Introduction
Esophageal squamous cell carcinoma (ESCC) is the
principal pathological subtype of thoracic esophageal cancer
reported in Asia in over the previous 30 years (1–3). Although
comprehensive therapeutic strategies, including surgery, radiation
therapy and chemotherapy, have been applied, the overall survival
(OS) rate of ESCC remains poor (4).
It has been widely recognized that lymph node metastasis (LNM)
usually occurs in the early stages of disease, and serves an
important role in the poor prognosis of patients with ESCC
(5).
Previous studies have published the distribution
pattern of LNM and investigated the influence of the surgical
removal of the LN on the prognosis of ESCC (6,7). The
American Joint Committee on the Tumor Node Metastasis (TNM) system
defined the N-staging classification using the number of positive
LN (PLN) collected during surgery (8). However, the number of negative LN (NLN)
identified pathologically following surgery has been considered to
be an even stronger predictor of OS rate in patients with ESCC
(9). The reason may be that the
number of NLN could reflect the extent and quality of the
individual surgery, and be considered a critical factor in the
prognosis of patients with ESCC. To the best of our knowledge, it
is unclear which LN station containing the NLN served a key role in
the prognosis of patients with ESCC; therefore, improving the
presently available knowledge would be helpful for the design of
treatment plans for postoperative radiotherapy. Research had proven
that radiotherapy serves a key role in decreasing the probability
of local tumor recurrence following surgery, and in prolonging OS
rate; however, there was an issue, in that the design of treatment
plans for radiotherapy was based on the metastasis pattern of PLN,
without considering the effect of NLN on the prognosis of patients
(10,11). In other words, it was necessary to
specify which LN station containing the NLN was associated with OS
rate in patients with ESCC. Therefore, the present study aimed to
investigate in which LN station the presence of NLN had a great
impact on OS rate, for patients with middle and lower thoracic
ESCC.
Patients and methods
Patient population
A retrospective review was performed on the medical
records of 216 patients with ESCC, who underwent esophagectomy and
lymphadenectomy at Linyi People's Hospital, Shandong University
(Linyi, China) between January 2009 and January 2013. The
Institutional Review Board of Linyi People's Hospital approved the
present study and all patients provided written informed consent.
The criterion for patient selection were as follows: i) The patient
received upper gastrointestinal endoscopy and upper
gastrointestinal barium swallow, and the site of primary tumor was
confirmed in the thoracic esophagus; ii) an ultrasound of the
cervical region, computed tomography (CT) of chest and upper
abdomen were performed to exclude distant metastasis of tumor; iii)
the patient did not receive neo-adjuvant radiotherapy or
chemotherapy prior to surgery; iv) the surgical specimen of the
patient was identified as ESCC by two pathologists in the
Department of Pathology in Linyi People's Hospital following
surgery, and discussed uncertain samples until they reached an
agreement; v) Tumor-free resection margin of the removed tissue
were confirmed following surgery by a pathologist.
Treatment method
All patients underwent an extended esophagectomy
with three-field or two-field LN dissection. Patients who underwent
three-field LN dissection with tumor metastasis in the cervical
region were considered based on an ultrasound or CT examination.
The three-field LN dissections consisted of a collar neck incision,
and a right thoracotomy and laparotomy, compared with the two-field
LN dissection (including only a right thoracotomy and laparotomy).
The dissection and labeling of LN stations was performed according
to the system of the Japanese Society for Esophageal Diseases
(12), as presented in Table I. The surgical specimens and LN marked
with site labels were collected at the end of surgery, and were
evaluated by two specialist pathologists from the Department of
Pathology in Linyi People's Hospital (Shangdong, China). All the
specimens and LN were fixed in 10% formalin for 24 h under room
temperature and embedded in paraffin, and then subjected to 0.5%
concentration of hematoxylin and 2% concentration of eosin staining
at room temperature for 0.5 h after sectioning (5-µm thick slices).
Metastasis in the LN was identified following a consensus from the
two pathologists using a light microscope with magnification ×200,
and the numbers of PLN and NLN in each LN station were recorded.
The pathological tumor stage of each case was evaluated according
to the American Joint Committee on Cancer (AJCC) TNM classification
(7th edition) (8). The post-operative
treatment was selected according to the TNM stage of the tumor in
each patient, and patients with an identical TNM stage received the
same post-operative treatment. In another word, for example,
patients with stage III of tumor were treated by chemo- and
radiotherapy.
 | Table I.Terminology of the regional lymph
nodes in esophageal cancer. |
Table I.
