Introduction
Ovarian cancer (OC) is ranked fifth among adults
worldwide while it is the most deadly gynecological disease
(1,2).
The incidence of OC has shown an increasing trend in China, over
50,000 new cases and the death toll exceeded 20,000 in 2015
(3). The current standard treatment
for OC is surgery and adjuvant chemotherapy with platinum and
taxane (4). Although many women with
epithelial OC incipiently respond to this regimen, the recurrence
rate is high, and the survival rate is less than 50% (5). Due to the frequently-occurring side
effect of the present chemotherapy, it is urgent to find novel
drugs for OC treatment (6).
A large proportion of cancers derive from epithelial
and endothelial cells have been reported to express muscarinic
acetylcholine receptors (mAChR), activation of which leads to
increase cell proliferation (7). It
has previously been reported that activation of muscarinic (mAChRs)
can promote the growth of tumor cells in colon, lung, glial cells
and prostate (8). Simultaneously, the
presence of mAChRs receptors in ovarian tumor has been confirmed,
and expression of mAChRs receptor relates with worse prognosis in
ovarian SKOV3 cancer (9). Conversely,
antimuscarinic drug potentially represses cancer cells growth
(10). Song et al found that
M3 muscarinic receptor antagonist could impede the proliferation of
lung cancer cells (11). In line with
this report, the presence of muscarinic receptors in OC is
correlated with reduced patient survival (9). Therefore, using muscarinic antagonist
might be promising for OC treatment.
Aclidinium (N-methyl-quinuclidinyl-benzylate), which
is commonly used to treat respiratory system diseases (12), is a typical muscarinic M3 antagonist.
Aclidinium has superiority of slightly quicker onset of action and
favorable safety profile with low toxicity. Yet, it is rarely
reported in the treatment of cancer, especially OC prognosis. Here,
using western blotting, Cell Counting Kit-8 (CCK-8) proliferation
assay, Transwell metastasis, invasion assay and apoptosis flow
assay, we found the inhibitory effects and underlying mechanism of
aclidinium on OC growth.
Materials and methods
Cell culture
The normal human OC line SKOV3 was provided from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were routinely cultivated in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with supplemented with 10% fetal bovine serum (FBS), containing
penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37°C in 5%
CO2 humidified atmosphere. Cells in exponential growth
phase (~1×106 cells/ml) were used for the following
experiment.
Cell proliferation assay
Proliferation of cells was tested by CCK-8 in
accordance with the manufacturer's instructions. Approximately
5×103 cells were seeded in a 96-well plate. After
overnight culture, experimental group cells admini-stered with 20
µM aclidinium (MCE Corporation, Dublin, CA, USA) and control groups
(NC) cells containing 1% DMSO (Amresco, LLC, Solon, OH, USA) in
culture media were cultured for 72 h, and then 10 µl CCK-8 reagent
(Solai Po Company, Beijing, China) was added to each well. Then the
plates were incubated for 1.5 h at 37°C. The cell viability was
measured every 24 h. CCK-8 detection steps were the same as above.
Absorbance value [optical density (OD)] was tested at 450 nm by a
microplate reader (Bio-Rad, Hercules, CA, USA).
Transwell metastasis and invasion
assay
Migration and invasion tests were executed using
24-well Transwell chambers (EMD Millipore, Billerica, MA, USA) with
membrane pore size of 8.0 µM and without/with Matrigel (both from
BD Biosciences, San Jose, CA, USA) following the manufacturer's
instructions. A total of 100 µl cell suspensions
(~1×105) were seeded to upper chamber, whereas 500 µl
culture medium containing with 10% FBS filled the lower chamber.
After incubation overnight at 37°C, 5% CO2,
cotton-tipped swabs were used to scrape off the non-migrating cells
on the top chamber and then cells migrated through the membrane
were fixed with 4% paraformaldehyde for 30 min. They were dyed with
0.1% crystal violet for 20 min. The migrated cells in the bottom of
the chamber were randomly selected for five visual field and were
counted under a microscope. For cell invasion detection, the steps
were similar to detection of cells metastasis except the Matrigel
was plated in the Transwell inserts.
