Introduction
The aging of Japan's population is advancing,
accordingly geriatric patients are often diagnosed to have
malignancy. Although radical surgery is appropriate in terms of
curativity, conservative procedures are often prefered in such
geriatric patients. Since the population in our medical area is
also continuing to age, urothelial carcinomas (UCs), which are
generally diseases of middle-aged and elderly people, are mainly
diagnosed in geriatric patients older than 60 years in our
institution.
Radical nephroureterectomy (RNU) is considered to be
the standard treatment for patients with non-metastatic upper tract
urothelial carcinomas (UTUCs), considering that UTUCs are
frequently multifocal and have high ipsilateral recurrence rate
(1,2).
Meanwhile, segmental ureterectomy (SU) is an interesting
alternative for the treatment of UTUCs because it allows a less
invasive procedure and guarantees the preservation of renal
units.
In the field of renal oncology, it has been
emphasized that the post-operative renal insufficiency may lead to
higher rates of dialysis, cardiovascular morbidity and overall
mortality (3,4). Therefore, partial nephrectomy is
currently recommended with respect to small renal masses (5).
Unlike renal tumors, SU for UTUCs has never been
popularized yet. One of the reasons is that, UTUCs are relatively
uncommon, accounting for only 5–6% of all UCs (6,7).
Accordingly, only limited data are available on the oncological
outcomes afforded by conservative management. However, SU has
gradually become more acceptable with recent data supporting the
importance of nephron-sparing. In our institution, SU for UTUCs has
been performed over the last decade. In this study, we
retrospectively analyzed the data of patients treated with SU in
comparison with patients treated with RNU. Through this study, we
would like to introduce this kidney-sparing procedures to
nephrologists and urologists.
Patients and methods
Patients
The subjects are comprised with consecutive 26
Japanese patients who had localized ipsilateral UTUCs who were
treated with SU or RNU between January 2004 and December 2016.
These 26 patients were retrospectively divided into 2 groups
according to therapy: The SU group (η=12), patients treated with
SU; and the RNU group (η=14), patients treated with RNU.
These patients had no previous history of radical
cystectomy for bladder tumor. Four patient in the SU group and 1
patient in the RNU group had previous histories of superficial
bladder tumor which had been curatively treated with transurethral
resection of bladder tumor (TURBT). No patients had co-existence of
bladder tumors at the time of SU or RNU. All these patients had no
metastatic disease at the time of diagnosis.
The present study conformed to the principles
outlined in the Declaration of Helsinki. The Ethics Committee of
Niigata Rousai Hospital (Niigata, Japan) approved the study (cat.
no. 2017-01) and waived the requirement for approval and informed
consent for patient participation in this study due to the
retrospective nature of this analysis of clinical data. The present
study was conducted using the opt-out method.
Treatment
Both SU and RNU were all performed through open
surgery. SU was performed to patients with clinically unifocal
disease without evidence of invasion in the preoperative imaging.
Surgical methods for the SU group patients depend on the tumor
location. For distal tumors, distal ureterectomy with a bladder
cuff resection plus ureteral re-implantation was carried out. As
re-implantation, direct ureterocystoneostomy was mostly performed.
When ureteral length is insufficient for direct re-implantation, a
Boari bladder flap or a psoas bladder hitch were then used
depending on the surgeon's preference. Meanwhile, for the mid or
proximal tumors, partial ureterectomy with ureteral end-to-end
anastomosis was performed. On the other hand, RNU with a bladder
cuff resection was performed in the patients for which SU may not
be appropriate, such as invasive and/or multifocal UTUCs and/or
renal pelvic tumors. Of 14 patients in the RNU group, 2 patients
had radiologically invasive disease. All patients were diagnosed as
clinically N0 disease for both SU and RNU, therefore abdominal or
pelvic lymph node dissection was not performed during this
period.
Preoperatively, preoperative T stratification was
established by Computed tomography (CT) and/or Magnetic Resonance
Imaging (MRI). Upper tract urinary cytology was performed as part
of a standard diagnostic work-up, and all patients were diagnosed
to have UCs preoperatively. Diagnostic ureteroscopy with biopsy was
not routinely practiced during this period. All histological
examinations were performed at the Institute of the Pathology at
our hospital. Tumors were staged according to the 2002
International Union Against Cancer TNM classification, clinical
stage and pathological stage are denoted by a small ‘c’ or ‘p’
before the stage (8). Tumor grading
was assessed according to the 1998 WHO/International Society of
Urological Pathology consensus classification (9).