Terminology of the regional lymph
nodes in esophageal cancer.
| LN station no. | JSED (location) |
|---|
| 100 | Cervical
compartment |
| 101 | Paraesophageal
nodes |
| 102 | Deep cervical
nodes |
| 103 | Retropharyngeal lymph
nodes |
| 104 | Supraclavicular lymph
nodes |
| 105 | Upper thoracic
paraesophageal |
| 106 | Thoracic paratracheal
lymph nodes |
| 107 | Bifurcational |
| 108 | Middle thoracic
paraesophageal |
| 109 | Main bronchus (R,
L) |
| 110 | Lower thoracic
paraesophageal |
| 111 | Diaphragmatic lymph
nodes |
| 1 | Right cardiac
nodes |
| 2 | Left cardiac
nodes |
| 3 | Nodes along the
lesser curvature |
| 4 | Nodes along the
greater curvature |
| 5 | Suprapyloric
nodes |
| 6 | Infrapyloric
nodes |
| 7 | Left gastric
artery |
Follow-up
All patients underwent follow-up every 3 months for
the first 2 years following surgical resection, and then every 6
months for the following 3 years. Upper gastrointestinal barium
swallow, and CT or magnetic resonance imaging of the chest and
upper abdomen, were used to evaluate the local recurrence and
remote metastasis of the tumors. Patients who could not return to
Linyi People's Hospital regularly were followed up by telephone.
The final follow-up was conducted in January 2016. OS rate was
evaluated as the time between surgery and patient mortality. The
time of censoring was calculated from the date of surgery to the
date of our last contact with the surviving patient.
Classification method of sub
groups
Patients were initially divided into two groups,
which included patients with PLN identified in the LN station 108,
and patients with NLN identified in the LN station 108. Secondly,
the patients were further divided into four groups, according to
the status of the LN in the LN station 108, which can be described
as follows: Group 1, patients whose PLN and NLN were identified in
LN station 108 following surgery; Group 2, patients whose PLN were
identified in LN station 108 following surgery; Group 3, patients
whose NLN were identified in the LN station 108 following surgery;
and Group 4, patients whose LN were not identified in the LN
station 108 following surgery.
Ratio of albumin to globulin (AGR) and
ratio of lymphocytes to neutrophils (LNR)
Information regarding albumin, globulin, lymphocytes
and neutrophils in the blood was collected from the medical records
of each patient. Four time points were selected to collect the
information at follow-up: The first time point was one week prior
to surgery (AGR1 and LNR1); the second was one week after surgery
(AGR2 and LNR2); the third was two weeks after surgery (AGR3 and
LNR3); and the fourth was one month after surgery (AGR4 and LNR4).
The ratio of AGR was calculated as the quantity of albumin divided
by the quantity of globulin in the blood. Accordingly, the ratio of
LNR was calculated using the number of lymphocytes divided by the
number of neutrophils in the blood. The AGR and LNR at the four
time points previously mentioned were calculated and used for
subsequent analysis between patient subgroups. The differences in
the ratios were investigated among the four groups prior to and
following surgery.
Statistical analysis
The data in the present study are presented as the
mean ± standard deviation. The relationship between clinical
parameters and the number of PLN or NLN were explored using the
χ2 test and a forward stepwise Cox regression model
analysis was further used to evaluate the associations between the
above parameters and prognosis. Survival analysis was performed
using Kaplan-Meier method and the differences of survival time
between patient subgroups were investigated by log rank test. All
the statistical analyses were computed using Stata/MP 13 (Stata
Corp LP, College Station, TX, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics and OS
rate
The number of censored case in this study was 24
(11.9%), which was lower than the average level in clinical study
(13). Excluding the patients who
failed to attend for follow-up (24 cases), the medical information
of the remaining 192 patients who completed follow-up was
retrospectively reviewed. The characteristics of patients are shown
in Table II. LNM were identified in
100 of the 192 patients, and the mean number of PLN was 0.874 (SD,
1.046; range, 1–5; median, 1) while the mean number of NLN was
3.168 (SD, 1.319; range, 1–7; median, 3). In the group of patients
without LNM, the mean number of NLN was 3.195 (SD, 1.297; range,
1–6; median, 3). The mean follow-up time was 27.2 months (SD, 18.8;
range, 3–73 months; median, 24 months). On the basis of the
analysis for the entire cohort (192 patients), the 1, 3 and 5-year
survival rates were 72.9, 38.9 and 23.8%, respectively.
 | Table II.Clinical and pathological variables
of the patients with pathologically confirmed positive lymph
node. |
Table II.
Clinical and pathological variables
of the patients with pathologically confirmed positive lymph
node.
