Introduction
Liver cancer, including hepatocellular carcinoma
(HCC) and intrahepatic cholangiocarcinoma, is one of the common
malignant tumors of the digestive tract in China, with mortality
rate as high as 42.5%, second only to esophageal and gastric
cancer. The most important risk factors for liver cancer are
hepatitis B virus and alcoholism. According to the results of an
epidemiological survey, the increased content of organic chlorides
and DDT in drinking water and aflatoxin in food are also major
factors inducing liver cancer (1). It
has also been reported that the occurrence of liver cancer may be
related to the difference in gene expression levels in patients, in
which the abnormal activation of Wnt/β-catenin signaling pathway
and expression deletion of the phosphatase and tensin homolog
deleted on chromosome ten (PTEN) and other tumor suppressor genes
can mediate the occurrence of liver cancer (2,3). PTEN,
discovered in 1997, is a tumor suppressor gene with
dual-specificity phosphatase activity located on chromosome 10,
which can dephosphorylate downstream phosphatidylinositol
3,4,5-trisphosphate in normal liver tissues, thus blocking the
downstream phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) signaling pathway, and negatively regulating the growth
of liver cells. In case of deletion or mutation of PTEN, however,
its negative regulatory effect on downstream pathway will disappear
or decline, and the normal regulatory mechanism for cell growth or
metastasis will be lost, thereby inducing the occurrence or
malignant transformation of tumors (4,5). At
present, it has been confirmed that the abnormal expression of PTEN
is associated with the occurrence and development of a variety of
malignant tumors, such as glioma, endometrial, liver and prostate
cancers (6,7). In this study, the expression of PTEN in
normal liver cells and liver cancer cells was retrospectively
analyzed to investigate the possible associations of PTEN with the
incidence of liver cancer, liver function grading and liver
function.
Patients and methods
General patient data
A total of 63 patients diagnosed with primary liver
cancer via preoperative puncture or postoperative pathological
diagnosis in Xiangya Hospital were enrolled, including 37 males and
26 females aged 30–82 years with an average of 48.5±8.6 years. The
hospitalization duration was 2.4–6.3 years with an average of
4.6±2.7 years. All patients underwent surgical resection after
preoperative chemotherapy. No abdominal visceral metastasis and
lymph node metastasis were found, and there were no other tumors,
except liver cancer, during operation. The study was approved by
the Ethics Committee of Xiangya Hospital, Central South University
(Changsha, China). Signed informed consents were obtained from the
patients or the guardians
Research methods
All patients enrolled in this study were treated
with preoperative chemotherapy using cisplatin (CDDP) once per
month (3–4 times as one course of treatment). After 2–3 courses of
chemotherapy, the patients that satisfied the surgical indications
received surgical resection. After operation, the specimens were
fixed in 40% formalin at 25°C for 24 h, collected, dehydrated, and
prepared into 3-µM sections, followed by routine hematoxylin and
eosin (H&E) staining and immunohistochemical staining. The
positive control was set. The tissue was blocked with 10% FBS at
20°C for 10 min, then incubated with rabbit anti-human PTEN
monoclonal antibody (cat. no. 9559, 1:50; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C for 12 h, and washed
with PBS for 10 min, 3 times. The tissue was then incubated with
secondary antibody SignalStain® Boost IHC Detection
Reagent (HRP, rabbit) (cat. no. 8114, 1:500; Cell Signaling
Technology, Inc.) at 20°C for 1 h. Serum tumor markers, blood
biochemical factors and inflammatory factors in patients were
detected via chemiluminescence. Fasting venous blood was drawn from
patients at 1 week before and after chemotherapy, and the
supernatant was taken after centrifugation at 6,000 × g at 4°C for
15 min for on-machine detection. Before chemotherapy, the liver
function of patients was scored according to the Child-Pugh
grading: grade A, 5–6 points; grade B, 7–9 points; and grade C,
10–15 points.
