Introduction
Hepatocellular carcinoma (HCC) is state of liver
cancer where hepatocytes lose the ability of controlled growth and
gain the ability to avoid apoptosis, due to a certain mutation. Its
prevalence being the sixth and the fact that it is the third most
frequent cause of cancer-related death explain the reason why it
grabs such attention (1). In addition
to having very poor prognosis, its incidence is going higher
worldwide mainly caused by the dissemination of hepatitis B and C
virus infection (2,3).
The methods for detection and treatment have been
greatly improved in the last decade. Despite such a success in
diagnosing HCC, it is mostly diagnosed after reaching a late-stage
tumour. At this point the survival time is quit short. The current
options for therapy might not be appropriate at that time to
intervene. Surgery is always the first choice, which is mostly
convenient in early stages with no metastasis of the tumour
(4). However, cancers in later stages
and high invasive tumours are difficult to cure, and the recurrence
chance is very high, thus the urgent need for novel treatment
methods that are biologically-developed (5,6).
There are 3 types of the Wnt/β-catenin pathway, and
only the canonical pathway will be discussed further in this study
The relation between the Wnt/β-Catenin pathway and cancer was first
found in 1993 when an interaction between β-Catenin and adenomatous
polyposis coli (APC) protein was observed. APC gene was previously
discovered in familial adenomatous polyposis (FAP), a case of
cancer (7).
In a screening for a wide variety of known drugs
including anti-inflammatory drugs (NSAIDs), EA was shockingly able
to inhibit the gene activity, while others could not. Although
being used as a loop-diuretic for years, the anti-tumor ability is
being well-used nowadays. It has been shown that EA can break apart
the LEF-1/β-catenin complex and has the ability to replace LEF-1,
thus stopping the Wnt target gene transcription in CLL cells. In
addition, the expression of LEF-1, cyclin D1 and fibronectin is
greatly suppressed after the treatment with EA (8). The other important chemical agent in
this study is CPX, which was originally used as an antifungal
agent. In addition to being an iron chelating agent and its
antibacterial and antimycotic abilities, it has been recently
reported that CPX might be a strong candidate to fight cancer. The
mechanism by which the inhibition works was revealed to be through
downregulation of cyclin proteins expression as well as
cyclin-dependent kinases (CDKs) (9).
In 1991, Ingo Schmidt-Wolf et al (10), developed a protocol which involves
expanding T-lymphocytes to a new kind of cells that phenotypically
express a mixture of T- and NK cells and having markers for both.
These new cells are called cytokine-induced killer cells (CIK)
cells. They are easily developed ex-vivo from peripheral blood
mononuclear cells (PBMCs) by adding the IFN-γ, anti-CD3 mAb, IL-2,
and IL-1β (10,11).
We aim to check if there is any increased killing
when combining CIK cells with either drug, EA or CPX, against liver
cancer cell lines using a cell viability assay.
Materials and methods
Cell lines and culture conditions
Hep3B and HepG2 cell lines (DSMZ, Braunschweig,
Germany) and CCD18-co cell line (ATCC, Wesel, Germany) were
incubated in aseptic optimal conditions as recommended; at 37°C
with 5% CO2 and 90% humidity in the incubator Cytoperm 2
(Thermo Fischer Scientific, Inc., Schwerte, Germany). The culture
medium used was different. For HepG2 cell line, 90% RPMI-1640
medium and 10% heat inactivated fetal bovine serum (FBS) was used.
For Hep3B and CCD18 cells, 90% EMEM containing 2 mM L-glutamine and
10% heat inactivated (FBS) combination was used. In addition, 1%
penicillin/streptomycin was added to each of the media.
CIK cells generation
Blood from healthy donors was acquired from
Blutspendedienst Bonn-Venusberg, Germany. Blood samples were
collected after approval by the Ethical Committee of the University
of Bonn. In all cases informed consent was obtained and the
experiments were conducted in agreement with the Declaration of
Helsinki. 25 ml of blood was added to 25 ml of PBS (Thermo Fischer
Scientific, Inc.) containing 1% BSA (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). After that, 30 ml of this mixture was pipetted
very slowly on 15 ml of Ficoll (Pan-Biotech, Aidenbach, Germany)
with a density of 1.077 g/ml. This new 45 ml containing tube was
then centrifuged for 30 min at 4°C without break, in order to
generate separate layers. The buffy coat later was aspirated using
a pipette and transferred to a new tube that contains 10 ml 1%
PBS/BSA, and filled up to 50 ml with the same solution. A second
centrifugation step at 320 × g for 7 min at room temperature was
performed. Next, the supernatant was discarded and 10 ml of the
lysis buffer. It was prepared by dissolving 8.29 g NH4Cl
(Merck KGaA), 1 g KHCO2, and 0.037 g EDTA (both from Sigma-Aldrich;
Merck KGaA) in 1 l distilled water. The pellet was resuspended and
the tube was then placed on ice for 10 min, in order to get rid of
the red blood cells. Then, the tube was filled with 1% PBS/BSA up
to 50 ml, and centrifuged at 320 × g for 7 min at RT. After that, 2
ml of CIK media was added, and the pellet was resuspended. CIK
media was prepared by adding 10% FBS, 1% Penicillin/Streptomycin
(Thermo Fischer Scientific, Inc.), and 12.5 ml of 1 M Hepes to RPMI
1640 media (both from Pan-Biotech). 10 µl of the suspension was
used to count the cells. First, a 10 fold dilution step with 90 µl
PBS was needed because the count is too high. Second, 10 µl of the
diluted suspension was added to 90 trypan blue (Biochrome, GmbH,
Berlin, Germany), which makes another 10 fold dilution.