|
Characteristics | Total number of
LN | Number of positive
LN | P-value | Number of negative
LN | P-value |
|---|
| Age (years) |
|
<60 | 970 | 117 | 0.630 | 853 | 0.893 |
|
≥60 | 1742 | 198 |
| 1544 |
|
| Sex |
|
Men | 2447 | 294 | 0.075 | 2153 | 0.627 |
|
Women | 265 | 21 |
| 244 |
|
| Length of tumor
(cm) |
| ≤4 | 1201 | 94 | <0.001 | 1107 | 0.429 |
|
4–6 | 775 | 118 |
| 657 |
|
| ≥6 | 736 | 95 |
| 641 |
|
| Tumor site |
|
Upper | 92 | 2 | <0.001 | 90 | 0.463 |
|
Middle | 1653 | 165 |
| 1488 |
|
|
Lower | 967 | 148 |
| 819 |
|
|
Differentiation |
|
Well | 778 | 47 | <0.001 | 731 | 0.419 |
|
Moderate | 1569 | 210 |
| 1359 |
|
|
Poor | 356 | 47 |
| 309 |
|
| Depth of tumor
invasion |
|
T1-T2 | 2091 | 35 | <0.001 | 2056 | 0.347 |
|
T3-T4 | 621 | 47 |
| 574 |
|
| Nerve invasion |
|
Yes | 119 | 31 | <0.001 | 88 | 0.195 |
| No | 2593 | 284 |
| 2309 |
|
| Lymph vessel
invasion |
|
Yes | 164 | 52 | <0.001 | 112 | 0.030 |
| No | 2548 | 263 |
| 2285 |
|
| LN dissention |
|
Two-field | 1389 | 178 | 0.006 | 1211 | 0.461 |
|
Three-field | 1323 | 121 |
| 1202 |
|
| Postoperative
radiochemotherapy |
|
Yes | 1246 | 156 | 0.228 | 1090 | 0.736 |
| No | 1466 | 159 |
| 1307 |
|
Association between the LN station
containing the NLN and patient survival
In the univariate analysis of the association
between patient clinicopathological features and the LN status, the
tumor length, tumor differentiation, depth of tumor invasion, tumor
site, nerve invasion and lymph vessel invasion were all risk
factors for the number of PLN, while only lymph vessel invasion was
identified as a risk factor for the number of NLN (Table II). All possible risk factors were
subsequently put into a stepwise Cox analysis model, and 108p (the
PLN number in LN station 108) and 109p (the PLN number in LN
station 109) were confirmed as independent prognostic factors for
OS rate (Table III). Conversely,
108n (the NLN number in LN station 108) was highlighted as a
protective factor for OS rate. In the analysis of the association
between 108n and tumor relapse, the former was proven to have
statistical effect against the latter (Table IV).
 | Table III.Association between the total number
of positive and negative of LN and overall survival. |
Table III.
Association between the total number
of positive and negative of LN and overall survival.
| Factor | Hazard ratio | Standard error | Z coefficient | P-value | [95% conf. | Interval] |
|---|
| 108P | 2.978 | 0.710 | 4.58 | <0.001 | 1.866 | 4.750 |