Observation indexes
Interpretation of results: PTEN displayed
brown-yellow particles in immunohistochemistry, and the expression
of PTEN in liver cancer cells was observed under high-power field
by a light microscope (×100): PTEN++, percentage of
positive cells >50%; PTEN+, percentage of positive
cells >10–50%; and PTEN−, percentage of positive
cells <10%. In this study, the percentage of positive cells
>10% indicted positive PTEN expression.
Statistical methods
GraphPad 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used for the statistical analysis of data.
t-test was used for measurement data and Chi-square test for
enumeration data. One-way analysis of variance was used for
multiple comparisons and Tukey's test was the post hoc test used.
P<0.05 suggested that the difference was statistically
significant.
Results
Expression of PTEN in normal liver
tissues and liver cancer tissues
In the 63 specimens of this study, the positive
expression rate of PTEN was 95.23% in normal liver tissues, but
only 46.03% in liver cancer tissues, suggesting that the positive
expression rate of PTEN in liver cancer tissues was significantly
decreased, and the difference was statistically significant
(P<0.05) (Table I).
 | Table I.Comparison of PTEN protein expression
in normal liver tissues and liver cancer tissues. |
Table I.
Comparison of PTEN protein expression
in normal liver tissues and liver cancer tissues.
|
| PTEN [n (%)] |
|---|
| Type of tissue | − | + |
|---|
| Normal liver tissues
(n=63) | 3 (4.76) | 60 (95.23) |
| Liver cancer tissues
(n=63) | 34 (53.97) | 29 (46.03) |
| χ2 |
| 34.43 |
| P-value |
| 0.0001 |
Comparison of serum tumor marker
levels between the two groups of patients
After chemotherapy, liver cancer patients were
divided into PTEN-positive and PTEN-negative groups according to
the expression of PTEN. There was no statistically significant
difference in the levels of serum tumor markers between the two
groups of patients after chemotherapy (P>0.05), but the level of
α-fetoprotein (AFP) in PTEN-positive group was obviously lower than
that in PTEN-negative group (P<0.05) (Table II).
 | Table II.Comparison of serum tumor marker
levels with PTEN expression before operation (mean ± SD). |
Table II.
Comparison of serum tumor marker
levels with PTEN expression before operation (mean ± SD).
| Serum tumor
marker | PTEN (−) | PTEN (+) |
|---|
| AFP (ng/ml) | 167.4±45.6 |
110.5±32.1a |
| CA125 (U/ml) | 143.5±25.7 | 139.7±13.6 |
| CEA (ng/ml) | 31.4±6.7 | 34.5±3.2 |
| CA199 (U/ml) | 51.3±9.7 | 48.6±4.8 |
| CA153 (U/ml) | 45.6±8.9 | 50.6±5.7 |
Comparison of changes in liver
function of patients before and after chemotherapy
After chemotherapy, the expression levels of serum
alanine transaminase (ALT) and aspartate transaminase (AST) in
patients were slightly increased, which might be related to
side-effects of chemotherapy drugs on the liver. The results were
not statistically different from those before chemotherapy. After
chemotherapy, the levels of albumin (ALB), alkaline phosphatase
(ALP) and prothrombin activity (PTA) in patients were obviously
decreased, and there were statistically significant differences
compared with those before chemotherapy (P<0.05) (Table III).
 | Table III.Comparison of changes in the liver
function of patients before and after chemotherapy (mean ± SD). |
Table III.
Comparison of changes in the liver
function of patients before and after chemotherapy (mean ± SD).
| Serum biochemical
index of liver | Before
chemotherapy | After
chemotherapy |
|---|
| ALT (U/l) | 387.5±103.4 | 413.6±110.7 |
| AST (U/l) | 396.4±98.4 | 428.7±104.3 |
| ALB (U/l) | 38.5±7.3 | 23.5±6.2a |
| ALP (g/l) | 237.8±84.8 |
165.4±57.1a |
| PTA (%) | 134.9±23.4 |
83.5±18.6a |
Association between PTEN expression
and liver function classification
There were varying degrees of changes in the liver
function of patients with liver cancer, among which the PTEN
positive rate was 78.57% in patients in grade A, 46.43% in patients
in grade B and 23.8% in patients in grade C. With the increase of
Child-Pugh grading of patients with liver cancer, the positive
expression rate of PTEN in patients was decreased, displaying a
negative association between them (Table
IV).
 | Table IV.Association between PTEN expression
and liver function classification. |
Table IV.