Immediately, using normal light microscope and the improved Neumann
chamber (Labor Optik, Lancing, UK) the cells were counted. Next,
cells were seeded in a T-175 culture flask (Greiner Bio-One,
Frickenhausen, Germany) at density of 75×106 cells, and
then 40 ml of CIK media was added. At the same day, 400 µl of IFNγ
(BioLegend GmBH, Koblenz, Germany) with a concentration of 100 U/µl
was added. On the next day, the cell suspension was removed and
transferred to a new culture flask, in order to get rid of the
adherent dendritic cells. In addition, 400 µl of α-CD3, 400 µl of
IL-1β (both from Thermo Fischer Scientific, Inc.) and 1,200 µl of
IL-2 (BioLegend GmBH) with working concentrations of 50 ng/ml, 10
U/µl, and 20 U/µl were added, respectively. All procedures were
performed under sanitary conditions under laminar flow hood (Hood
Series 1300 Class A2; Thermo Fisher Scientific, Inc.). CIK cells
were stained with antibodies against CD3 and CD56 to prove the
subset of NKT cells within the CIK cell culture (data not
shown).
Drugs
EA was ordered from Sigma-Aldrich; Merck KGaA, while
CPX was obtained from BIOZOL Diagnostica Vertrieb GmbH (Eching,
Germany). The drugs were dissolved in 100% Ethanol (Thermo Fischer
Scientific, Inc.). Aliquots of the original drug were prepared
using PBS and frozen at −20°C. The range of concentrations was from
31.25 µM till 3.0 mM. During the titration experiment, 10 µl of the
aliquot was pipetted into 90 µl of media, which resulted in a
10-fold dilution of the concentration.
Cell viability assay
The most common method to determine the viability of
the cell has used to be the MTT assay. However, recently a new
method has been introduced, which is much more sensitive than its
preceding methods. This method is called WST-8 assay (Water-Soluble
Tetrazolium). In the presence of viable cells, the dehydrogenase
enzyme converts the WST to a yellow-colored media-soluble formazan
dye. In addition to being more sensitive, WST reagent is not toxic
to the cells and does not require any preparation procedure
(12). The cell counting kit was
bought from Dojindo Molecular Technologies, Inc., (Munich,
Germany). The protocol was followed as recommended in the manual.
2000 of each cell line were seeded in a 96-well plate 24 h before
the treatment. The measurement of the absorbance was performed 3–4
h after the addition of the WST reagent at 450 nm.
IFN-γ Enzyme-linked immunosorbent
assay (ELISA)
5×105 cells were seeded in a 6 well
plate, and 1 ml of the media was added. Next day the media was
aspirated, and replaced with fresh 750 µl media. Then
5×106 CIK cells were added as well as 750 µl of CIK
media, making the total volume of each well 1.5 ml. The plate was
incubated for 2 days. After that the media was aspirated into a 1.5
ml Eppendorf tube and centrifuged at 14,000 g for 15 min. Then the
supernatant was transferred to a new tube and stored at −20°C for
later analysis. ELISA was performed using Human IFN-gamma DuoSet
ELISA (R&D Systems, Inc., Minneapolis, MN, USA). The protocol
was followed as provided in the manual and nothing was changed.
Data analysis
WST assay data were exported as an excel file, where
initial analysis took place. The cellular viability was calculated
according to the following equation: (Absorbance of treated
cells-absorbance of the blank) ×100 Absorbance of untreated
cells-absorbance of the blank
The resulting viability values were transferred to
GraphPad Prism 6 v.6.01 (GraphPad Software, Inc., La Jolla, CA,
USA), and the res ults were further analysed using two-way ANOVA
with Tukey's multiple comparisons test as a post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. The number of experiments or measurements performed is
indicated as (n). Data are represented as mean ± standard deviation
(SD).