Flow cytometry apoptosis assay
Apoptotic SKOV3 cells were analyzed by an Annexin
V-fluorescein isothiocyanate (Annexin V-FITC)/propidium iodide (PI)
apoptosis detection kit (Beijing 4A Biotech, Co., Ltd., Beijing,
China) according to the manufacturer's instructions. SKOV3 cells
were treated with aclidinium and harvested by trypsinization
without EDTA. Then, they were rinsed twice with cold PBS,
centrifuged at 1111 × g at room temperature for 5 min and the
supernatant was removed, the pellet was resuspended in 500 µl 1X
binding buffer, and the cell density were adjusted to
3×106 cells/ml. Then, FITC-conjugated Annexin V and PI
were added. After incubation for 5 min in the dark at room
temperature, flow cytometry was acquired on a FACSCalibur (BD
Biosciences) and analyzed using Flowjo 7.6 software (Tree Star,
Ashland, OR, USA).
Western blot analysis
After treatment with aclidinium or vehicle for 24 h,
the cells were added with RIPA lysis buffer (CWBIO, Beijing, China)
for the cleavage and extraction of protein. Proteins concentrations
were analyzed by BCA protein assay kit (CWBIO). Proteins with equal
amount 20 µg were separated on 8–10% Tris-glycine gradient gels via
dodecyl sulfate-polyacrylamide gel electrophoresis. Then these
proteins were tranferred onto PVDF membrane for 2 h and blocked
