Introduction
Biliary tract cancer, including cancers of the
gallbladder and extrahepatic bile duct, is rare but highly fatal
(1), and complete surgical resection
is the only treatment that offers a chance of cure in patients
(2). Although preoperative diagnosis
has been improved by modern imaging techniques, it is still
difficult to diagnose the extent of cholangiocarcinoma infiltration
(3). Currently, the only method to
confirm microscopic infiltration of cancer into ductal resection
margins during surgery is intraoperative histological examination.
However, some reports have revealed that prognosis for patients
with positive bile duct stumps does not differ significantly from
that of negative patients (4).
Moreover, recent studies have shown that additional resection of a
positive proximal bile duct margin does not offer any survival
advantage (4,5). It remains controversial whether
additional resection of the bile duct has a favorable impact on the
patient outcomes (5). The impact of
remnant carcinoma in situ (CIS) at the bile duct stump on on
postoperative course remain unclear (4). In this study, surgical margin status and
postoperative course were evaluated in 93 patients with biliary
tract cancer to determine any correlation between remnant carcinoma
and postoperative survival. Positive margin cases were divided into
two groups, invasive carcinoma and carcinoma in situ, and
the postoperative survival and recurrence-free survival were
evaluated. Immunohistochemical staining targeting Ki67 and p53 for
positive margins and invasion portion was conducted to evaluate the
correlation between expression of these proteins and prognosis.
Patients and methods
Patients
All consecutive patients who underwent resection for
biliary tract cancer at our institution between January 2004 and
May 2012 were identified from a database. The number of cases was
93. Patients with carcinoma of the ampulla of Vater and
intrahepatic cholangiocarcinoma were excluded. Data collection and
analysis were performed according to institution guidelines in
conformance with the ethical standards of the Declaration of
Helsinki. The present study was approved by the Ethics Committee of
Nippon Medical School Tamanagayama Hospital (Tokyo, Japan), and a
consent form signed by patients prior to the start of the
study.
Margin status
In practice, margins of at least 5 mm are
recommended to achieve curative resection. Intraoperative
histological examination is routinely used to assess the bile duct
margin. If evidence or possibility of a tumour-positive margin is
observed during this examination, additional resection of the
proximal duct is performed, but only as far as it is technically
feasible. All duct margins submitted for intraoperative
histological examination are then re-evaluated later by permanent
pathology. Margin status is classified as negative, positive with
CIS, or positive with invasive carcinoma.
Immunostaining and scoring
In this study we used the cancer tissues in ductal
margin. The paraffin-embedded serial tissue sections (3.5-µm thick)
were subjected to immunostaining using Histofine Simple Stain
Max-PO kits. Antigen Activation Liquid (pH 9.0; Nichirei
Biosciences, Inc., Tokyo, Japan) at 121°C for 15 min. Endogenous
peroxidase was blocked in 0.3% hydrogen peroxide and methanol for
30 min. Sections were then incubated with antibodies for p53 (cat.
no. 20050705; dilution, 1:50) and Ki-67 (MIB1; cat. no. 20049476;
dilution, 1:500; both Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) in phosphate-buffered saline containing 1% bovine
serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
16 h at 4°C. The sections were further incubated with the Histofine
Simple Stain™ MAX-PO (R; Nichirei Biosciences, Inc.) for
30 min, and peroxidase activity was visualized by
3,3′-diaminobenzidine. The sections were then counterstained with
hematoxylin. Evaluation of the degree and positive ratio of p53 and
ki-67 immunostaining was conducted using the following scale: 0, no
staining; 1+, mild staining; 2+, moderate staining; and 3+, intense
staining; and 0, no staining; 1+, 1–30% positive ratio in cancer
cells; 2+, 31–60%; and 3+, >60% (6).
Clinicopathological features
Clinicopathological data included age, sex, location
of the main tumour, surgical dissection margin, pathological depth
of invasion (pT), major vessel invasion (A, PV), minor vein
invasion (v), lymph duct invasion (ly), pathological lymph node
metastasis category (pN), pancreas invasion (Panc), duodenal
invasion (Du), hepatic invasion (Hinf), neural invasion (ne), bile
duct distal margin (pDM), horizon margin (pHE), patholoical
dissected margin (pEM), stage characterized according to the
Japanese Society of Biliary Surgery Classification (7), date of recurrence, recurrence site, and
date and cause of death. Sites of disease recurrence were confirmed
by computed tomography and/or magnetic resonance imaging and/or
positron emission tomography/computed tomography. When new findings
of suspected cancer appeared, and cancer progression was observed
by serial imaging, radiologic evidence of tumour recurrence was
accepted without a biopsy. The date of the first radiologic finding
of suspected cancer was recorded as the date of initial disease
recurrence.