| 108N | 0.475 | 0.102 | −3.47 | 0.001 | 0.312 | 0.723 |
| 108P+105P | 5.606 | 4.226 | 2.29 | 0.022 | 1.279 | 24.563 |
| 105P | 14.165 | 11.427 | 3.29 | 0.001 | 2.914 | 68.848 |
| 109P | 10.756 | 11.545 | 2.21 | 0.027 | 1.312 | 88.163 |
| 7P | 1.771 | 0.436 | 2.32 | 0.020 | 1.094 | 2.868 |
| Diff | 1.322 | 0.199 | 1.86 | 0.064 | 0.984 | 1.776 |
 | Table IV.Association between the total number
of positive and negative of LN and tumor relapse. |
Table IV.
Association between the total number
of positive and negative of LN and tumor relapse.
| Factor | Hazard ratio | Standard error | Z coefficient | P-value | [95% conf. | Interval] |
|---|
| 108P | 2.506 | 0.777 | 2.96 | 0.003 | 1.364 | 4.602 |
| 105P | 9.768 | 11.002 | 2.02 | 0.043 | 1.074 | 88.826 |
| 109P | 13.539 | 14.754 | 2.39 | 0.017 | 1.599 | 114.601 |
| 108N | 0.501 | 0.139 | −2.48 | 0.013 | 0.290 | 0.864 |
| 107N | 0.546 | 0.139 | −2.37 | 0.018 | 0.331 | 0.900 |
| Diff | 1.559 | 0.312 | 2.21 | 0.027 | 1.052 | 2.309 |
LN status of LN station 108 and OS
rate of patients
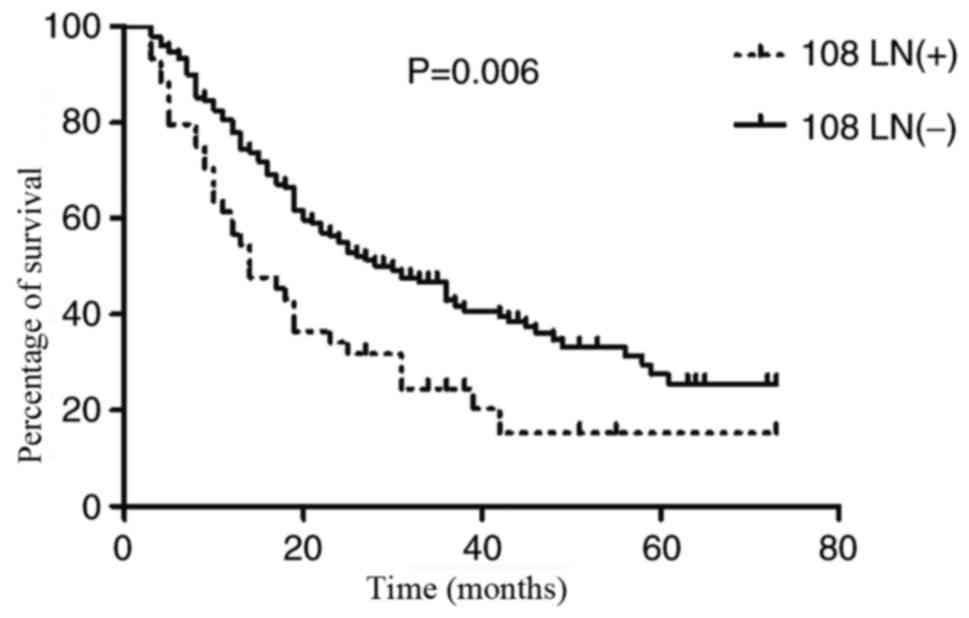
The results of log-rank tests revealed that the
subgroup of patients with NLN identified in LN station 108 had an
improved OS rate than those with PLN identified at LN station 108
(P=0.006). The mean OS times of the two sub-groups were 30.0 months
(SD, 18.197; range, 3–73 months; median, 28 months) and 27.436
months (SD, 19.129; range, 3–73 months; median, 24 months). The 1-,
3- and 5-year survival rates for the patients with NLN identified
in LN station 108 were 80.4, 46.9 and 27.5%, respectively, while
the rates for the patients with PLN identified in LN station 108
were 62.5, 27.0 and 21.2%, respectively (Fig. 1).
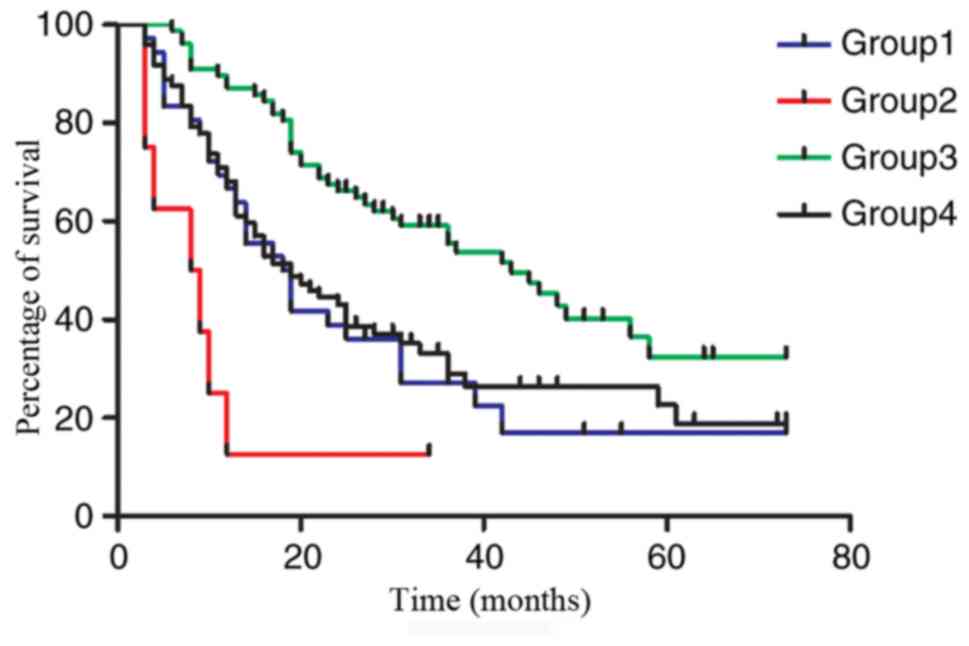
Further comparative analysis confirmed the presence
of significant differences in OS rate among the four groups. Group
3 had the best outcome with a mean OS time of 34.208 months when
compared with the other groups (Group 3 vs. Group 1: P<0.001;
Group 3 vs. Group 2: P<0.001; Group 3 vs. Group 4: P=0.001).
Group 2 had the worst outcome with a mean OS time of 10.375 months
of any group (Group 2 vs. Group 1: P=0.018; Group 2 vs. Group 3:
P<0.001; Group 2 vs. Group 4: P=0.007.). However, there was no
significant difference between Group 4 and Group 1 of which the OS
times were 23.847 and 22.389 months, respectively (P=0.667;
Fig. 2). Group 2 was the group of
patients with only PLN identified in LN station 108 after surgery
and was used as the control.