with 5% non-fat milk in TBST buffer (pH 7.4 Tris-buffered saline
buffer containing 0.1% Tween-20) for 1 h at room temperature. Next,
membranes were probed with primary antibodies including AKT (cat.
no. 4691), p-AKT (cat. no. 4060), mTOR (cat. no. 2972), p-mTOR
(cat. no. 2974) at 1:1,000 dilution (Cell Signaling Technology,
Inc., Danvers, MA, USA); Bcl-2 (cat. no. 12789-1-AP), Bax (cat. no.
50599-2-Ig), active-caspase-3 (cat. no. 19677-1-AP), cyclin D1
(cat. no. 60186-1-Ig), P-70 (cat. no. 14485-1-AP) at 1:1,000
dilution (ProteinTech Group, Inc., Wuhan, China); tubulin at
1:5,000 dilution (ProteinTech Group, Inc.) overnight in 4°C at room
temperature, and were detected using anti-rabbit (cat. no.
66467-1-Ig)/mouse (cat. no. 10283-1-AP) horseradish
peroxidase-conjugated secondary antibodies (PTG 1:5,000). Primary
and secondary antibodies are from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Protein expression was visualized using a
chemiluminescence reagent (ECL) system (PTG, Chicago, IL, USA).
Protein bands were analyzed by measuring densitometry using Image J
6.0 software (National Institutes of Health (NIH), Stapleton, NY,
USA) and quantitative densitometry of bands were expressed by bar
charts.
Statistical analysis
Statistical analysis was carried out by using
SPSS18.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). The