Results
The effect of EA and CPX on the
cellular viability
Fig. 1 demonstrates
the relationship between the concentration of the drug and the
viability of the cells. An inversely proportional relationship
between the concentration of EA and the viability of Hep3B cells
can be seen. Furthermore, by increasing the incubation time from 1
to 3 days, more cells were killed, with the exception of 100 and
200 µM, since all the cells died in the first day (1A). In
comparison to EA, lower CPX concentrations are needed to kill the
same number of Hep3B cells. Starting from 50 µM up to 200 µM almost
all the cells were killed during the first day, with no viable
remaining cells in the second or third day. CPX acted in a dose and
time dependent manner as well, but much more potent than EA (1B).
In regard to the control cell line CCD18 and the drugs, it is
evident that EA did not affect the cells in concentration under 75
µM. Nevertheless, it has caused cellular death at extremely higher
concentrations (1C). In contrast, CPX sustained the killing ability
regardless of the fact that the cells are not cancerous. CPX even
in very low concentration resulted in decreased viability (1D).
Furthermore, it can be concluded that EA had a killing effect on
HepG2 cell line after 24 h of incubation starting from 75 µM and
higher and resulted in almost total death. During the next 48 h,
the lower concentration 50 and 25 µM had mild killing ability while
concentration lower than 25 barely had a noticeable effect (1E).
Compared to EA, CPX expressed more time and dose dependent behavior
and maintained constant killing increment. However, even with very
high concentration of CPX (200 µM), total cell death has not been
achieved (1F). The IC50 values were gathered and presented in
Table I.
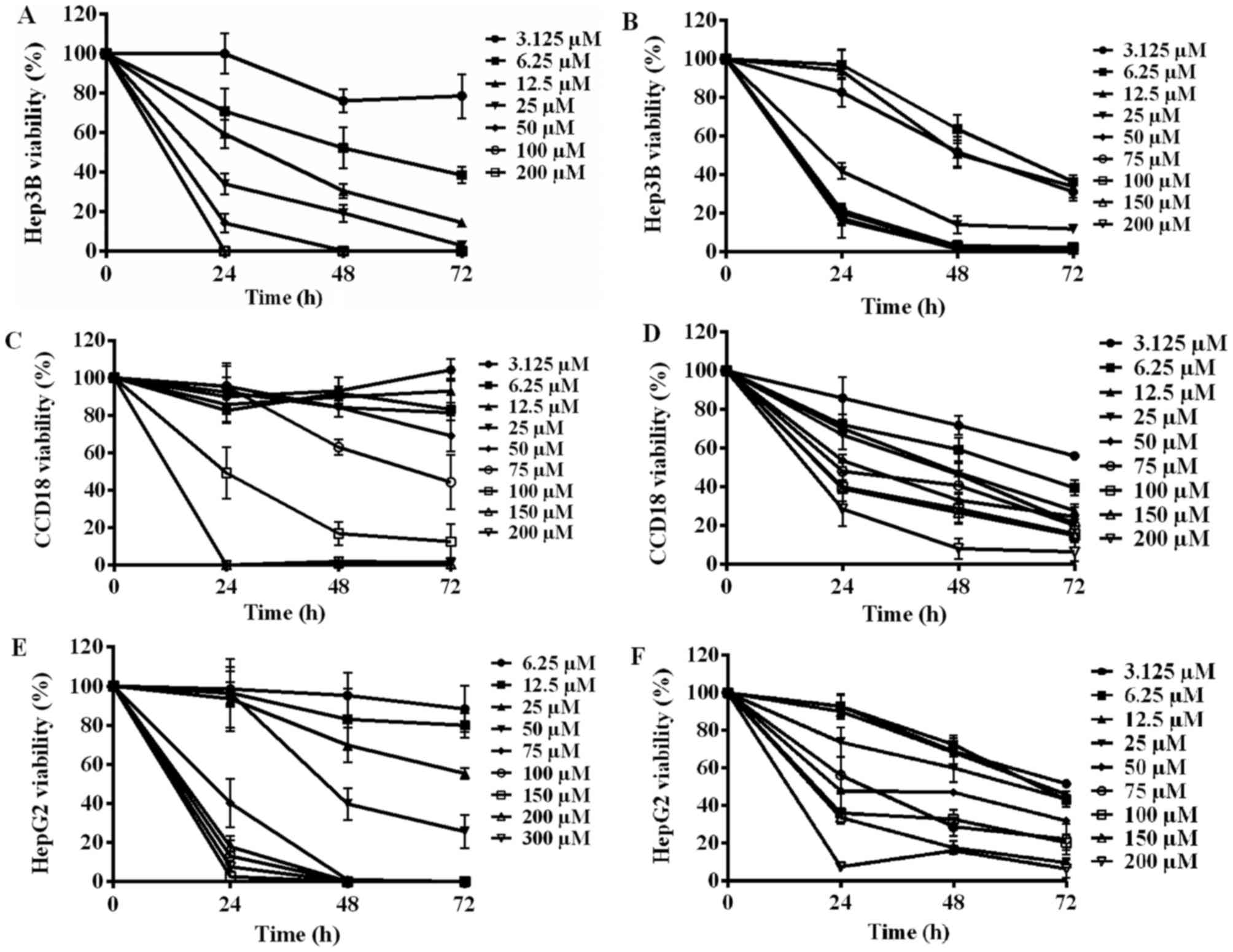 | Figure 1.The effect of EA (Left side) and CPX
(Right side) against (A and B) Hep3B, (C and D) HepG2 and (E and F)
CCD18 cell lines. 2,000 cells were incubated for various amounts of
time with increasing concentrations (µM) of one drug, in a 37°C, 5%
CO2 incubator. A reversely proportional relationship can
