BRAF-V600E-negative as well as-positive melanoma in patient-derived
orthotopic xenograft (PDOX) mouse models. Oncotarget. 9:915–923.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami T, Igarashi K, Kawaguchi K,
Kiyuna T, Zhang Y, Zhao M, Hiroshima Y, Nelson SD, Dry SM, Li Y, et
al: Tumor-targeting Salmonella typhimurium A1-R regresses an
osteosarcoma in a patient-derived xenograft model resistant to a
molecular-targeting drug. Oncotarget. 8:8035–8042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Igarashi K, Kawaguchi K, Murakami T,
Kiyuna T, Miyake K, Nelson SD, Dry SM, Li Y, Yanagawa J, Russell
TA, et al: Intra-arterial administration of tumor-targeting
Salmonella typhimurium A1-R regresses a cisplatin-resistant
relapsed osteosarcoma in a patient-derived orthotopic xenograft
(PDOX) mouse model. Cell Cycle. 16:1164–1170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Igarashi K, Murakami T, Kawaguchi K,
Kiyuna T, Miyake K, Zhang Y, Nelson SD, Dry SM, Li Y, Yanagawa J,
et al: A patient-derived orthotopic xenograft (PDOX) mouse model of
a cisplatinum-resistant osteosarcoma lung metastasis that was
sensitive to temozolomide and trabectedin: Implications for
precision oncology. Oncotarget. 8:62111–62119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Igarashi K, Kawaguchi K, Kiyuna T, Miyake
K, Miyake M, Li Y, Nelson SD, Dry SM, Singh AS, Elliott IA, et al:
Temozolomide combined with irinotecan regresses a
cisplatinum-resistant relapsed osteosarcoma in a patient-derived
orthotopic xenograft (PDOX) precision-oncology mouse model.
Oncotarget. 9:7774–7781. 2017.PubMed/NCBI
|
|
20
|
Mirabello L, Pfeiffer R, Murphy G, Daw NC,
Patino-Garcia A, Troisi RJ, Hoover RN, Douglass C, Schuz J, Craft
AW and Savage SA: Height at diagnosis and birth-weight as risk
factors for osteosarcoma. Cancer Causes Control. 22:899–908. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arora RS, Alston RD, Eden TO, Geraci M and
Birch JM: The contrasting age-incidence patterns of bone tumours in
teenagers and young adults: Implications for aetiology. Int J
Cancer. 131:1678–1685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greil H and Kahl H: Assessment of
developmental age: Cross-sectional analysis of secondary sexual
characteristics. Anthropol Anz. 63:63–75. 2005.PubMed/NCBI
|
|
23
|
Longhi A, Pasini A, Cicognani A, Baronio
F, Pellacani A, Baldini N and Bacci G: Height as a risk factor for
osteosarcoma. J Pediatr Hematol Oncol. 27:314–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gelberg KH, Fitzgerald EF, Hwang S and
Dubrow R: Growth and development and other risk factors for
osteosarcoma in children and young adults. Int J Epidemiol.
26:272–278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Endicott AA, Morimoto LM, Kline CN,
Wiemels JL, Metayer C and Walsh KM: Perinatal factors associated
with clinical presentation of osteosarcoma in children and
adolescents. Pediatr Blood Cancer. 64:2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Homa DM, Sowers MR and Schwartz AG:
Incidence and survival rates of children and young adults with
osteogenic sarcoma. Cancer. 67:2219–2223. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petrilli AS, Gentil FC, Epelman S, Lopes
LF, Bianchi A, Lopes A, Figueiredo MT, Marques E, De Bellis N and
Consentino E: Increased survival, limb preservation, and prognostic
factors for osteosarcoma. Cancer. 68:733–737. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smeland S, Muller C, Alvegard TA, Wiklund
T, Wiebe T, Bjork O, Stenwig AE, Willen H, Holmstrom T, Folleras G,
et al: Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII:
prognostic factors for outcome and the role of replacement salvage
chemotherapy for poor histological responders. Eur J Cancer.
39:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saeter G, Elomaa I, Wahlqvist Y, Alvegard
TA, Wiebe T, Monge O, Forrestier E and Solheim OP: Prognostic
factors in bone sarcomas. Acta Orthop Scand Suppl. 273:156–160.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guillon MA, Mary PM, Brugiere L,
Marec-Berard P, Pacquement HD, Schmitt C, Guinebretiere JM and
Tabone MD: Clinical characteristics and prognosis of osteosarcoma
in young children: A retrospective series of 15 cases. BMC Cancer.