Follow-up procedures
Follow-up consisted of routine blood work and serum
chemistry studies, urinary cytology, cystoscopy, ultrasound
sonography and CT scan. Cystoscopy and urinary cytology were
examined every 3 months up to the 5th year, then every 6 months
thereafter. Ultrasound sonography was done every 6 months up to the
5th year, and annually thereafter. A CT evaluating the chest and
abdomen was performed annually, and additionally done when
clinically indicated. Elective bone scan and MRI were also
performed when clinically indicated.
When the recurrence without distant metastasis has
developed, curative surgery was added whenever possible. TURBT was
performed when bladder only recurrences occurred. Radical
cystectomy was subsequently carried out when the bladder recurrence
proved to be invasive disease. If ipsilateral localized recurrence
occurred, RNU for the residual kidney and ureter was performed.
Statistical analysis
Statistical analysis of the data was performed using
StatView version 5 software (SAS Institute, Inc., Cary, NC, USA).
The significance of any differences in patient characteristics
among groups was tested using Student's t-test or Mann-Whitney U
test. Statistical comparison of estimated glomerular filtration
rates (eGFRs) between groups was made by two-way repeated measure
analysis of variance (ANOVA) with Fisher's protected least
significant difference (PLSD) post hoc test. Repeated measure
one-way ANOVA followed fisher's PLSD for multiple comparisons, in
which each value was compared with the preoperative control value,
was also used.
The survival curves were determined using the
Kaplan-Meier method, and differences were evaluated using the
log-rank test. A univariate Cox regression model was used to
evaluate the risk factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Clinical and pathological characteristics are shown
in Table I. Mean age at diagnosis was
72.5 (range, 62–78) years for the SU group and 73.7 (range, 65–82)
years for the RNU group, respectively. No significant differences
between treatment groups were observed with respect to age, sex,
affected side, previous bladder cancer history and tumor
multiplicity. Meanwhile, patients treated with SU tend to have
smaller tumor when compared to patients treated with RNU (20.6±9.7
cm vs. 33.4±19.7 cm, P=0.05). And there was obvious difference in
tumor locations between the two groups (P<0.01). It is because
RNUs are indicated for all site of tumors including renal pelvic
tumors, for which SU is scarcely indicated.
 | Table I.Subject characteristics. |
Table I.
Subject characteristics.
| Characteristic | SU group | RNU group | P-value |
|---|
| Total n | 12 | 14 |
|
| Age, years [mean ± SD
(range)] | 72.5±4.4 (62–78) | 73.7±5.8 (65–82) | 0.54 |
| Sex |
|
| 0.76 |
| Male | 7 | 9 |
|
|
Female | 5 | 5 |
|
| Affected side |
|
| 0.99 |
| Righ | 6 | 7 |
|
| Left | 6 | 7 |
|
| Tumor location |
|
| <0.01 |
| Renal
pelvis | 0 | 8 |
|
| Upper
ureter | 1 | 1 |
|
| Middle
ureter | 2 | 3 |
|
| Distal
ureter | 9 | 1 |
|
|
Throughout upper tract | 0 | 1 |
|
| Tumor length, mm
[mean ± SD (range)] | 20.6±9.7
(6–35) | 33.4±19.7
(10–84) | 0.05 |
| Tumor
multiplicity |
|
| 0.18 |
|
Solitary | 12 | 12 |
|
|
Multiple | 0 | 2 |
|
| Previous bladder
cancer history |
|
| 0.09 |
| No | 8 | 13 |
|
|
Yes | 4 | 1 |
|
| Clinical tumor
stage |
|
| 0.18 |
|
≤cT2 | 12 | 12 |
|
|
≥cT3 | 0 | 2 |
|
| Pathological tumor
stage |
|
| 0.91 |
|
pTa | 4 | 4 |
|
|
pT1 | 1 | 4 |
|
|
pT2 | 3 | 1 |
|
|
pT3 | 3 | 4 |
|
|
pTis | 1 | 1 |
|
| Tumor grade |
|
| 0.83 |
| Low
grade | 6 | 7 |
|
| High
grade | 5 | 7 |
|
| High
grade with squamous differentiation | 1 | 0 |
|
Among the SU group, 11 of 12 patients had elective
indications with normal contralateral kidney and the remaining 1
patient had imperative indication. The only imperative case was
that of a 74 year old male who had a solitary left kidney due to
past right nephrectomy for renal tuberculosis. He was presented
with acute renal failure due to obstructive hydronephrosis in the
solitary kidney. Emergently, percutaneous nephrostomy was inserted
in order to preserve renal function. He was then diagnosed with
proximal ureter cancer. Subsequently, subtotal ureterectomy with
ureteropelvic junction ligation was carried out, and permanent
nephrostomy was required thereafter.