measurement data are the mean ± standard deviation (SD). The
Student's t test was performed to compare the mean between two
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Aclidinium inhibits the proliferation
of OC SKOV3 cells
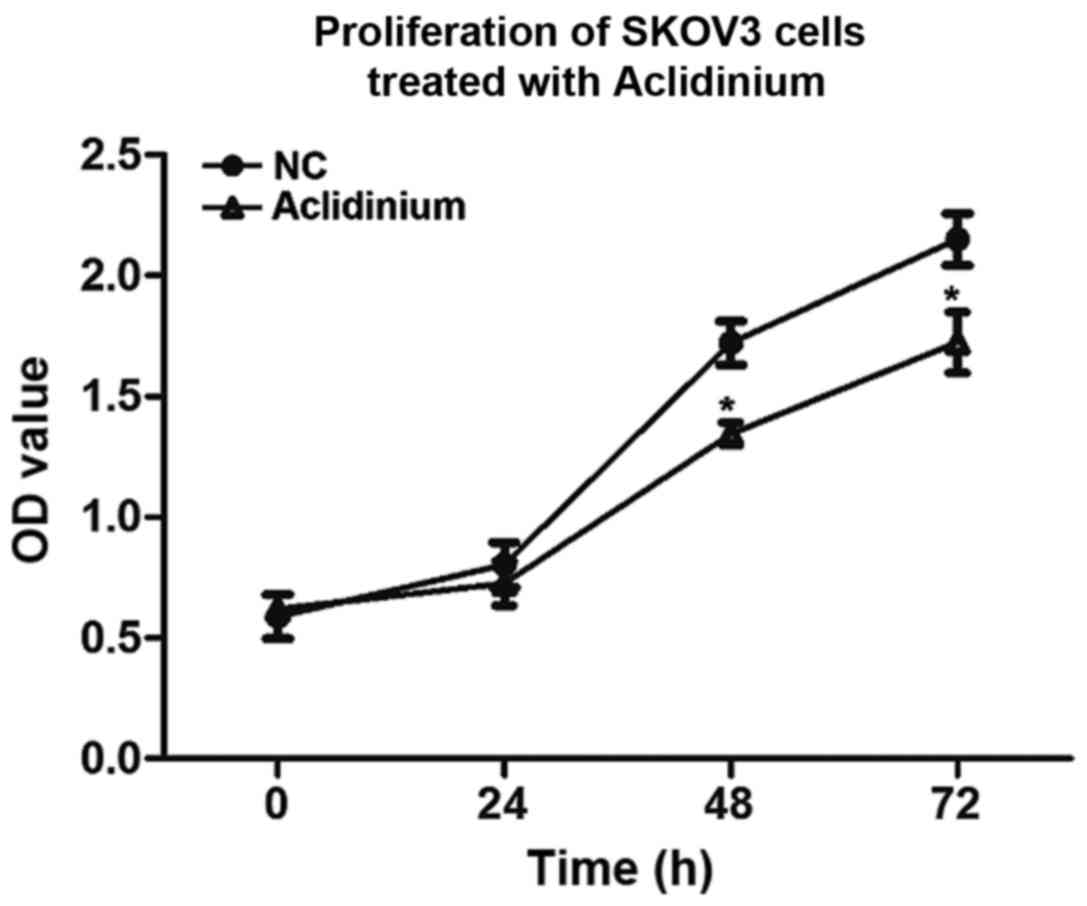
To investigate the influence of aclidinium on SKOV3
cell proliferation, CCK-8 was used to analyze cell viability in
vitro. We observed that prohibitive action on SKOV3 cells after
treatment with aclidinium was in an obvious time-dependent mode and
the OD value was significantly decreased compared to the NC group
48 and 72 h after administration (Fig.
1; P<0.05). These results suggested that the proliferation
abilities of SKOV3 cells decreased evidently after treatment with
aclidinium.
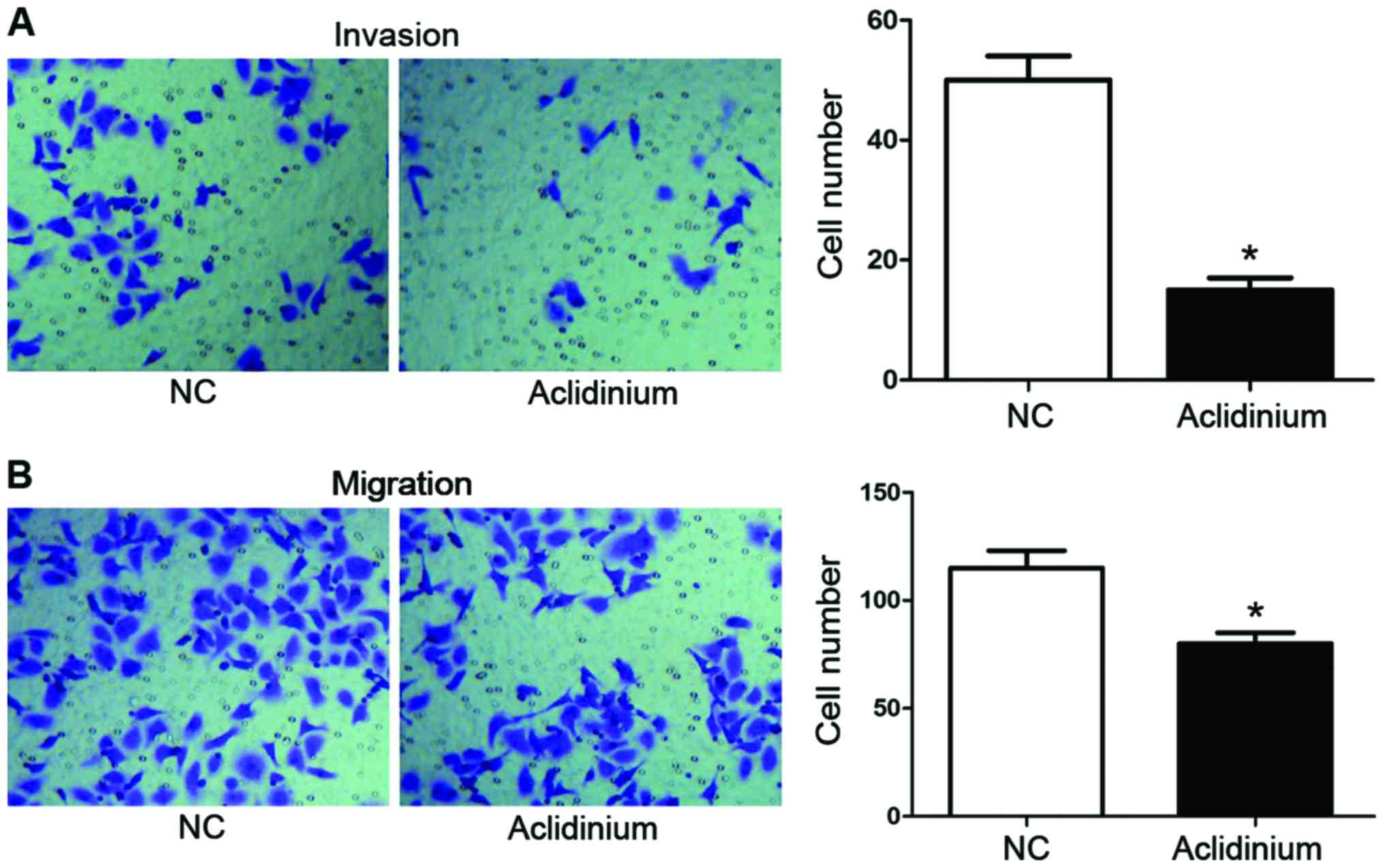
Aclidinium inhibits metastasis and
invasion of OC SKOV3 cells
Transwell metastasis and invasion were used to
measure the potential effect of aclidinium on SKOV3 cells migration
and invasion. As shown in Fig. 2A,
the numbers of invaded cells after aclidinium treatment were
markedly decreased (15±2) compared with the NC groups (50±4)
(P<0.05). Likewise, as shown in Fig.
2B, the metastasis ability in the aclidinium treatment group
was also inhibited compared with the NC group (80±5 <115±8)