Statistical analysis
Statistical analysis was performed using the
statistical software package SPSS, version 16.0 (SPSS, Chicago, IL,
USA). Categorical variables were compared using the χ2
test or Fisher's exact test. Survival was calculated using the
Kaplan-Meier method and compared by the log-rank test and
Cox-regression analysis. P<0.05 was considered to indicate a
statisticaly significant difference.
Results
During the study, 93 patients underwent curative
intent resection. The clinical and pathological characteristics of
the study participants are shown in Table
I. The mean age of the patients was 69 years. The age range was
35–85 years and 61 patients were male (65.6%) and 32 patients were
female (34.4%). There were 20 cases (21.5%) of positive-margin
intraoperative histological examination and permanent pathological
findings. The distribution of disease was as follows: 46 cases
(49.5%) of common bile duct cancer (CBD), 25 cases (26.9%) of
hiller cholangiocarcinoma (HCCA), and 22 cases (23.6%) of gall
bladder cancer (GB). Operative procedures included 34 cases of
pancreaticoduodenectomy, 32 cases of hemihepatectomy with bile duct
resection, 16 cases of partial hepatectomy with bile duct
resection, 8 cases cholecystectomy with bile duct resection, and 3
cases of hepatopancreatoduodenectomy. Next, patients were divided
into two groups, a positive margin group (positive) and a negative
margin group (negative). Clinicopathological findings were
comparatively evaluated between the positive and negative groups
(Table II). Cases with major vessel
invasion were significantly higher in the positive group than in
the negative group (P=0.0218). We focused on the positive group and
further divided these patients into two subgroups according to
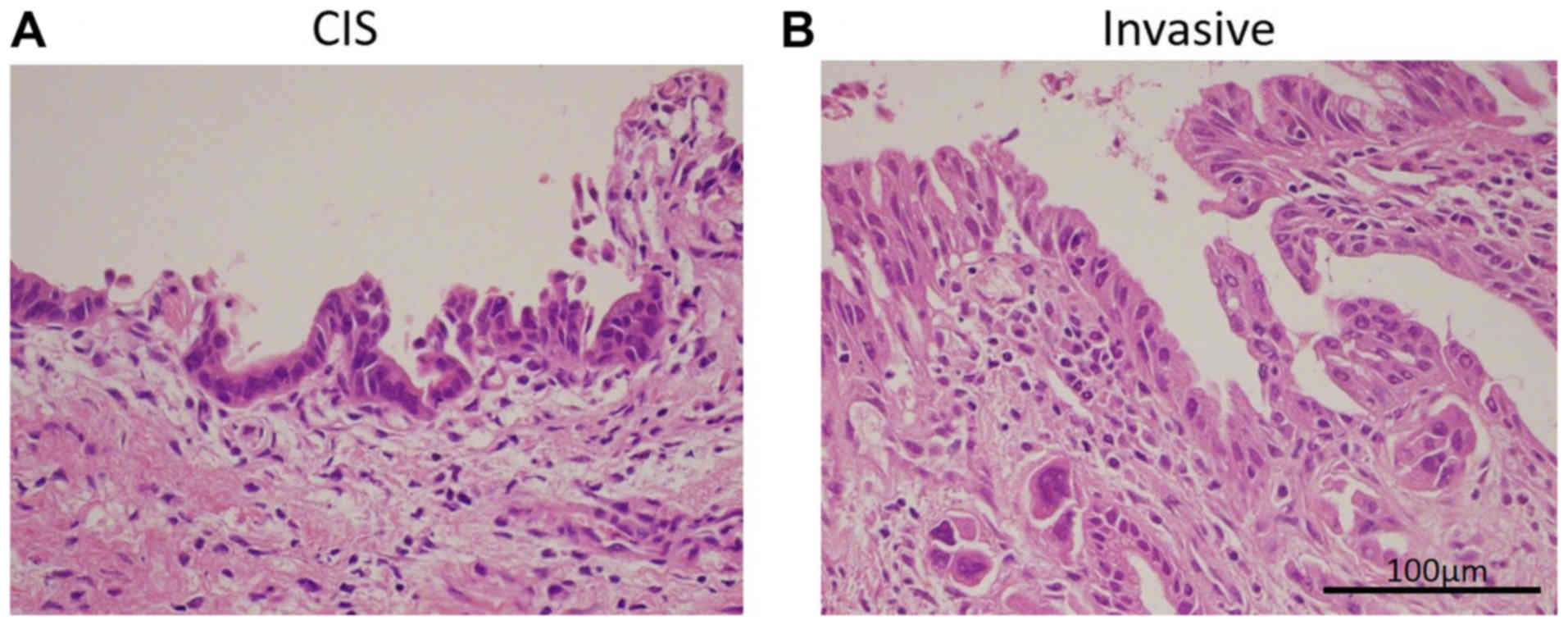
resected margin status: CIS at the bile duct stump (CIS group, n=8)
(Fig. 1A) and invasive carcinoma at
any surgical margin (Invasive group, n=12) (Fig. 1B). No significant difference in
clinicopathological findings between the two groups was observed.