be observed in all graphs, although they differ in the intensity of
responsiveness to the drugs. (A) With the exception of 100 and 200
µM, increasing the incubation time with EA resulted in lower
viability of Hep3B cells. (B) CPX killed most of the Hep3B cells
within the first day, and even low concentration caused significant
cellular death. (C) Below 75 µM, EA did not affect CCD18 cells,
affecting the viability at higher concentrations. (D) CPX, even in
low concentrations resulted in severe CCD18 cellular loss. (E) EA
had a killing effect on HepG2 cell line after 24 h of incubation
starting from 75 µM and higher and resulted in almost total death.
During the next days, lower concentrations started having mild
killing effects while concentration lower than 25 hardly had any
effect. (F) CPX, even with very high concentration of CPX (200 µM),
was not efficient in achieving total cell death. The results shown
are derived from measurements done in quadruplicates (n=4). EA,
ethacrynic acid, CPX, ciclopirox olamine. |
 | Table I.IC50 for EA and CPX against
hepatocellular carcinoma cell lines and a control (CCD18). |
Table I.
IC50 for EA and CPX against
hepatocellular carcinoma cell lines and a control (CCD18).
|
| IC50
µM |
|---|
|
|
|
|---|
| Cell line | EA | CPX |
|---|
| Hep3B | 6.4 | 6.8 |
| HepG2 | 14.8 | 32.7 |
| CCD18 | 64 | 19.7 |
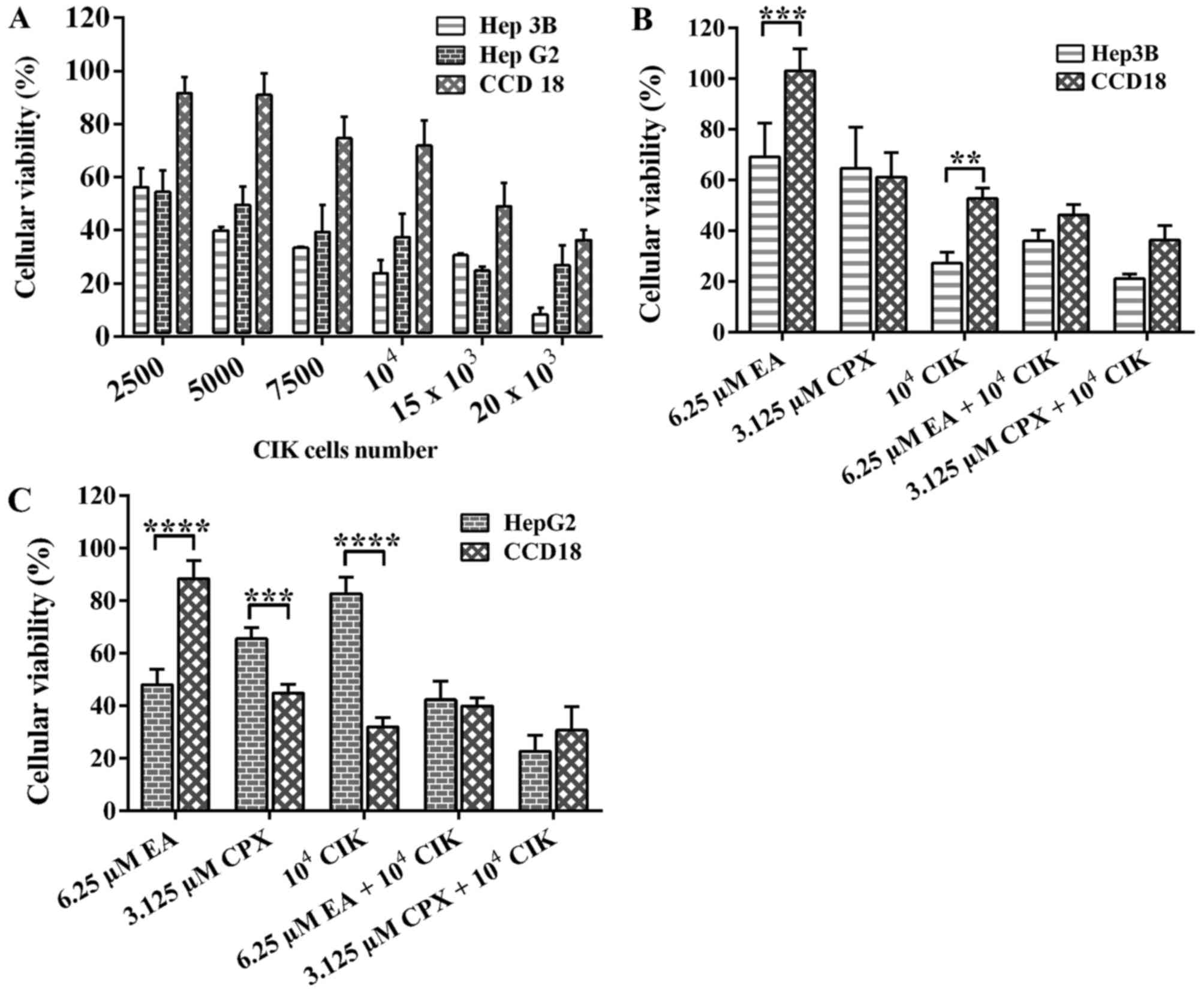
The effect of CIK cells on the
cellular viability
After incubating different numbers of CIK cells with
each cell line for 48 h, the results were collected and depicted in
Fig. 2A. As seen, Hep3B cell line was
the least resistant and the one to have more dead cells.
Nevertheless, HepG2 were also killed in an increasing manner as
well. In contrast, CCD18 normal human fibroblasts, used as a
control, were slightly affected and did not die significantly until
incubated with very high numbers of CIK cells (20 K).
The effect of EA on CIK cells
viability
It has been pointed out by Schmidt et al
(13), that 30 µM of EA was
sufficient to significantly affect cancer cell lines, while having