Association between PTEN expression
and liver function classification.
| Child-Pugh
grading | Positive PTEN
expression [n (%)] | n |
|---|
| A | 11 (78.57) | 14 |
| B | 13
(46.43)a | 28 |
| C | 5 (23.8)a | 21 |
Comparison of serum inflammatory factors in patients
with liver cancer before and after chemotherapy. Detection results
of serum biochemical indexes in patients at 1 week before and after
chemotherapy manifested that the levels of inflammatory factors in
peripheral blood of patients after chemotherapy were remarkably
lower than those before chemotherapy, and differences were
statistically significant (P<0.05 or P<0.001) (Table V).
 | Table V.Comparison of serum inflammatory
factors in patients with liver cancer before and after chemotherapy
(mean ± SD). |
Table V.
Comparison of serum inflammatory
factors in patients with liver cancer before and after chemotherapy
(mean ± SD).
| Inflammatory
factor | Before
chemotherapy | After
chemotherapy |
|---|
| IL-1 (ng/l) | 5.46±0.23 |
3.27±0.19b |
| IL-6 (ng/l) | 183.47±29.36 |
26.83±9.26b |
| IL-2 (µg/ml) | 217.45±76.21 |
165.43±91.3a |
| IL-12 (pg/ml) | 256.56±98.34 |
182.17±86.47a |
| IL-10 (ng/ml) | 274.65±79.28 |
173.54±69.7b |
| MIF (ng/ml) | 25.42±6.95 |
10.32±4.31b |
Association between PTEN expression
and liver cancer tissues
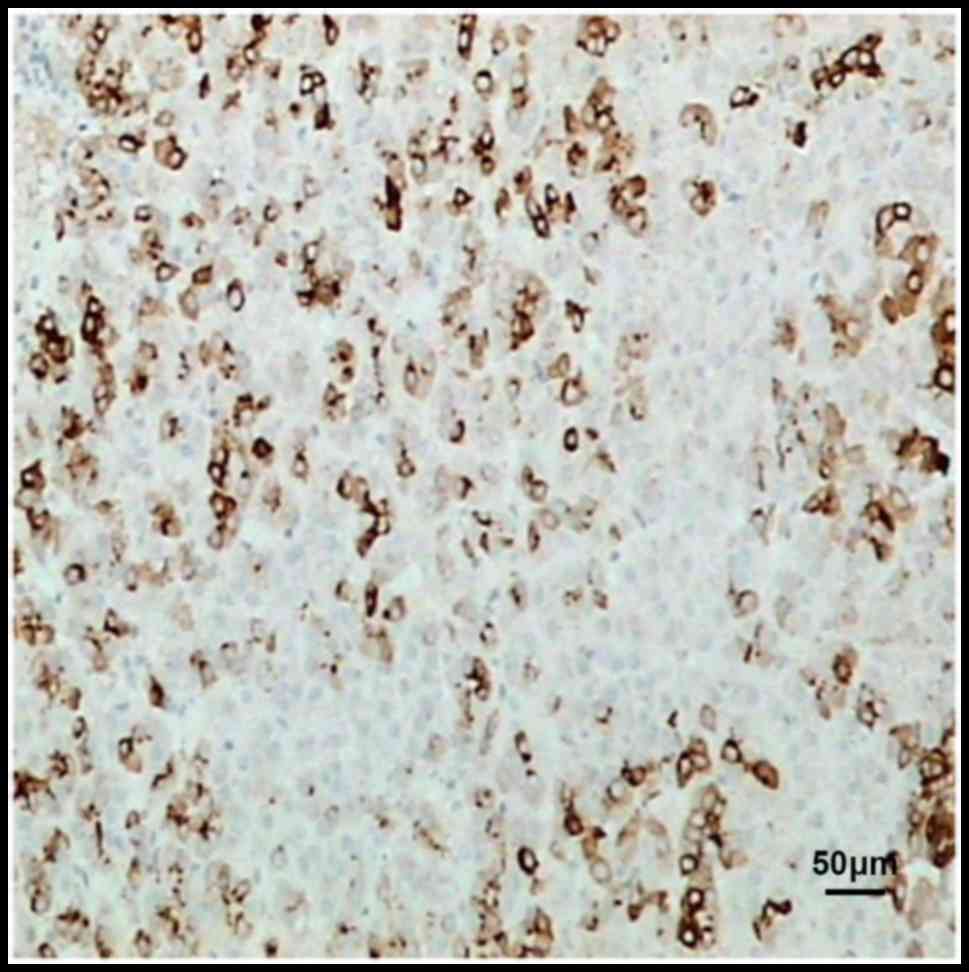
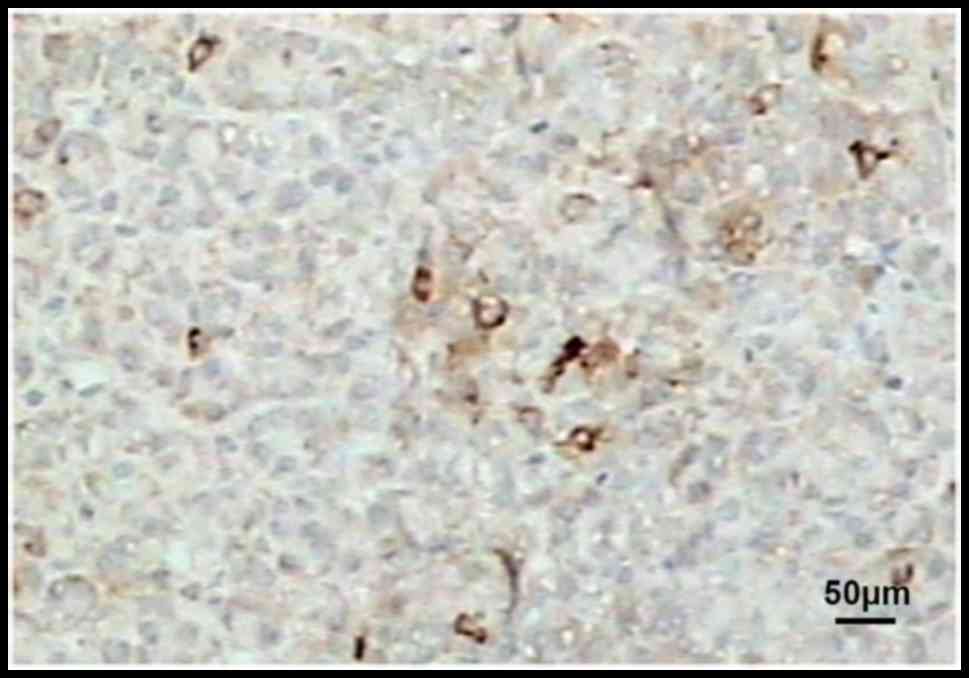
Immunohistochemical results revealed that PTEN was
mainly localized in the cytoplasm, and also slightly in the
nucleus. Compared with those in normal liver tissues, the
expression of PTEN in liver cancer tissues before and after
chemotherapy was obviously reduced (Figs.
1–3).