Among the patients in the SU group, the tumor
location was in the distal ureter in 9 of 12 patients (75%),
followed by the middle ureter in 2 patients (16.6%) and proximal
ureter in 1 patient (8.3%). In 9 patients with the distal tumor,
distal ureterectomy was performed and the following re-implantation
was carried out, and divided as follows: direct
ureterocystoneostomy, 7 patients; reimplantation on psoas hitch
bladder, 1 patient; reimplantation on Boari flap bladder, 1
patient. Meanwhile, SU with ureteral end-to-end anastomosis was
performed in 2 patients with mid ureter tumors. The remaining one
patient with proximal tumor underwent permanent nephrostomy plus
subtotal ureterectomy as stated above.
Pathological findings
In the SU group, tumors were low grade UC in 6
(50.0%), high grade UC in 5 (41.7%), and high grade UC with
squamous cell differentiation in 1 (8.3%) patient, as well as ≤pT1
in 5 (41.7%), ≥pT2 in 6 (50.0%) and pTis in 1 patient(8.3%). While
in the RNU group, tumors were low grade UC in 7 (50.0%) and high
grade UC in 7 (50.0%), as well as ≤pT1 in 8 (57.1%), ≥pT2 in 5
(35.7%) and pTis in 1 patient (7.1%).
Survival
Univariate Cox regression analysis of prognostic
factors for the overall survival (OS), cancer-specific survival
(CSS), recurrence-free survival (RFS) and metastasis free survival
(MFS) are shown in Table II.
Although the sample size is small, patients with high grade and/or
invasive tumors had significantly worse OS rates than patients with
low grade and stage tumors. There also was a trend towards lower
CSS and MFS, which did not reach significance. On the other hand,
there is no obvious difference in these survival rates between the
SU group and the RNU group.
 | Table II.Univariate cox regression analysis
for survival outcomes. |
Table II.
Univariate cox regression analysis
for survival outcomes.
|
| Overall
survival | Cancer-specific
survival | Recurrence-free
survival | Metastasis free
survival |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.01 | 0.86–1.17 | 0.90 | 1.01 | 0.83–1.23 | 0.88 | 0.92 | 0.83–1.03 | 0.14 | 0.92 | 0.77–1.11 | 0.41 |
| Sex:
Male/female | 0.86 | 0.20–3.84 | 0.84 | 0.36 | 0.06–2.28 | 0.28 | 1.03 | 0.34–3.11 | 0.94 | 0.28 | 0.05–1.75 | 0.17 |
| Affected side:
Right/left | 0.51 | 0.12–2.13 | 0.35 | 0.58 | 0.10–3.50 | 0.55 | 0.77 | 0.27–2.22 | 0.64 | 0.57 | 0.10–3.43 | 0.54 |
| Tumor location:
Pelvic/ureter | 1.10 | 0.26–4.76 | 0.90 | 1.19 | 0.19–7.19 | 0.85 | 1.36 | 0.45–4.08 | 0.57 | 1.20 | 0.19–7.69 | 0.84 |
| Tumor length | 0.99 | 0.95–1.03 | 0.80 | 0.99 | 0.94–1.05 | 0.81 | 0.97 | 0.93–1.01 | 0.17 | 0.99 | 0.94–1.05 | 0.93 |
| Tumor grade: High
grade/low grade | 10.1 | 1.22–83.4 | 0.03 | 6.44 | 0.70–59.0 | 0.09 | 0.67 | 0.23–1.96 | 0.47 | 5.80 | 0.63–54.3 | 0.11 |
| Pathological tumor
stage: ≥pt2/≤pt1 | 5.55 | 1.02–29.4 | 0.04 | 7.69 | 0.76–76.9 | 0.08 | 1.14 | 0.39–3.31 | 0.80 | 8.06 | 0.86–7.69 | 0.06 |
| Treatment:
SU/RNU | 0.63 | 0.15–2.65 | 0.52 | 0.70 | 0.11–4.34 | 0.69 | 1.03 | 0.36–2.94 | 0.95 | 0.76 | 0.12–4.76 | 0.76 |
Mean follow-up period was 48.5 (range, 7–148) months
for the SU group and 46.9 (range, 6.0–122) months for the RNU
group, respectively. Fig. 1
demonstrate the OS, CSS, RFS and MFS for patients treated with SU
or RNU. The 5-year OS, CSS, RFS and MFS in the SU group were 77.8,
87.5, 34.4 and 80.8%, respectively, which all showed no significant
differences compared with those of the RNU group. The 5-year OS,
CSS, RFS and MFS in the RNU group were 60.1, 71.9, 50.0 and 73.5%,
respectively.