(P<0.05). These results showed that aclidinium could inhibit
metastasis and invasion of OC SKOV3 cells.
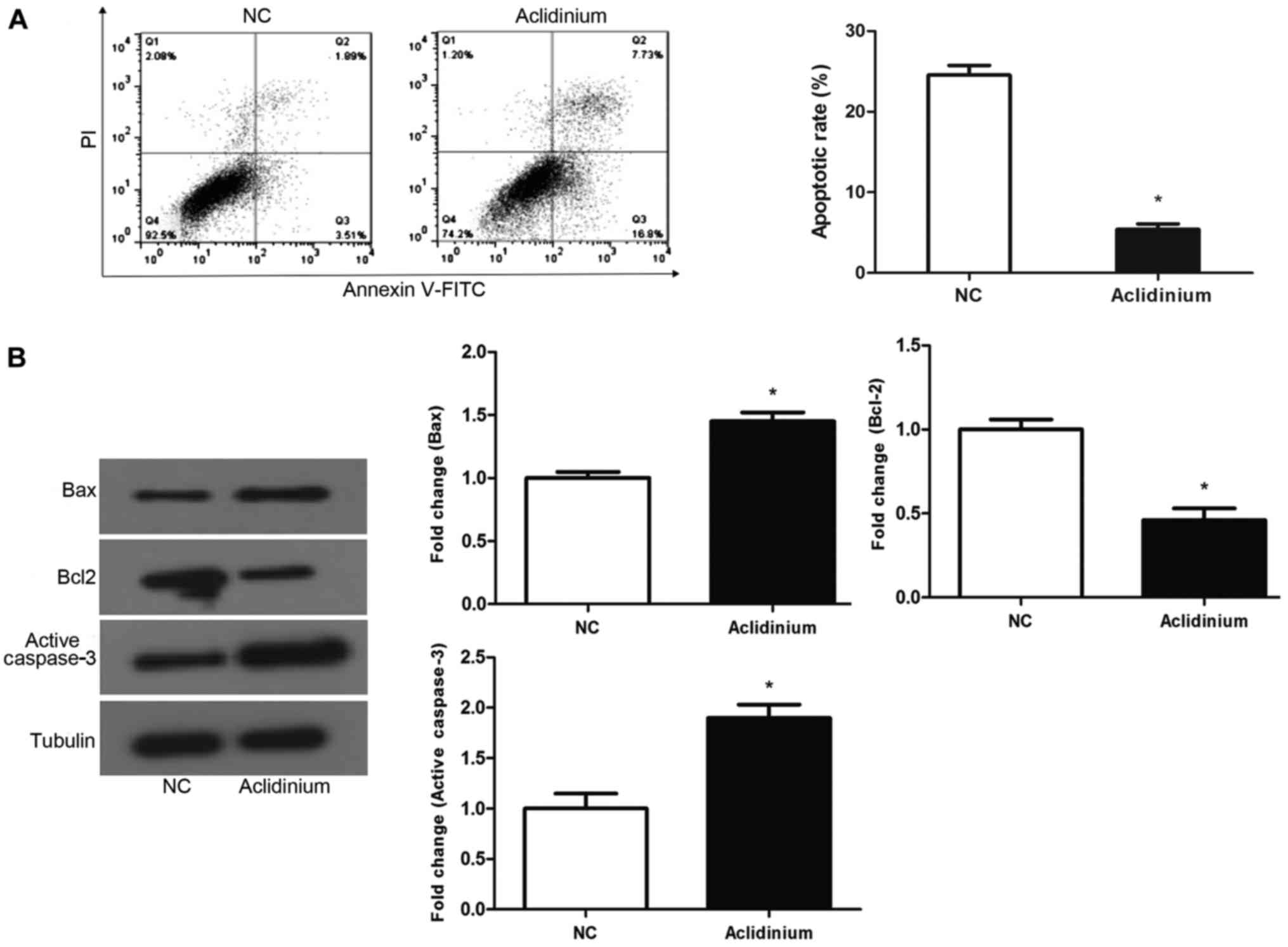
Aclidinium promotes apoptosis of OC
SKOV3 cells
To examine whether aclidinium influences SKOV3 cell
apoptosis, flow cytometry assay with staining Annexin-V-FITC/PI was
applied to test the SKOV3 cell apoptotic ratio. Results discovered
that cell apoptotic rate in aclidinium group was significantly
increased compared with NC groups (24.53 >5.4%) (Fig. 3A; P<0.05). Western blotting was
used to examine the proteins closely related with apoptosis.
Results revealed that after treatment with aclidinium, the
expression of the anti-apoptotic protein, Bcl-2, was decreased
while the level of pro-apoptotic protein Bax and active-caspase-3
were increased significantly (Fig.
3B; P<0.05). These results showed that aclidinium could
effectively promote apoptosis of OC SKOV3 cells.
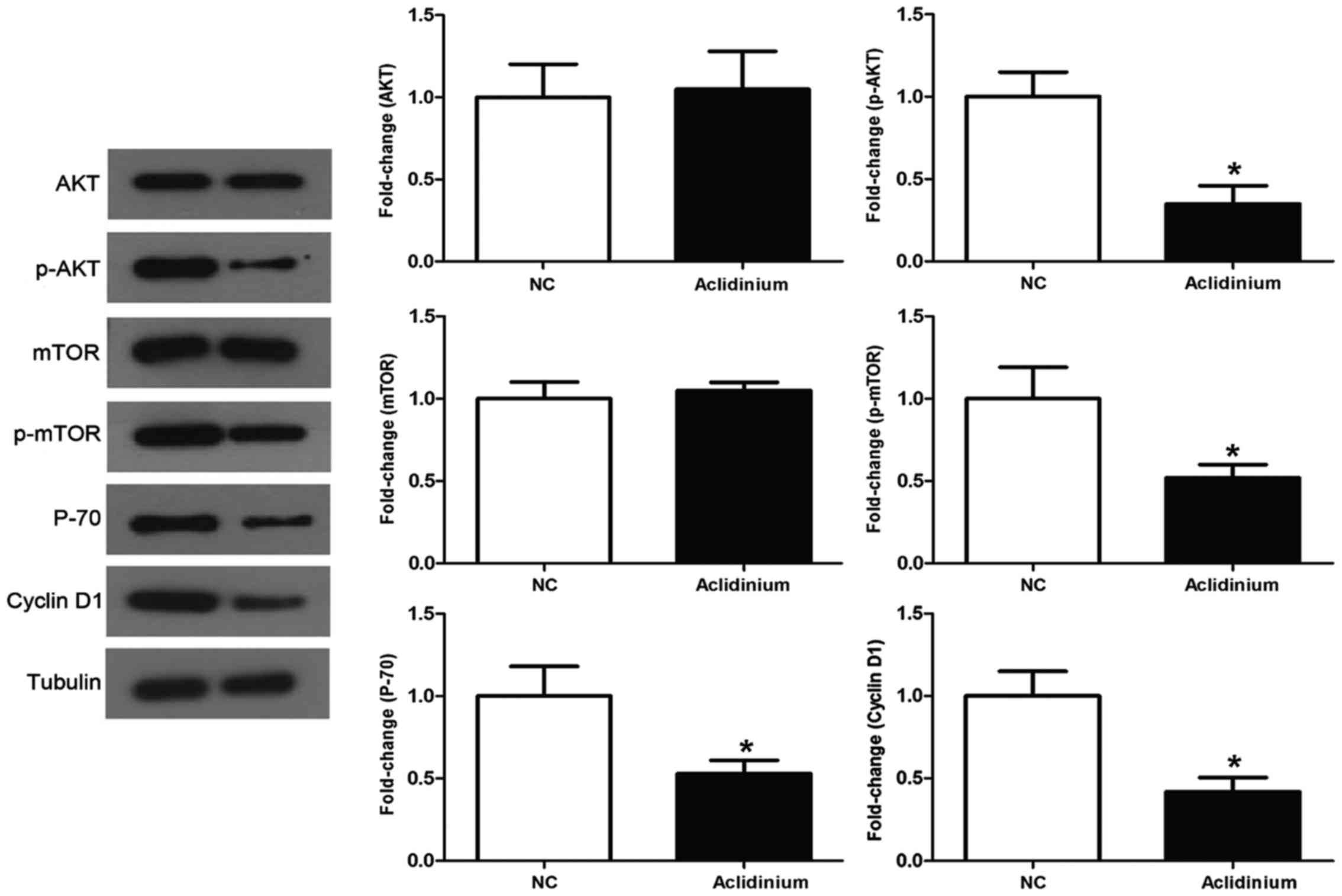
Aclidinium induces inhibition of the
activation of PI3K/AKT/mTOR signaling pathway in OC SKOV3
cells
It has been demonstrated that PI3K/AKT signaling
pathway plays a vital role in numerous tumors (13). Thus, to assess the role of aclidinium
in the inhibition of PI3K/AKT signaling, we detected a series of
specific key proteins with this vital pathway. The results in