minumun effect on PBMCs. Since CIK cells are a specific type of
PBMCs, we expected the same result in our experiment. On the other
hand, the effect of CPX has never been reported or tested.
The effect of either drug combined
with CIK cells
As shown in Fig. 2B,
the effect of EA and CPX incubated alone on Hep3B cell line results
in 60 to 70% viability, while CIK cells results in viability around
30%. Furthermore, once added in combination, EA and CIK cells
resulted in decreased viability as low as 40%, while CPX and CIK
cells yielded only 20%. In other words, the killing was increased
once added in combination rather than separately. The figure also
shows that there is a significant difference between the effect on
cancer cells and normal human fibroblasts. They were not affected
by EA alone, and a combination of EA and CIK cells resulted in 55%
viability. Regarding HepG2 cell line, EA and CPX caused cellular
death giving 60 and 70% viable cells respectively (Fig. 2C). CIK cells were not as effective in
killing because they killed only 20% of the cells. However, once
combined with either EA or CPX, the viability dropped drastically
to 40 and 20% respectively. Regarding CCD18 normal fibroblasts, EA
had no significant killing. In contrast, CPX had a severe effect
and killed 60% of the cells. Tables
II and III show the mean and
the standard deviation values for the parameters presented in
Figs. 2B and C, respectively.
 | Table II.Mean and SD of cell viability of HCC
and control group cells treated with EA, CPX and CIK. |
Table II.
Mean and SD of cell viability of HCC
and control group cells treated with EA, CPX and CIK.
|
| Hep3B cell
viability | CCD18 cell
viability |
|---|
|
|
|
|
|---|
| Treatment | Mean | SD | Mean | SD |
|---|
| 25 µM EA | 69.17 | 13.3 | 103.05 | 8.65 |
| 12.5 µM CPX | 64.65 | 16.2 | 61.10 | 9.78 |
| 104
CIK | 27.28 | 4.2 | 52.79 | 4.00 |
| 25 µM EA +
104 CIK | 36.08 | 4.1 | 46.22 | 4.14 |
| 12.5 µM CPX +
104 CIK | 21.11 | 1.8 | 36.32 | 5.74 |
 | Table III.Mean and SD of cell viability of HCC
and control group cells treated with EA, CPX and CIK. |
Table III.