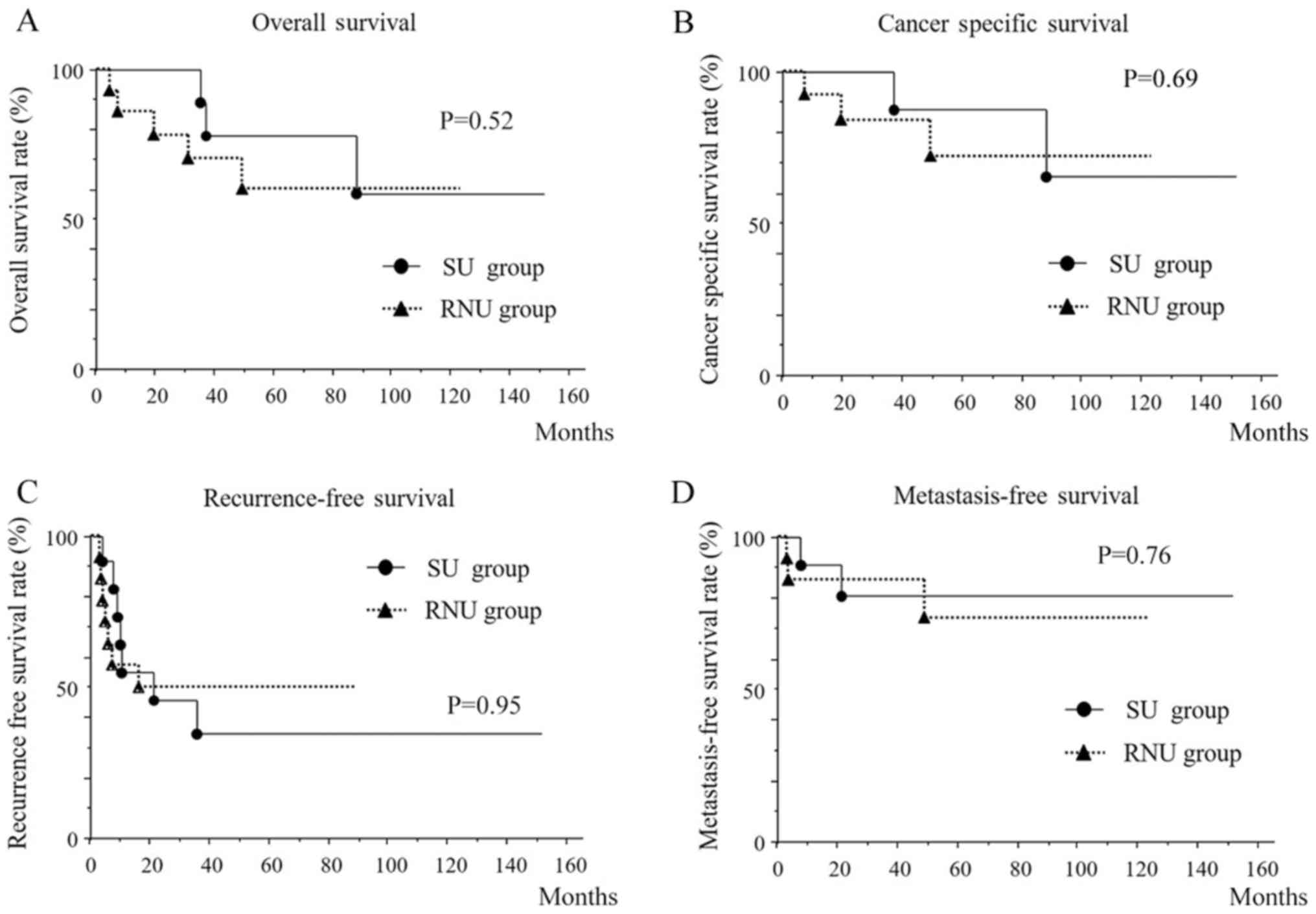 | Figure 1.Patient survival. (A) 5-year overall,
(B) cancer-specific, (C) recurrence free and (D) metastasis free
survival for patients treated with SU or RNU. The 5-year overall,
cancer-specific, recurrence free and metastasis free survival in
the SU group were 77.8, 87.5, 34.4 and 80.8%, respectively, which
all exhibited no significant differences when compared with those
of the RNU group. SU, segmental ureterectomy; RNU, radical
nephroureterectomy. |
The incidence of recurrence and/or metastasis was
similar between the patients undergo SU and RNU. In the SU group,
recurrences and/or metastasis were observed in 7/12 cases during
the follow-up period. For details, bladder recurrences, upper
urinary tract recurrences, lymph node metastasis and visceral
metastasis occur in 7, 2, 2 and 2 cases, respectively. While those
were observed in 7/14 cases in the RNU group. For details, bladder
recurrences, local recurrences, lymph node metastasis and visceral
metastasis occur in 6, 1, 2 and 3 cases, respectively.
In the SU group, recurrences and/or metastasis did
not occur in 5 cases during the follow-up period. Meanwhile,
although 4 patients in the SU group developed recurrences, they
were all successfully treated with adjuvant surgical procedures and
have become free of disease at the time of their last follow-up as
stated below. Bladder-only recurrence was found in 3 of these 4
patients. Among them, 2 cases who developed superficial bladder
recurrence had undergone curative TURBT, while one who developed
invasive bladder recurrence was curatively treated with radical
cystectomy. The other one patient experienced repeated superficial
bladder recurrence treated with several TURBT and consequently