Fig. 4 show that administration of
SKOV3 cells with aclidinium results in dramatic decrease in the
phosphorylation levels of AKT and mTOR, while no obvious changes
were observed in the total AKT and mTOR level. Besides, aclidinium
administration attenuated activity of PI3K/AKT/mTOR signaling
downstream proteins such as P70 and cyclin D1 related to cell
growth. Together, these results showed that aclidinium could
inhibit activation of PI3K/AKT/mTOR signaling pathway.
Discussion
In this investigation, we explored the potential
antitumor effect of aclidinium in OC. Before the formal
experiments, the antiproliferative effects of various
concentrations of aclidinium, including 2, 5, 10 and 20 µM, were
evaluated. The results showed that when the concentration of
aclidinium was up to 20 µM, the cell viability was inhibited
significantly. So in the following experiments, 20 µM aclidinium
was used. We found aclidinium was able to inhibit SKOV3 cell
proliferation, migration and invasion while induced apoptosis of
SKOV3 cells. Furthermore, it revealed that the above
antitumorigenic potent effects of aclidinium may be regulated by
inhibition of the PI3K/AKT/mTOR signaling pathway.
It has been demonstrated that aclidinium is
muscarinic M3 antagonist drug, and may help chronic obstructive
pulmonary disease (COPD) (14).