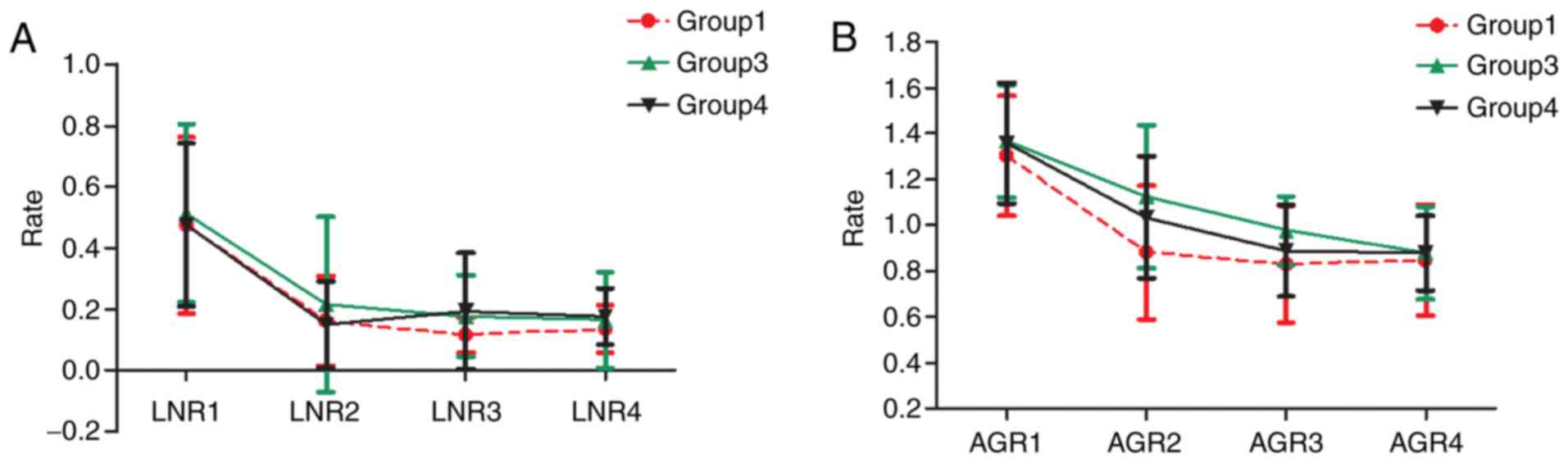
Change in AGR and LNR in the subgroups
following surgery
The AGR and LNR of patients decreased following
surgery in all except Group 2, for which insufficient information
regarding the AGR and LNR was obtained (data not shown). At the
initial diagnosis of the disease, the number of AGR was similar
amongst the three groups. However, following the operation, the
number of AGR in Group 3 was higher than that of any other group
until three months later. Similarly, the number of LNR were similar
to one another amongst the three groups prior to the operation,
however, the number of LNR in Groups 3 was higher compared with the
other two groups until two months later (Fig. 3).
Discussion
Previous studies have demonstrated that a greater
number of NLN identified during surgery indicated an improved OS
rate (9,14). The underlying mechanism of the
important role of NLN in prognosis remains uncertain. Interpreting
this has led to two possible hypotheses (15,16).
The first hypothesis is ‘stage migration’. The
extent of lymphadenectomy is insufficient if only PLN are removed
in ESCC. The N stage of a tumor can be precisely evaluated only
when sufficient NLN and PLN are removed via lymphadenectomy;
otherwise, late-stage ESCC may be erroneously classified as
early-stage ESCC. The second hypothesis is that the collection of
more NLN during surgery may decrease the false-negative error rate
in the pathological examination of LN. Researchers have previously
demonstrated that tumor metastasis may be present in NLN, as
determined by immunohistochemical staining methods, which may
explain the aforementioned false-negative error rate (17,18).
Increased identification of NLN by a pathologist may decrease the
possibility of an incorrect evaluation of the N stage of a
tumor.
Previous studies have stated that the site of LNM is
a more important prognostic factor than the number of LNM, and it
was necessary to clarify which LN station containing the NLN would
most significantly influence the outcome of patients (19,20).
The present study identified that the presence of
NLN in LN station 108 could significantly influence the outcomes of
patients, on the basis of multivariate Cox regression analysis and
survival analysis. Furthermore, patients had a better prognosis if
only NLN were identified in LN station 108, and these patients were
primarily in the early N stage, which supports the ‘stage
migration’ theory. The present study also identified that the LN
stations 107 and 7 were high-efficiency LN stations containing NLN
in patients with ESCC at an early N stage, which was in accordance
with our previously published results (21).
As the LN station 108 was categorically removed in
the lymphadenectomy, regardless of whether three-field or two-field
LN dissection was performed, and was used to assess the prognosis
of patients and/or the extent of lymphadenectomy. The present study
demonstrated that patients with only NLN identified in LN station
108 had an improved prognosis when compared with the other
subgroups, indicating it should be seriously considered whether
postoperative radiation therapy or chemotherapy treatment is
appropriate for this group of patients. On the other hand, since
the extent of lymphadenectomy remains controversial at present, it
was worth exploring whether this group of patients could benefit
from surgery wherein more LNs are removed via lymphadenectomy
(22,23).