ipsilateral ureteral recurrence appeared 9 years after SU which
curatively treated with RNU for residual urinary tract. In summary,
UTUCs were well-controlled in these 9 (75%) cases in the SU
group.
On the other hand, 3 of the 12 patients in the SU
group have developed the metastatic disease and, subsequently, 2 of
them died from the disease. One patient who died was 70 years old
at diagnosis, female and initially treated with right SU plus
ureteral end-to-end anastomosis for a mid-ureter tumor, which
proved to be pT1 high grade UC. The ipsilateral ureteral recurrence
was confirmed at 9 months after SU which was re-operated with RNU
for residual urinary tract. After a while, at 61 months after SU,
invasive bladder recurrence occurred and she died from the disease
at 86 months after initial SU. The other patient who died was
75-years at diagnosis, male and initially treated with right distal
ureterectomy for a distal tumor, proved to be a pT3 high grade UC.
Left pelvic lymph node metastasis had occurred at 20 months after
SU and died from the disease at 35 months after SU. The remaining
one patient who developed distant metastasis was 62 years old at
diagnosis, female and initially treated with right distal
ureterectomy for a distal tumor, proved to be a pT3 high grade UC
with positive surgical margin. Multiple liver metastasis had
occurred at 7 months after SU. Thereafter, chemotherapy with
methotrexate, vinblastine, adriamycin and cisplatin was initiated
at the last study follow-up date.
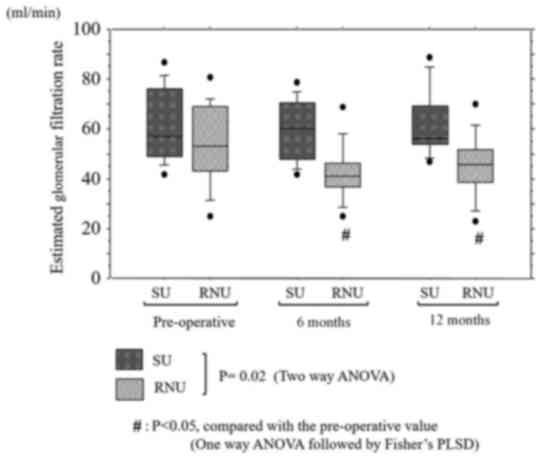
Renal function
Time course of the changes in eGFRs in the SU and
RNU group are shown in Fig. 2. Mean
preoperative serum eGFRs were 61.1±14.2 ml/min in the SU group and
52.2±16.3 ml/min in the RNU group, with no significant differences
between groups (P=0.19).