11:4072011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Worch J, Matthay KK, Neuhaus J, Goldsby R
and DuBois SG: Osteosarcoma in children 5 years of age or younger
at initial diagnosis. Pediatr Blood Cancer. 55:285–289. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sugalski AJ, Jiwani A, Ketchum NS, Cornell
J, Williams R, Heim-Hall J, Hung JY and Langevin AM:
Characterization of localized osteosarcoma of the extremity in
children, adolescents, and young adults from a single institution
in south texas. J Pediatr Hematol Oncol. 36:e353–e358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hagleitner MM, Hoogerbrugge PM, van der
Graaf WT, Flucke U, Schreuder HW and te Loo DM: Age as prognostic
factor in patients with osteosarcoma. Bone. 49:1173–1177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neel MD, Wilkins RM, Rao BN and Kelly CM:
Early multicenter experience with a noninvasive expandable
prosthesis. Clin Orthop Relat Res. 1–81. 2003.
|
|
36
|
Miller BJ, Cram P, Lynch CF and Buckwalter
JA: Risk factors for metastatic disease at presentation with
osteosarcoma: An analysis of the SEER database. J Bone Joint Surg
Am. 95:e892013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jawad MU, Cheung MC, Clarke J, Koniaris LG
and Scully SP: Osteosarcoma: Improvement in survival limited to
high-grade patients only. J Cancer Res Clin Oncol. 137:597–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JA: Osteosarcoma in Korean children
and adolescents. Korean J Pediatr. 58:123–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kantar M, Cetingul N, Azarsiz S, Kansoy S,
Sabah D, Memis A, Basdemir G and Burak Z: Treatment results of
osteosarcoma of the extremity in children and adolescents at ege
university hospital. Pediatr Hematol Oncol. 19:475–482. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakajima H, Sim FH, Bond JR and Unni KK:
Small cell osteosarcoma of bone. Review of 72 cases. Cancer.
79:2095–2106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mankin HJ, Hornicek FJ, Rosenberg AE,
Harmon DC and Gebhardt MC: Survival data for 648 patients with
osteosarcoma treated at one institution. Clin Orthop Relat Res.
1–291. 2004.PubMed/NCBI
|
|
43
|
Bacci G, Bertoni F, Longhi A, Ferrari S,
Forni C, Biagini R, Bacchini P, Donati D, Manfrini M, Bernini G and
Lari S: Neoadjuvant chemotherapy for high-grade central
osteosarcoma of the extremity. Histologic response to preoperative
chemotherapy correlates with histologic subtype of the tumor.
Cancer. 97:3068–3075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Janinis J, McTiernan A, Driver D, Mitchell
C, Cassoni AM, Pringle J, Kilby A and Whelan JS: London Bone and
Soft Tissue Tumour Service: A pilot study of short-course intensive
multiagent chemotherapy in metastatic and axial skeletal
osteosarcoma. Ann Oncol. 13:1935–1944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meazza C, Luksch R, Daolio P, Podda M,
Luzzati A, Gronchi A, Parafioriti A, Gandola L, Collini P, Ferrari
A, et al: Axial skeletal osteosarcoma: A 25-year monoinstitutional
experience in children and adolescents. Med Oncol. 31:8752014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akyuz C, Ilhan I, Kutluk T and
Buyukpamukcu M: Primary osteosarcoma presenting in axial bones in
childhood. Turk J Pediatr. 37:375–381. 1995.PubMed/NCBI
|
|
47
|
Han G, Bi WZ, Xu M, Jia JP and Wang Y:
Amputation versus limb-salvage surgery in patients with
osteosarcoma: A meta-analysis. World J Surg. 40:2016–2027. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li X, Zhang Y, Wan S, Li H, Li D, Xia J,
Yuan Z, Ren M, Yu S, Li S, et al: A comparative study between
limb-salvage and amputation for treating osteosarcoma. J Bone
Oncol. 5:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grimer RJ: Surgical options for children
with osteosarcoma. Lancet Oncol. 6:85–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wafa H and Grimer RJ: Surgical options and
outcomes in bone sarcoma. Expert Rev Anticancer Ther. 6:239–248.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Postma A, Kingma A, De Ruiter JH, Koops
Schraffordt H, Veth RP, Goeken LN and Kamps WA: Quality of life in
bone tumor patients comparing limb salvage and amputation of the
lower extremity. J Surg Oncol. 51:47–51. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aksnes LH, Bauer HC, Jebsen NL, Folleras
G, Allert C, Haugen GS and Hall KS: Limb-sparing surgery preserves
more function than amputation: A Scandinavian sarcoma group study
of 118 patients. J Bone Joint Surg Br. 90:786–794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Picci P, Sangiorgi L, Bahamonde L, Aluigi
P, Bibiloni J, Zavatta M, Mercuri M, Briccoli A and Campanacci M:
Risk factors for local recurrences after limb-salvage surgery for
high-grade osteosarcoma of the extremities. Ann Oncol. 8:899–903.
1997. View Article : Google Scholar : PubMed/NCBI
|