Although the mechanism underlying the prognostic
role of NLN in LN station 108 is complex, it could be interpreted
from interactions between the patient immune system and tumor
metastasis. LN station 108 is the closest LN station to the
esophagus, and may be the first station, or the foothold, for tumor
metastasis (12). Patients with only
NLN identified in LN station 108 may be in a stable condition due
to immune system resistance to tumor metastasis. Dynamic
investigation of the change in AGR and LNR in the present study
supported this assumption. AGR and LNR were higher in patients with
NLN identified in LN station 108 than those with PLN identified in
LN station 108, and the AGR was significantly higher in patients
with only NLN identified in LN station 108 compared with those in
the other groups. This was consistent with prior reports that AGR
and LNR have a close association with the prognosis of patients
(24–26). Based on the results of the present
study, the AGR may be a more reliable predictor of outcomes
inpatients, compared with the LNR. The reason for this may be that
AGR may provide a true reflection of the condition of a patient
that the AGR provides the combined information of immune and
nutritional situation of patients (27,28).
According to previous studies, AGR was considered to be closely
associated with the patient immune system, and may be used as an
indicator of the immune condition of patients in the future
(29,30). Based on the results of other reports,
the change in LNR could reflect the T-cell-dependent immune
response to a tumor (31,32). However, one previous study produced
the opposite results (33). The
reason for this might be that LNR is less stable than AGR, which
was confirmed in the present study. Based on the aforementioned
results the LN status of LN station 108 has the potential to be
used as an indicator for the selection of postoperative treatment.
Chemo-radiotherapy would not be recommended for patients with only
NLN identified in LN station 108, compared with the recommended
treatment based on the tumor stage.
In the univariate analysis performed in the present
study, the length of the tumor, tumor differentiation, depth of
tumor invasion, tumor site, nerve invasion and lymph vessel
invasion were risk factors for tumor metastasis, which was
consistent with previous results (34). However, only the parameter of lymph
vessel invasion influenced the number of NLN, which supported the
interpretation, that tumor metastasis usually occurs through the
lymph vessels in ESCC. Multivariate analysis revealed that the
presence of negative LN in LN station 108 served a key role in
tumor relapse and patient outcome, which supported the survival
analysis results of the present study. The 1-, 3- and 5-year
survival rates of patients were similar to those in reports
published previously, and therefore, the selection bias of patients
could be ignored in this study (35).
There were several limitations to this study. First,
this study was not an example of large-scale research; however, the
inclusion criterion was strict and the pathological type was
limited to the thoracic squamous cell carcinoma in order to exclude
confounders. Furthermore, the statistical results were
cross-validated using several methods to avoid statistical bias.
The present study has been completed according to the research plan
supervised by the Linyi People's Hospital ethics committee (Linyi,
China); however, another larger-scale study is currently being
conducted. Additionally, the results of the present study could be
repeated based on the preliminary analysis (data not shown), and
due to the size limitation of the cohort, it was impossible to
further investigate the influence of NLN on prognosis in the
sub-groups classified by T stage or the type of LN dissection. As
the number of patients with only PLN identified in the LN station
108 was small, classified as group 2 in this study, it was
difficult to dynamically explore the LNR and AGR change patterns in
this group. Secondly, the inclusion criteria of the patients did
not set a threshold on the LN number removed in the surgery in this
study. However, in the present study, the majority of cases
experienced systematic lymphectomy. Furthermore, results without an
artificial threshold for the LN number are more valuable for
popularized application in the future. Thirdly, the site of 108 LN
station was adjacent to the 105 LN station in anatomy, and it was
even difficult for experienced surgeons to differentiate where the
swollen and syncretic positive LN where located in some cases
(36). To combat this, the present
study labeled the positive LN in this situation as 108P+105P.
To conclude, the results of this study demonstrated
that the presence of NLN in LN station 108 could be used as a
predictor of prognosis; it may also be used as an indicator to
recommend the extent of LN dissection. A prospective study is
required to explore whether the presence of NLN in LN station 108
may be used as an indicator for the selection of post-operative
treatment, compared with the present method instructed by the TNM
system, which neglected the reported impact of negative LN on the
prognosis of patients. Although the present study preliminarily
identified the potential underlying mechanisms and the key role of
NLN in LN station 108 in prognosis, further research is still
required in order to explore and validate these mechanisms. Our
further test program includes two additional studies. The first one
aims to demonstrate that the negative LN inside thoracic cavity
serves an important role in the prognosis of patients using a
larger scale of patients (which at the time of publication is
ongoing, data not shown). The second study aims to explore whether
the status of LN in station 108 serves a key role in patients with
ESCC in a perspective study, and whether it may be used a marker to
indicate the extent and efficacy of lymphadenectomy.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shandong
Provincial Medical and Health Development Plan (grant no.
2013WSA13018, Dr Jinling Zhang), the Natural Science Foundation of
Shandong Province (grant no. ZR2013HL018 and ZR2014HL062, Dr
Jinling Zhang).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL, FC, YL, JZ and HZ designed the study. XH and LL
collected the information of patients and analyzed the data. JZ and
HZ wrote the paper. FC, YL and BL reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All procedures used in the present study involving
human participants were in accordance with the ethical standards of
the institutional research committee, and with the 1964 Helsinki
declaration and its later amendment or comparable ethical
standards. The present study was approved by the Ethics Review
Board of Linyi People's Hospital (Shangdong, China) and written
informed consent was collected from each patient.