Mean and SD of cell viability of HCC
and control group cells treated with EA, CPX and CIK.
|
| HepG2 cell
viability | CCD18 cell
viability |
|---|
|
|
|
|
|---|
| Treatment | Mean | SD | Mean | SD |
|---|
| 25 µM EA | 48.05 | 5.94 | 88.44 | 6.77 |
| 12.5 µM CPX | 65.55 | 4.27 | 44.80 | 3.42 |
| 104
CIK | 82.62 | 6.38 | 31.90 | 3.63 |
| 25 µM EA +
104 CIK | 42.32 | 7.00 | 39.95 | 3.13 |
| 12.5 µM CPX +
104 CIK | 22.66 | 6.20 | 30.74 | 8.96 |
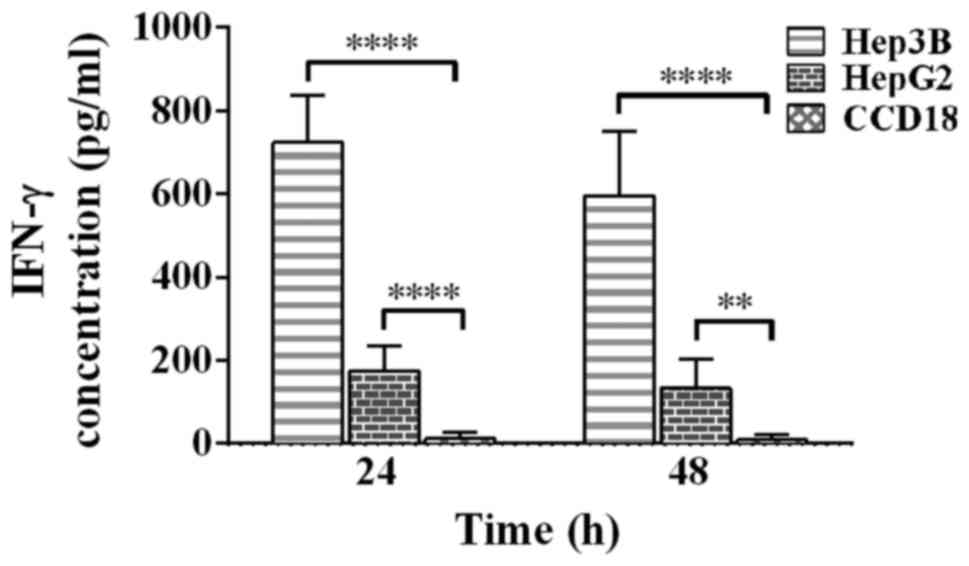
IFN-γ ELISA analysis
Fig. 3 shows the
increase in IFN-γ that is released from CIK cells once incubated
with each cell line. After 24 h, a massive surge is noticed once
incubated with Hep3B cell line. On the other hand HepG2 caused
less, yet significant, response. Moreover, levels or IFN-γ have
dropped as time passes, as the concentrations falls from 724 to
595, and from 175 to 133 pg•ml−1 once incubated with
Hep3B and HepG2 respectively. In contrast, a response can barely be
seen against CCD18 control cell line reaching a peak of 12
pg•ml−1 after 24 h, and falling after that to almost
zero.
Discussion
The vast majority of liver cancer cases worldwide
are HCC. Its fast spread is due to viruses like Hepatitis B and C
(2,11). Although huge progress in terms of
diagnosis has been achieved, the mystery of treating liver cancer
once and for all remains unsolved. However, studies suggest that
abrupt activation of the canonical Wnt/β-Catenin pathway plays an
important role in the pathogenesis process of liver cancer
(14). This pathway is involved in
cell division and proliferation. Mutations in any component
belonging to this pathway may lead to cancer, as reported by Cieply
et al (15). They proved that
tumors with mutated β-catenin gene 1 (CTNNB1) had larger size and
were more aggressive once compared to those without mutation. This
is an indicator that this pathway is a promising target for future
possible therapy. In previous studies, it was shown that both EA
and CPX have the ability to eliminate cancer cells such as renal
cell carcinoma and multiple myeloma by interfering with
Wnt/β-Catenin pathway (15–17). Identifying what kind of drugs and
which concentration to use is essential and plants the primary seed
to developing successful non-invasive treatment. For that reason we
tried to do the same but with different type of cancer cells,
namely HCC cell line Hep3B and hepatoblastoma cell line (HB)
HepG2.
EA is being used as a loop diuretic and possesses
antitumoral effects, presumably by breaking down the β-catenin-TCF
complex in the nucleus (8). CPX has
been approved as a topically-administered drug that has anti-fungal
abilities. Furthermore, systemic administration of CPX had
promising effects in countering tumor cells in many blood cancer
cases, as proven in recent studies (18). Although the exact mechanism is still
unravelled, it is thought to halt the cell cycle in the G1/S phase
by inhibiting metal-dependent enzymes like the cyclin-dependent
kinases (9). There was a strong
induction of cellular apoptosis once EA and CPX were added to HCC
and HB cell lines. Similarly, our findings agree with those of Lu
et al (19), regarding
lymphoma and myeloma cell lines. Also, similar results were
obtained from drugs that have analogous structure to EA and CPX
(20,21).