In the SU group, mean postoperative serum eGFRs were
59.8±12.1 and 61.6±13.9 ml/min at 6 and 12 months, respectively,
which showed no significant decrease from the preoperative eGFRs.
While in the RNU group, mean postoperative serum eGFRs were
42.3±11.4 and 45.1±12.1 ml/min at 6 and 12 months, respectively,
both of which were lower than the preoperative eGFRs (P<0.01 and
P=0.02, respectively).
Discussion
For non-metastatic UTUCs, RNU is still the standard
treatment, which generally results in loss of renal function. It is
demonstrated that patients who underwent nephrectomy experienced
renal insufficiency with a reduction in mean eGFR by ~24% (10,11). The
solitary kidney status is suggested to induce renal insufficiency,
higher rates of dialysis, cardiovascular morbidity and overall
mortality (3,4). With such a background, conservative
therapy has gradually developed. Conservative approaches for UTUCs
including SU have originally been developed in patients with
imperative indications, such as chronic renal insufficiency,
solitary kidneys, or comorbidity (12,13).
Gradually, SU has become the most considered alternative procedure
for patients who has elective indications with normal contralateral
kidney (1,14,15). It is
partly because the selection of candidates for SU become safer than
before with recent developments of imaging techniques which plays
important roles in characterizing UTUCs preoperatively (16,17).
Needless to say, renal function was well preserved in the SU group.
Preservation of renal function may protect patients from
non-cancer-related mortality (3). In
addition, it may allow the administration of adjuvant chemotherapy
for patients who has borderline renal function which may not be
administrated once RNU is performed (18).
With regard to oncological outcomes, previous
reports retrospectively demonstrated that the incidence of local
recurrence was similar between the patient who underwent RNU and
distal ureterectomy, although relatively small number of patients
were involved (19). The recurrence
rate of SU for low stage and low grade UTUCs was estimated at
between 10 and 25% (20,21). Recently, retrospective multicentre
study of 2299 patients showed no significant difference in survival
between patients treated with SU compared with those treated with
RNU (22). Subsequently, Bagrodia
et al (23) analyzed a large
cohort of 835 patients with UTUC in detail and concluded that
partial ureterectomy was not an independent risk factor for
recurrence or disease-specific survival on multivariable regression
analyses. More recently, Huang et al (24) investigated the oncological and renal
outcomes after SU (n=24) and RNU (n=39) who had at least one
high-risk factor and demonstrated similar oncological outcomes
between SU and RNU, with better preservation of renal function
after SU. Our series also demonstrated similar oncologic and renal
outcomes to these literatures. SU seems to have comparable
oncologic outcomes to RNU, with better preservation of renal
function.
In our study, 3 of 12 patients in the SU group had
developed metastatic disease and 2 of them died from the disease.
These 3 patients had high grade UC and 2 of which had ≥pT3 disease.
It is probable that they could be cured with appropriate surgery
(RNU). Preoperative exact diagnosis for appropriate indication for
surgery is really needed. Since recent ureteroscopy has good
quality and can be performed easily, histological evaluation by
ureteroscopic biopsy may be useful.
With regard to tumor location of UTUCs, distal
tumors which accounts for almost 70% are most common than mid and
proximal ureter tumors (25). Distal
tumors are more frequently solitary, smaller and of lower stage and
grade than upper UTUCs (2,26). Distal tumors are also known to be less
frequently associated with local recurrence than upper UTUCs
(27). Moreover, recurrences tend to
be lower stage and grade, as well as occur distal to the primary
tumor site in conservatively treated patients (15,28,29). Based
on these findings, distal ureterectomy is thought to be the most
safe and acceptable option among various conservative therapies
(2,15). The open procedure is thought to be the
standard for distal ureterectomy so far. However, laparoscopic or
robotic distal ureterectomy has been currently developed (1,30).
It is remarkable that our study population comprised
geriatric patients aged 62 years and above. For the elderly
patients with shorter life expectancies, radical surgery may have
minimal impact on eventual outcomes. For geriatric patients, less
invasive surgical options should be developed and cancer control
should not be considered of supreme importance.