Discussion
PTEN is a tumor suppressor gene with
dual-specificity phosphatase activity, which is involved in the
growth, proliferation, adhesion, migration, differentiation and
apoptosis of normal cells and plays important roles in these
processes (8,9). Therefore, the role of PTEN gene deletion
and mutation in tumorigenesis has attracted the attention of many
researchers. At present, a certain progress has been made in the
research on the phosphorylase activity and substrate of PTEN in
tumor cells and the role of PTEN in signal transduction pathway in
various tumor cells (10). Through
the in vitro nerve cell experiment, Weng et al have
confirmed that PTEN leads to the decline in the activation and
phosphorylation of glycogen synthase kinase 3 (GSK3) through the
PI3K/AKT signaling pathway. Due to the decreased phosphorylation of
GSK3 for downstream cyclin D1, more cyclin D1 is accumulated in
cells, thus arresting cells in G1 phase (11). According to results of research on
glioblastoma, PTEN can inhibit cell growth and migration via
regulating focal adhesion kinase (FAK) and p130cas
tyrosine phosphorylation (12,13). In
breast cancer cells, PTEN, through affecting the insulin receptor
IRS-1 phosphorylation and the formation of IRS-1/Grb2/SOS complex,
blocking Gab1 migration, and other pathways, can inhibit
mitogen-activated protein kinase (MAPK) activity, and reduce the
positive regulatory effects of MAPK signaling pathway on cell
growth, proliferation and differentiation (14). It has also been reported that PTEN
plays a role in prostate cancer, in which PTEN can increase the
sensitivity of prostate cancer cells to apoptosis receptor- and
drug-mediated Fas-associated protein with death domain
(FADD)-dependent apoptosis signaling pathway, making a breakthrough
in the treatment of prostate cancer (15). Therefore, PTEN gene has a close
association with the occurrence and development of tumors. It has
been reported that the positive expression rate of PTEN protein in
HCC tissues is markedly lower than that in para-carcinoma normal
tissues, and even not expressed in cancer tissues (16). The expression of PTEN is negatively
associated to HCC pathological grading and the presence of cancer
thrombus. The positive expression rates of PTEN in patients in
Child-Pugh grade C and B are significantly lower than that in
patients in grade A, and HCC patients with low or negative PTEN
protein expression are often accompanied by the elevated level of
AFP and the metastasis of cancer cells. However, there are no
definite reports on the relationships of the positive expression
rate of PTEN with HCC size, serum AFP level and pathological typing
(17). The results of this study
manifested that the expression of PTEN in liver cancer tissues was
mainly located in the cytoplasm, and slightly in the nucleus, which
are consistent with the results previously reported in the
literature (18–20). Compared with that in normal liver
tissues, the expression of PTEN in HCC was remarkably decreased,
and the difference was statistically significant (P<0.05). No
significant differences were found in the expression levels of
serum tumor markers, except AFP, in HCC patients with negative PTEN
expression compared with those in HCC patients with positive PTEN
expression, indicating that PTEN expression basically has no effect
on the levels of serum tumor markers in HCC, but the expression of
AFP is significantly increased in HCC patients with negative PTEN
expression. Whether PTEN is related to the expression level of AFP
and the way in which it affects the expression of AFP remain to be
further investigated. Moreover, there was no significant change in
blood biochemical indexes in patients after chemotherapy. The
levels of ALT and AST in HCC patients were slightly increased after
chemotherapy, which was possibly due to the side-effects of
chemotherapy drugs on the liver. The expression levels of ALB, ALP
and PTA and inflammatory factors (IL-1 and IL-2) were obviously
decreased after chemotherapy, and there were statistically
significant differences compared with those before chemotherapy
(P<0.05). The expression of PTEN was negatively associated with
the Child-Pugh grading of liver function, which was obviously lower
in HCC patients in grade B and C than that in patients in grade A,
indicating that the expression of PTEN has a certain effect on the
liver function of HCC patients. In conclusion, the association of
the expression of PTEN with the liver function classification,
serum tumor markers and liver function of HCC patients is described
above, providing experimental data for predicting the clinical
progression and prognosis of HCC. PTEN can serve as a potential
gene in the biologically targeted therapy of liver cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Hunan Natural
Sciences Foundation Key Program (no. 2017JJ3504).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and XL drafted the manuscript. They also recorded
and analyzed the expression of PTEN and the liver function. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xiangya Hospital, Central South University (Changsha, China).
Signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gravitz L: Liver cancer. Nature.