Patient consent for publication
Informed consent was obtained from all participants
included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li B, Chen H, Xiang J, Zhang Y, Kong Y,
Garfield DH and Li H: Prevalence of lymph node metastases in
superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc
Surg. 146:1198–1203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merkow RP, Bilimoria KY, Keswani RN, Chung
J, Sherman KL, Knab LM, Posner MC and Bentrem DJ: Treatment trends,
risk of lymph node metastasis, and outcomes for localized
esophageal cancer. J Natl Cancer Inst. 106:pii: dju1332014.
View Article : Google Scholar
|
|
3
|
McCormack VA, Menya D, Munishi MO,
Dzamalala C, Gasmelseed N, Roux Leon M, Assefa M, Osano O, Watts M,
Mwasamwaja AO, et al: Informing etiologic research priorities for
squamous cell esophageal cancer in Africa: A review of
setting-specific exposures to known and putative risk factors. Int
J Cancer. 140:259–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du D, Song T, Liang X, Fang M and Wu S:
Concurrent chemoradiotherapy with elective lymph node irradiation
for esophageal cancer: A systemic review and pooled analysis of the
literature. Dis Esophagus. 30:1–9. 2017.
|
|
5
|
Liu J, Liu Q, Wang Y, Xia Z and Zhao G:
Nodal skip metastasis is associated with a relatively poor
prognosis in thoracic esophageal squamous cell carcinoma. Eur J
Surg Oncol. 42:1202–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dubecz A, Kern M, Solymosi N, Schweigert M
and Stein HJ: Predictors of lymph node metastasis in surgically
resected T1 esophageal cancer. Ann Thorac Surg. 99:1879–1886. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren X, Zhao Z, Huang W, Liu H, Dong C and
Li Y: Analysis of the characteristics and factors influencing lymph
node metastasis in thoracic esophageal carcinoma and cancer of the
gastric cardia. Hepatogastroenterology. 62:73–76. 2015.PubMed/NCBI
|
|
8
|
Zhang D, Zheng Y, Wang Z, Huang Q, Cao X,
Wang F and Liu S: Comparison of the 7th and proposed 8th editions
of the AJCC/UICC TNM staging system for esophageal squamous cell
carcinoma underwent radical surgery. Eur J Surg Oncol.
43:1949–1955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baba Y, Watanabe M, Shigaki H, Iwagami S,
Ishimoto T, Iwatsuki M and Baba H: Negative lymph-node count is
associated with survival in patients with resected esophageal
squamous cell carcinoma. Surgery. 153:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KH, Chang JS, Cha JH, Lee IJ, Kim DJ,
Cho BC, Park KR and Lee CG: Optimal adjuvant treatment for
curatively resected thoracic esophageal squamous cell carcinoma: A
radiotherapy perspective. Cancer Res Treat. 49:168–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Wang Z, Yang Z, Liu Y, Liu X,
Shang B and Jiang WP: Postoperative radiotherapy improves survival
in stage pT2N0M0 esophageal squamous cell carcinoma with high risk
of poor prognosis. Ann Surg Oncol. 23:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajiyama Y: New Japanese classification of
esophageal cancer (11th Edition). Gan To Kagaku Ryoho.
43:1049–1052. 2016.(In Japanese). PubMed/NCBI
|
|
13
|
Cheng J, Kong L, Huang W, Li B, Li H, Wang
Z, Zhang J, Zhou T and Sun H: Explore the radiotherapeutic clinical
target volume delineation for thoracic esophageal squamous cell
carcinoma from the pattern of lymphatic metastases. J Thorac Oncol.
8:359–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenstein AJ, Litle VR, Swanson SJ,
Divino CM, Packer S and Wisnivesky JP: Effect of the number of
lymph nodes sampled on postoperative survival of lymph
node-negative esophageal cancer. Cancer. 112:1239–1246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Z, Chen H, Yu W, Fu X, Xiang J, Li H,
Zhang Y, Sun M, Wei Q, Zhao W and Zhao K: Number of negative lymph
nodes is associated with survival in thoracic esophageal squamous
cell carcinoma patients undergoing three-field lymphadenectomy. Ann
Surg Oncol. 21:2857–2863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu PK, Huang CS, Wang BY, Wu YC, Chou TY
and Hsu WH: The prognostic value of the number of negative lymph
nodes in esophageal cancer patients after transthoracic resection.