The limitations of this study include its
retrospective design, being performed at a single center using a
single arm, and the relatively small number of patients. The
results may have been biased by the patient selection for SU or
RNU. Especially, substantial selection bias for SU group might be
present which cannot be overlooked due to our positive
recommendation for patients with solitary UTUCs. The results could
also have been biased by a long period of time to accrue 2004 to
2016. During the last decade, imaging techniques and diagnostic
endoscopic managements have expanded considerably which play
important roles in selecting patients. Our series include previous
cases wherein imaging techniques were not well improved yet.
Moreover, reimplantation on psoas hitch bladder or on Boari flap
bladder were used in 2 patients in our series, which may lead
difficulty in detecting recurrence via cystoscopy or cause
impossibility in performing TURBT. It may result to secondary
effect on oncologic outcome. Nevertheless, even with these
limitations, the current results suggest that SU is an acceptable
alternative for low grade, non-invasive ureteric tumors in selected
patients. Further prospective studies with large populations are
necessary in order to clarify this issue, which is difficult to
conduct due to the low incidence rate of ureteric tumors.
This study demonstrates the efficacy of SU for UTUCs
in geriatric patients. SU can preserve renal function and is
thought to be an acceptable alternative to RNU in selected
patients. We believe that the results of this study could provide
useful information on geriatric oncology.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK and RN designed the study, contributed to the
analysis and interpretation of data, and wrote the initial draft of
the manuscript. TH, MK, AK and HK contributed to the analysis and
assisted in the preparation of the manuscript. MK performed the
pathological diagnoses. All authors contributed to clinical
management of the reported case. All authors critically reviewed
the manuscript and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Niigata Rousai Hospital
approved the present study (cat. no. 2017-01) and waived the
requirement for approval and written informed consent for patient
participation in the study due to the retrospective nature of this
analysis of clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SU
|
segmental ureterectomy
|
|
RNU
|
radical nephroureterectomy
|
|
UTUC
|
upper tract urothelial carcinoma
|
|
UC
|
urothelial carcinoma
|
|
TURBT
|
transurethral resection of bladder
tumor
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
SD
|
standard deviation
|
References
|
1
|
Rouprêt M, Zigeuner R, Palou J, Boehle A,
Kaasinen E, Sylvester R, Babjuk M and Oosterlinck W: European
guidelines for the diagnosis and management of upper urinary tract
urothelial cell carcinomas: 2011 update. Eur Urol. 59:584–594.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tawfiek ER and Bagley DH: Upper-tract
transitional cell carcinoma. Urology. 50:321–329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zini L, Perrotte P, Capitanio U, Jeldres
C, Shariat SF, Antebi E, Saad F, Patard JJ, Montorsi F and
Karakiewicz PI: Radical versus partial nephrectomy: Effect on
overall and noncancer mortality. Cancer. 115:1465–1471. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Go AS, Chertow GM, Fan D, McCullouch CE
and Hsu CY: Chronic kidney disease and the risks of death,
cardiovascular events and hospitalization. N Engl J Med.
351:1296–1305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell SC, Novick AC, Belldegrun A,
Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ,
Matin SF, et al: Guideline for management of the clinical T1 renal
mass. J Urol. 182:1271–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Munoz JJ and Ellison LM: Upper tract
urothelial neoplasms: Incidence and survival during the last 2
decades. J Urol. 164:1523–1525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czito B, Zietman A, Kaufman D, Skowronski
U and Shipley W: Adjuvant radiotherapy with and without concurrent
chemotherapy for locally advanced transitional cell carcinoma of
the renal pelvis and ureter. J Urol. 172:1271–1275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epstein JI, Amin MB, Reuter VR and Mostofi
FK: The World Health Organization/International Society of
Urological Pathology consensus classification of urothelial
(transitional cell) neoplasms of the urinary bladder. Bladder
consensus conference committee. Am J Surg Pathol. 22:1435–1448.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaag MG, O'Malley RL, O'Malley P, Godoy G,
Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G,
et al: Changes in renal function following nephroureterectomy may