Nevertheless, with incidence of various cancers and advancement of
abundant research, muscarinic antagonist drug was considered as an
antitumor agent in several cancers. Studies reported by Fritz et
al (15) and Mayerhofer and Kunz
(16) have suggested that a new
cholinergic autocrine loop was expressed in normal ovary, implying
muscarinic antagonists is likely to control OC. There are more
studies showing that muscarinic antagonist drug could inhibit tumor
cell proliferation and migration such as lung carcinoma (17), urothelial bladder cancer (18) and colon cancer (19), and our results were consistent with
these studies. Furthermore, the report depicted that muscarinic
antagonist drug inhibited cell growth in vitro, and in
transplanted nude mice decreased levels of MAPK phosphorylation was
observed (20). It is well
established that the mAChRs harbor five genetically different
subtypes: M1-5. Among these mAChRs, M1, M3, and M5 are combined
with the Gq type of G proteins which irritate phospholipase C to
initiate the phosphatidylinositol triphosphate-signaling cascade
(17). There is research that [3H]
quinuclidinyl benzilate (QNB), emerged as radioligand, was used to
examine muscarinic cholinergic receptor sites in separated plasma
membrane fractions from human OC, the final results showed that the
emergence of muscarinic receptors in ovarian adenocarcinoma by
bonding profile were optimum coherent with M3 receptors (21). In our study, the results showed
aclidinium could inhibit SKOV3 cell proliferation, migration and
invasion as well as promote apoptosis which were consistent with
the above point of view. Collectively, all the results indicated
that aclidinium (a muscarinic M3 antagonist) acts on
antiproliferative and antimetastatic characteristics in OC.
The PI3K/AKT/mTOR signaling is supposed to be a
vital player in cancer proliferation, metastasis and carcinoge
nesis (22). The activation of the
PI3K/AKT/mTOR signaling pathway is of importance in OC
tumorigenesis, chemotherapy resistance and progression (23,24). PIK3
and AKT2 overexpression has been observed in OC (25,26) and
the signaling is emerging as an important and viable therapeutic
target in OC (27). Importantly,
cyclin D1 has been documented to affect OC cell growth (28,29), and
it is the indispensable downstream protein of PI3K/AKT/mTOR
signaling. Consistent with this result, our finding revealed that
key factors of signaling, p-AKT and p-mTOR were reduced after
administration with aclidinium, downstream factors like cyclin D1
and P70 were also reduced by aclidinium treatment. Altogether, the
above data indicated that aclidinium exerted potent prohibitory
activity against SKOV3 cell proliferation, invasion, metastasis and
this might be via mediating PI3K/AKT/mTOR pathway inactivation.
In conclusion, this study elucidated that aclidinium
could inhibit SKOV3 cell proliferation, migration and invasion as
well as promote apoptosis. Furthermore, these effects might be
achieved by downregulating PI3K/AKT/mTOR signaling pathway. The
above studies have provided experimental basis for the future
development of clinical anticancer drugs. However, there still
exist limitations in this subject. It is unclear if there are more
signaling pathways mediating the functional role of aclidinium.
These questions still require future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived the study and drafted the manuscript.
JQ and WJW performed the experiments, analyzed the data and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Minlikeeva AN, Freudenheim JL, Cannioto
RA, Szender JB, Eng KH, Modugno F, Ness RB, LaMonte MJ, Friel G,
Segal BH, et al: Australian Ovarian Cancer Study Group; Ovarian
Cancer Association Consortium: History of hypertension, heart
disease, and diabetes and ovarian cancer patient survival: Evidence
from the ovarian cancer association consortium. Cancer Causes
Control. 28:469–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schorge JO, Clark RM, Lee SI and Penson
RT: Primary debulking surgery for advanced ovarian cancer: Are you
a believer or a dissenter? Gynecol Oncol. 135:595–605. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi T, Wang P, Xie C, Yin S, Shi D, Wei C,
Tang W, Jiang R, Cheng X, Wei Q, et al: BRCA1 and BRCA2 mutations
in ovarian cancer patients from China: Ethnic-related mutations in
BRCA1 associated with an increased risk of ovarian cancer. Int J
Cancer. 140:2051–2059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogel TJ, Goodman MT, Li AJ and Jeon CY:
Statin treatment is associated with survival in a nationally
representative population of elderly women with epithelial ovarian
cancer. Gynecol Oncol. 146:340–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA;
Gynecologic Oncology Group, : Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel S, Kumar L and Singh N: Metformin
and epithelial ovarian cancer therapeutics. Cell Oncol (Dordr).
38:365–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spindel ER: Muscarinic receptor agonists
and antagonists: Effects on cancer. Handb Exp Pharmacol.