516:S12014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibata T, Chuma M, Kokubu A, Sakamoto M
and Hirohashi S: EBP50, a beta-catenin-associating protein,
enhances Wnt signaling and is over-expressed in hepatocellular
carcinoma. Hepatology. 38:178–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alioui A, Dufour J, Leoni V, Loregger A,
Moeton M, Iuliano L, Zerbinati C, Septier A, Val P, Fouache A, et
al: Liver X receptors constrain tumor development and metastasis
dissemination in PTEN-deficient prostate cancer. Nat Commun.
8:4452017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo JH, Ahn Y, Lee SR, Yeo Yeol C and Hur
Chung K: The major target of the endogenously generated reactive
oxygen species in response to insulin stimulation is phosphatase
and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase)
in the PI-3 kinase/Akt pathway. Mol Biol Cell. 16:348–357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furnari FB, Lin H, Huang HS and Cavenee
WK: Growth suppression of glioma cells by PTEN requires a
functional phosphatase catalytic domain. Proc Natl Acad Sci USA.
94:12479–12484. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dahia PL: PTEN, a unique tumor suppressor
gene. Endocr Relat Cancer. 7:115–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steck PA, Pershouse MA, Jasser SA, Yung
WKA, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shearn CT and Petersen DR: Understanding
the tumor suppressor PTEN in chronic alcoholism and hepatocellular
carcinoma. Adv Exp Med Biol. 815:173–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu DD, Zhang XR and Cao XR: Expression and
significance of new tumor suppressor gene PTEN in primary liver
cancer. J Cell Mol Med. 7:67–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weng L, Brown J and Eng C: PTEN induces
apoptosis and cell cycle arrest through
phosphoinositol-3-kinase/Akt-dependent and -independent pathways.
Hum Mol Genet. 10:237–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamura M, Gu J, Takino T and Yamada KM:
Tumor suppressor PTEN inhibition of cell invasion, migration, and
growth: Differential involvement of focal adhesion kinase and
p130Cas. Cancer Res. 59:442–449. 1999.PubMed/NCBI
|
|
13
|
Zhang L, Yu Q, He J and Zha X: Study of
the PTEN gene expression and FAK phosphorylation in human
hepatocarcinoma tissues and cell lines. Mol Cell Biochem.
262:25–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng LP, Smith WM, Brown JL and Eng C:
PTEN inhibits insulin-stimulated MEK/MAPK activation and cell
growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos
complex formation in a breast cancer model. Hum Mol Genet.
10:605–616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan XJ and Whang YE: PTEN sensitizes
prostate cancer cells to death receptor-mediated and drug-induced
apoptosis through a FADD-dependent pathway. Oncogene. 21:319–327.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rahman MA, Kyriazanos ID, Ono T, Yamanoi
A, Kohno H, Tsuchiya M and Nagasue N: Impact of PTEN expression on
the outcome of hepatitis C virus-positive cirrhotic hepatocellular
carcinoma patients: Possible relationship with COX II and inducible
nitric oxide synthase. Int J Cancer. 100:152–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan XW, Jiang M, Cao HF, He YQ, Liu SQ,
Qiu XH, Wu MC and Wang HY: The alteration of PTEN tumor suppressor
expression and its association with the histopathological features
of human primary hepatocellular carcinoma. J Cancer Res Clin Oncol.
129:100–106. 2003.PubMed/NCBI
|
|
18
|
Ginn-Pease ME and Eng C: Increased nuclear
phosphatase and tensin homologue deleted on chromosome 10 is
associated with G0-G1 in MCF-7 cells. Cancer
Res. 63:282–286. 2003.PubMed/NCBI
|
|
19
|
Yeh KT, Chang JG, Chen YJ, Chen ST, Yu SY,
Shih MC, Perng LI, Wang JC, Tsai M and Chang CP: Mutation analysis
of the putative tumor suppressor gene PTEN/MMAC1 in hepatocellular
carcinoma. Cancer Invest. 18:123–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao YJ, Ping XL, Zhang H, Chen FF, Lee PK,
Ahsan H, Chen CJ, Lee PH, Peacocke M, Santella RM, et al:
PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene.
18:3181–3185. 1999. View Article : Google Scholar : PubMed/NCBI
|