affect the use of perioperative chemotherapy. Eur Urol. 58:581–587.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lane BR, Smith AK, Larson BT, Gong MC,
Campbell SC, Raghavan D, Dreicer R, Hansel DE and Stephenson AJ:
Chronic kidney disease after nephroureterectomy for upper tract
urothelial carcinoma and implications for the administration of
perioperative chemotherapy. Cancer. 116:2967–2973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lehmann J, Suttmann H, Kovac I, Hack M,
Kamradt J, Siemer S, Wullich B, Zwergel U and Stöckle M:
Transitional cell carcinoma of the ureter: Prognostic factors
influencing progression and survival. Eur Urol. 51:1281–1288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arancibia MF, Bolenz C, Michel MS, Keeley
FX Jr and Alken P: The modern management of upper tract urothelial
cancer: Surgical treatment. BJU Int. 99:978–981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iborra I, Solsona E, Casanova J, Ricós JV,
Rubio J and Climent MA: Conservative elective treatment of upper
urinary tract tumors: A multivariate analysis of prognostic factors
for recurrence and progression. J Urol. 169:82–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pohar KS and Sheinfeld J: When is partial
ureterectomy acceptable for transitional-cell carcinoma of the
ureter? J Endourol. 15:405–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida S, Masuda H, Ishii C, Saito K,
Kawakami S and Kihara K: Initial experience of functional imaging
of upper urinary tract neoplasm by diffusion-weighted magnetic
resonance imaging. Int J Urol. 15:140–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sudakoff GS, Dunn DP, Guralnick ML,
Hellman RS, Eastwood D and See WA: Multidetector computerized
tomography urography as the primary imaging modality for detecting
urinary tract neoplasms in patients with asymptomatic hematuria. J
Urol. 179:862–867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lucas SM, Svatek RS, Olgin G, Arriaga Y,
Kabbani W, Sagalowsky AI and Lotan Y: Conservative management in
selected patients with upper tract urothelial carcinoma compares
favourably with early radical surgery. BJU Int. 102:172–176. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazeman E: Tumors of the upper excretory
urinary tract, calices, renal pelvis and ureter. J Urol. 78 Suppl
9:S1–S219. 1972.(In French).
|
|
20
|
Zincke H and Neves RJ: Feasibility of
conservative surgery for transitional cell cancer of the upper
urinary tract. Urol Clin North Am. 11:717–724. 1984.PubMed/NCBI
|
|
21
|
Zungri E, Chechile G, Algaba F, Diaz I,
Vilá F and Castro C: Treatment of transitional cell carcinoma of
the ureter: Is the controversy justified? Eur Urol. 17:276–280.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lughezzani G, Jeldres C, Isbarn H, Sun M,
Shariat SF, Alasker A, Pharand D, Widmer H, Arjane P, Graefen M, et
al: Nephroureterectomy and segmental ureterectomy in the treatment
of invasive upper tract urothelial carcinoma: A population-based
study of 2299 patients. Eur J Cancer. 45:3291–3297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bagrodia A, Kuehhas FE, Gayed BA, Wood CG,
Raman JD, Kapur P, Derweesh IH, Bensalah K, Sagalowsky AI, Shariat
SF, et al: Comparative analysis of oncologic outcomes of partial
ureterectomy vs. radical nephroureterectomy in upper tract
urothelial carcinoma. Urology. 81:972–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Z, Zhang X, Zhang X, Li Q, Liu S, Yu
L and Xu T: Segmental ureterectomy is acceptable for high-risk
ureteral carcinoma comparing to radical nephroureterectomy. J
Invest Surg. 25:1–8. 2018. View Article : Google Scholar
|
|
25
|
Ho KL and Chow GK: Ureteroscopic resection
of upper-tract transitional-cell carcinoma. J Endourol. 19:841–848.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anderström C, Johansson SL, Pettersson S
and Wahlqvist L: Carcinoma of the ureter: A clinicopathological
study of 49 cases. J Urol. 142:280–283. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giannarini G, Schumacher MC, Thalmann GN,
Bitton A, Fleischmann A and Studer UE: Elective management of
transitional cell carcinoma of the distal ureter: Can
kidney-sparing surgery be advised? BJU Int. 100:264–268. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mazeman E: Tumors of the upper urinary
tract calyces, renal pelvis and ureter. Eur Urol. 2:120–126. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leitenberger A, Beyer A and Altwein JE:
Organ-sparing treatment for ureteral carcinoma? Eur Urol.
29:272–278. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uberoi J, Harnisch B, Sethi AS, Babayan RK
and Wang DS: Robot-assisted laparoscopic distal ureterectomy and
ureteral reimplantation with psoas hitch. J Endourol. 21:368–373.
2007. View Article : Google Scholar : PubMed/NCBI
|