208:451–468. 2012. View Article : Google Scholar
|
|
8
|
Hua N, Wei X, Liu X, Ma X, He X, Zhuo R,
Zhao Z, Wang L, Yan H, Zhong B, et al: A novel muscarinic
antagonist R2HBJJ inhibits non-small cell lung cancer cell growth
and arrests the cell cycle in G0/G1. PLoS One. 7:e531702012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oppitz M, Möbus V, Brock S and Drews U:
Muscarinic receptors in cell lines from ovarian carcinoma: Negative
correlation with survival of patients. Gynecol Oncol. 85:159–164.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah N, Khurana S, Cheng K and Raufman JP:
Muscarinic receptors and ligands in cancer. Am J Physiol Cell
Physiol. 296:C221–C232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song P, Sekhon HS, Lu A, Arredondo J,
Sauer D, Gravett C, Mark GP, Grando SA and Spindel ER: M3
muscarinic receptor antagonists inhibit small cell lung carcinoma
growth and mitogen-activated protein kinase phosphorylation induced
by acetylcholine secretion. Cancer Res. 67:3936–3944. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni H, Soe Z and Moe S: Aclidinium bromide
for stable chronic obstructive pulmonary disease. Cochrane Database
Syst Rev. 19:CD0105092014.
|
|
13
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reid DJ and Carlson AA: Clinical use of
aclidinium in patients with COPD. Int J Chron Obstruct Pulmon Dis.
9:369–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fritz S, Wessler I, Breitling R,
Rossmanith W, Ojeda SR, Dissen GA, Amsterdam A and Mayerhofer A:
Expression of muscarinic receptor types in the primate ovary and
evidence for nonneuronal acetylcholine synthesis. J Clin Endocrinol
Metab. 86:349–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayerhofer A and Kunz L: A non-neuronal
cholinergic system of the ovarian follicle. Ann Anat. 187:521–528.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ami N, Koga K, Fushiki H, Ueno Y, Ogino Y
and Ohta H: Selective M3 muscarinic receptor antagonist inhibits
small-cell lung carcinoma growth in a mouse orthotopic xenograft
model. J Pharmacol Sci. 116:81–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pacini L, De Falco E, Di Bari M, Coccia A,
Siciliano C, Ponti D, Pastore AL, Petrozza V, Carbone A, Tata AM,
et al: M2 muscarinic receptors inhibit cell proliferation and
migration in urothelial bladder cancer cells. Cancer Biol Ther.
15:1489–1498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park YS and Cho NJ: Enhanced proliferation
of SNU-407 human colon cancer cells by muscarinic acetylcholine
receptors. BMB Rep. 41:803–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russo P, Del Bufalo A, Milic M, Salinaro
G, Fini M and Cesario A: Cholinergic receptors as target for cancer
therapy in a systems medicine perspective. Curr Mol Med.
14:1126–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Batra S, Popper LD and Iosif CS:
Characterisation of muscarinic cholinergic receptors in human
ovaries, ovarian tumours and tumour cell lines. Eur J Cancer.
29A:1–1306. 1993.
|
|
22
|
Dobbin ZC and Landen CN: The importance of
the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int
J Mol Sci. 14:8213–8227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cazzola M: Aclidinium bromide, a novel
long-acting muscarinic M3 antagonist for the treatment of COPD.
Curr Opin Investig Drugs. 10:482–490. 2009.PubMed/NCBI
|
|
25
|
Shayesteh L, Lu Y, Kuo WL, Baldocchi R,
Godfrey T, Collins C, Pinkel D, Powell B, Mills GB and Gray JW:
PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet.
21:99–102. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng JQ, Ruggeri B, Klein WM, Sonoda G,
Altomare DA, Watson DK and Testa JR: Amplification of AKT2 in human
pancreatic cells and inhibition of AKT2 expression and
tumorigenicity by antisense RNA. Proc Natl Acad Sci USA.
93:3636–3641. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia B, Yang S, Liu T and Lou G: miR-211
suppresses epithelial ovarian cancer proliferation and cell-cycle
progression by targeting Cyclin D1 and CDK6. Mol Cancer. 14:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu W, Slomovitz BM, Soliman PT, Schmeler
KM, Celestino J, Milam MR and Lu KH: Correlation of cyclin D1 and
cyclin D3 overexpression with the loss of PTEN expression in
endometrial carcinoma. Int J Gynecol Cancer. 16:1668–1672. 2006.
View Article : Google Scholar : PubMed/NCBI
|