CIK cells were able to bind and kill cancer cells,
while reacting less to normal fibroblasts. They also secreted
higher levels of IFN-γ once incubated with liver cancer cells,
which argues that they are activated and responsive. IFN-γ is one
of the most important chemokines which is produced by effector
T-cells and NK cells. It has antiviral property, and it is involved
in the immune-regulation process. It has been already found by
Yelei Guo (22) that IFN-γ is
secreted with and without stimulation by cancer cells. Our results
confirm these statements.
As for the approach of combining EA and CPX with CIK
cells, our data prove that EA and CIK cells work synergistically
and as a consequence can be used against liver cancer cells.
Although CPX showed a similar response, it also caused apoptosis of
a high number of normal non-cancerous cells. This is a big
disadvantage which could counteract the idea of using it as a
candidate for therapy. The reason why CPX killed normal cells is
unknown. However, it is possible that after prolonged culture of
normal cells few changes occur, which might lead to them being
recognized as abnormal, and thus being attacked. Nevertheless,
clinical trials that combine CIK cells with either dendritic cells
or chemotherapy are currently being conducted (22).
It was proven that iron is crucial in the signaling
pathway, and blocking it is an effective measure to hinder
down-stream signaling. Not Only CPX but also various chemicals such
as desferrioxamine, deferasirox and an identified series of acyl
hydrazones have the ability to bind iron molecules and thus have
the same inhibiting effect. They all decreased the levels of free
cytoplasmic β-catenin. It was also mentioned that leukemic cells
were more susceptible to iron chelation than normal cells (23). In our study, we did not assess the
iron level in the cells. That's why further investigation is
required.
As pointed out by Jiang et al (24), other important factors come to play a
role such as RNF43 and ZNRF3, which decrease Wnt/β-Catenin activity
by inhibiting Frizzled. Furthermore, it has been proven that both
RNF43 and ZNRF3 are indeed target genes for β-Catenin. This means
that this process is a negative feedback loop. Also, they showed
that inhibition of these factors resulted in higher levels of
β-catenin in the cytosol in a pancreatic adenocarcinoma cell line.
There are other attempts to target this pathway, as was
demonstrated by Bar-Yehuda et al (25), that the drug CF102 increased GSK-3β
expression, which in turn inhibited the down-stream components of
the Wnt/β-catenin pathway. A phase I/II clinical trial suggests
that CF102 is safe to be used against HCC (26). Finally, there is certainly more about
this pathway to be discovered; the more we learn about it, the more
complex it appears to be.
In a study about CLL (18), it has been elucidated that each member
of the Wnt/β-Catenin pathway is involved in the pathogenesis.
Despite investigation a different type of cells (liver cancer), the
evidence is strong and supports the theory that the target genes
for this pathway, like TCF/LEF, are drivers of malignancy and
apoptosis survival. Pharmacological actions against the faulty
members should be taken.
In the present study, EA and CPX have been used for
the first time in the context of liver cancer. Although being
preliminary results, it is concluded that at least EA could be used
in lower doses, as a result of its low toxicity on CCD18 normal
cell.
One limitation of this study is the fact that the
effects of IFN-γ in terms of cell growth inhibition have not been
discussed. In addition, HepG2 cells were misidentified as HCC
cells, while in fact they are Hepatoblastoma cells (27). Despite this error and that HCC and HB
have several differences in their genetic characteristics, the
pathway discussed here, Wnt/β-Catenin, is aberrantly activated in
both conditions. It has been proven that β-Catenin accumulates in
the cytoplasm and the nucleus in HB and HCC (28). This supports the idea that having
misidentified HepG2 as a HCC cell line will not affect the results
of this study.
EA has strong synergistic anti-tumorous effect with
CIK cells against HCC cells, while CPX affects normal cells as
well. This implies that EA is a great drug for future usage, while
CPX is not. There are many studies on multiple myeloma or leukemia,
and rather a low number of studies on HCC and HB. More studies are
warranted.
Acknowledgements
The authors would like to thank Ms. Anja Schmidt,
Mrs. Isabel Cornez and Dr. Savita Bisht-Feldmann for providing
general advice in the lab.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AA performed the experiment, analysed the data, and
wrote the manuscript. ISW designed the experiment, analysed the
data and corrected the manuscript. HW analysed the data and
corrected the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Blood samples were collected once ethical approval
was obtained from the Ethical Committee of the University of Bonn
(Bonn, Germany). In all cases written informed consent was obtained
and the experiments were conducted in agreement with the
Declaration of Helsinki.