Ann Thorac Surg. 96:995–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luketich JD, Kassis ES, Shriver SP, Nguyen
NT, Schauer PR, Weigel TL, Yousem SA and Siegfried JM: Detection of
micrometastases in histologically negative lymph nodes in
esophageal cancer. Ann Thorac Surg. 66:1715–1718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komukai S, Nishimaki T, Watanabe H, Ajioka
Y, Suzuki T and Hatakeyama K: Significance of immunohistochemically
demonstrated micrometastases to lymph nodes in esophageal cancer
with histologically negative nodes. Surgery. 127:40–46. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Meer SG, Dauwan M, de Keizer B, Valk
GD, Rinkes Borel IH and Vriens MR: Not the number but the location
of lymph nodes matters for recurrence rate and disease-free
survival in patients with differentiated thyroid cancer. World J
Surg. 36:1262–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka H, Ohira M, Kubo N, Muguruma K,
Yamashita Y, Sawada T and Hirakawa K: Association of location of
lymph node metastases with postoperative recurrence of esophageal
squamous cell carcinoma. Anticancer Res. 32:3421–3426.
2012.PubMed/NCBI
|
|
21
|
Yang HX, Xu Y, Fu JH, Wang JY, Lin P and
Rong TH: An evaluation of the number of lymph nodes examined and
survival for node-negative esophageal carcinoma: Data from China.
Ann Surg Oncol. 17:1901–1911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lagergren J, Mattsson F, Zylstra J, Chang
F, Gossage J, Mason R, Lagergren P and Davies A: Extent of
lymphadenectomy and prognosis after esophageal cancer surgery. JAMA
Surg. 151:32–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Schaaf M, Johar A, Wijnhoven B,
Lagergren P and Lagergren J: Extent of lymph node removal during
esophageal cancer surgery and survival. J Natl Cancer Inst.
107:pii: djv0432015.
|
|
24
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharaiha RZ, Halazun KJ, Mirza F, Port JL,
Lee PC, Neugut AI, Altorki NK and Abrams JA: Elevated preoperative
neutrophil: Lymphocyte ratio as a predictor of postoperative
disease recurrence in esophageal cancer. Ann Surg Oncol.
18:3362–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang F, Sun P, Wang ZQ, de Wang S, Wang
Y, Zhang DS, Wang FH, Fu JH, Xu RH and Li YH: Low preoperative
albumin-globulin score predicts favorable survival in esophageal
squamous cell carcinoma. Oncotarget. 7:30550–30560. 2016.PubMed/NCBI
|
|
27
|
Fukushima H, Kobayashi M, Kawano K and
Morimoto S: Prognostic value of albumin/globulin ratio in patients
with upper tract urothelial carcinoma patients treated with radical
nephroureterectomy. Anticancer Res. 38:2329–2334. 2018.PubMed/NCBI
|
|
28
|
He J, Pan H, Liang W, Xiao D, Chen X, Guo
M and He J: Prognostic effect of Albumin-to-Globulin ratio in
patients with solid tumors: A systematic review and meta-analysis.
J Cancer. 8:4002–4010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suh B, Park S, Shin DW, Yun JM, Keam B,
Yang HK, Ahn E, Lee H, Park JH and Cho B: Low albumin-to-globulin
ratio associated with cancer incidence and mortality in generally
healthy adults. Ann Oncol. 25:2260–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Iseki Y, Ikeya T, Sugano K and Hirakawa K: The pretreatment
albumin to globulin ratio predicts chemotherapeutic outcomes in
patients with unresectable metastatic colorectal cancer. BMC
Cancer. 15:3472015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji WH, Jiang YH, Ji YL, Li B and Mao WM:
Prechemotherapy neutrophil : lymphocyte ratio is superior to the
platelet : Lymphocyte ratio as a prognostic indicator for locally
advanced esophageal squamous cell cancer treated with neoadjuvant
chemotherapy. Dis Esophagus. 29:403–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yutong H, Xiaoli X, Shumei L, Shan S, Di L
and Baoen S: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with esophageal cancer in a high
incidence area in China. Arch Med Res. 46:557–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie X, Luo KJ, Hu Y, Wang JY and Chen J:
Prognostic value of preoperative platelet-lymphocyte and
neutrophil-lymphocyte ratio in patients undergoing surgery for
esophageal squamous cell cancer. Dis Esophagus. 29:79–85. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsuda S, Tsubosa Y, Niihara M, Sato H,
Takebayashi K, Kawamorita K, Mori K, Tsushima T, Yasui H, Takeuchi
H and Kitagawa Y: Distribution of lymph node metastasis and
clinical validity of gastric tube reconstruction in lower thoracic
esophageal squamous cell carcinoma with gastric invasion. Ann Surg
Oncol. 22:617–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilke TJ, Bhirud AR and Lin C: A review of
the impact of preoperative chemoradiotherapy on outcome and
postoperative complications in esophageal cancer patients. Am J
Clin Oncol. 38:415–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuasa Y, Seike J, Yoshida T, Takechi H,
Yamai H, Yamamoto Y, Furukita Y, Goto M, Minato T, Nishino T, et
al: Sentinel lymph node biopsy using intraoperative indocyanine
green fluorescence imaging navigated with preoperative CT
lymphography for superficial esophageal cancer. Ann Surg Oncol.
19:486–493. 2012. View Article : Google Scholar : PubMed/NCBI
|