Patient consent for publication
The manuscript does not include any identifying
information, including names, initials, date of birth or hospital
numbers, images or statements from any patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15 Suppl
4:S5–S13. 2010. View Article : Google Scholar
|
|
4
|
Bialecki ES and Di Bisceglie AM: Diagnosis
of hepatocellular carcinoma. HPB Oxf. 7:26–34. 2005. View Article : Google Scholar
|
|
5
|
Lin S, Hoffmann K and Schemmer P:
Treatment of hepatocellular carcinoma: A systematic review. Liver
Cancer. 1:144–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang B, Tang F, Wang Z, Qi G, Liang X, Li
B, Yuan S, Liu J, Yu S and He S: Overexpression of CTNND1 in
hepatocellular carcinoma promotes carcinous characters through
activation of Wnt/β-catenin signaling. J Exp Clin Cancer Res.
35:822016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu D, Liu JX, Endo T, Zhou H, Yao S,
Willert K, Schmidt-Wolf IG, Kipps TJ and Carson DA: Ethacrynic acid
exhibits selective toxicity to chronic lymphocytic leukemia cells
by inhibition of the Wnt/beta-catenin pathway. PLoS One.
4:e82942009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou H, Shen T, Luo Y, Liu L, Chen W, Xu
B, Han X, Pang J, Rivera CA and Huang S: The antitumor activity of
the fungicide ciclopirox. Int J Cancer. 127:2467–2477. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Technologies DM: Inc., . Cell Counting
Kit-8. Jan 24–2017http://www.dojindo.com/store/p/456-Cell-Counting-Kit-8.html
|
|
13
|
Schmidt M, Kim Y, Gast SM, Endo T, Lu D,
Carson D and Schmidt-Wolf IG: Increased in vivo efficacy of
lenalidomide and thalidomide by addition of ethacrynic acid. In
Vivo. 25:325–334. 2011.PubMed/NCBI
|
|
14
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cieply B, Zeng G, Proverbs-Singh T, Geller
DA and Monga SP: Unique phenotype of hepatocellular cancers with
exon-3 mutations in beta-catenin gene. Hepatology. 49:821–831.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Von S, chulz-Hausmann SA, Schmeel LC,
Schmeel FC and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin
pathway in renal cell carcinoma. Anticancer Res. 34:4101–4108.
2014.PubMed/NCBI
|
|
17
|
Schmeel LC, Schmeel FC, Kim Y, Endo T, Lu
D and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin pathway in
multiple myeloma. Anticancer Res. 33:4719–4726. 2013.PubMed/NCBI
|
|
18
|
Weir SJ, Patton L, Castle K, Rajewski L,
Kasper J and Schimmer AD: The repositioning of the anti-fungal
agent ciclopirox olamine as a novel therapeutic agent for the
treatment of haematologic malignancy. J Clin Pharm Ther.
36:128–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M,
Leoni LM, Kipps TJ, Corr M and Carson DA: Activation of the Wnt
signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 101:3118–3123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim Y, Alpmann P, Blaum-Feder S, Krämer S,
Endo T, Lu D, Carson D and Schmidt-Wolf IG: Increased in vivo
efficacy of lenalidomide by addition of piroctone olamine. In Vivo.
25:99–103. 2011.PubMed/NCBI
|
|
21
|
Kim Y, Alpmann P, Blaum-Feder S, Krämer S,
Endo T, Lu D, Carson D and Schmidt-Wolf IG: In vivo efficacy of
griseofulvin against multiple myeloma. Leuk Res. 35:1070–1073.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Y and Han W: Cytokine-induced killer
(CIK) cells: From basic research to clinical translation. Chin J
Cancer. 34:99–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song S, Christova T, Perusini S, Alizadeh
S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, et
al: Wnt inhibitor screen reveals iron dependence of β-catenin
signaling in cancers. Cancer Res. 71:7628–7639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Hao HX, Growney JD, Woolfenden S,
Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, et al:
Inactivating mutations of RNF43 confer Wnt dependency in pancreatic
ductal adenocarcinoma. Proc Natl Acad Sci USA. 110:12649–12654.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bar-Yehuda S, Stemmer SM, Madi L, Castel
D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle
L, et al: The A3 adenosine receptor agonist CF102 induces apoptosis
of hepatocellular carcinoma via de-regulation of the Wnt and
NF-kappaB signal transduction pathways. Int J Oncol. 33:287–295.
2008.PubMed/NCBI
|
|
26
|
Stemmer SM, Benjaminov O, Medalia G,
Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z,
Farbstein M, Cohen S, et al: CF102 for the treatment of
hepatocellular carcinoma: A phase I/II, open-label, dose-escalation
study. Oncologist. 18:25–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Human
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
28
|
Buendia MA: Genetic alterations in
hepatoblastoma and hepatocellular carcinoma: Common and distinctive
aspects. Med Pediatr Oncol. 39:530–535. 2002. View Article : Google Scholar : PubMed/NCBI
|