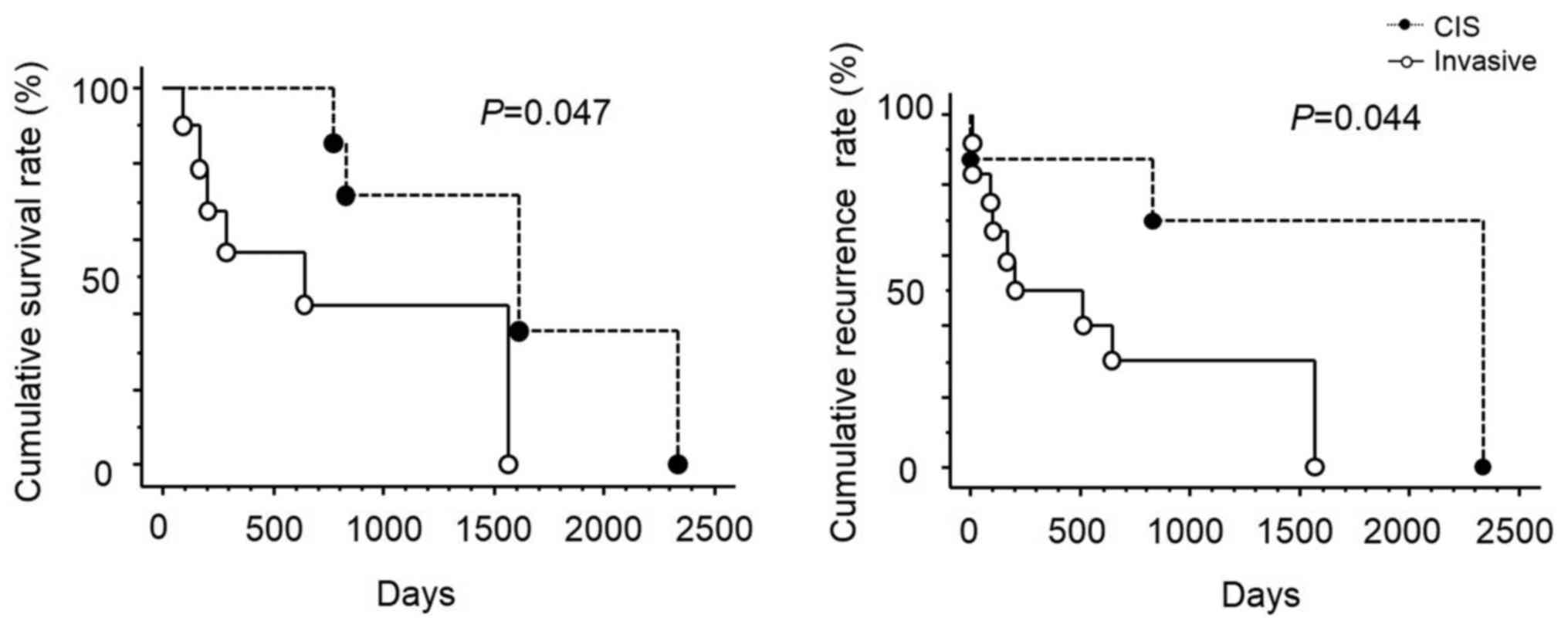
Recurrence and survival were significantly lower and higher,
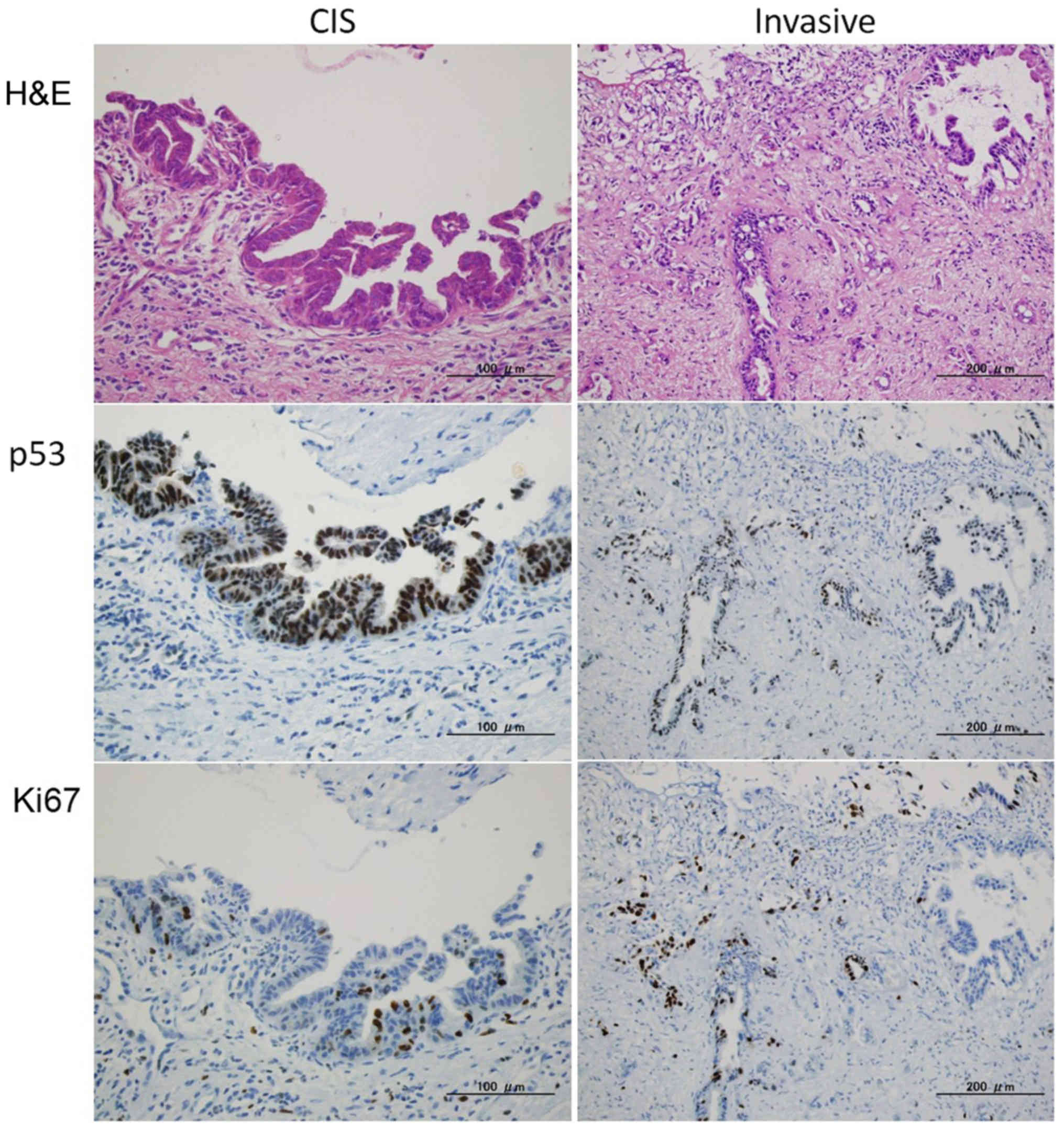
respectively, in the CIS group than in the Invasive group (Fig. 2). Immunohistochemical staining
targeting p53 and Ki67 was conducted in the positive margins
(Fig. 3). The expression levels of
p53 and Ki67 in ductal margins were significantly higher in the
Invasive group (P=0.035) (Table
III). However, the expression levels in the invasion portion
were not significantly different between the two groups. Moreover,
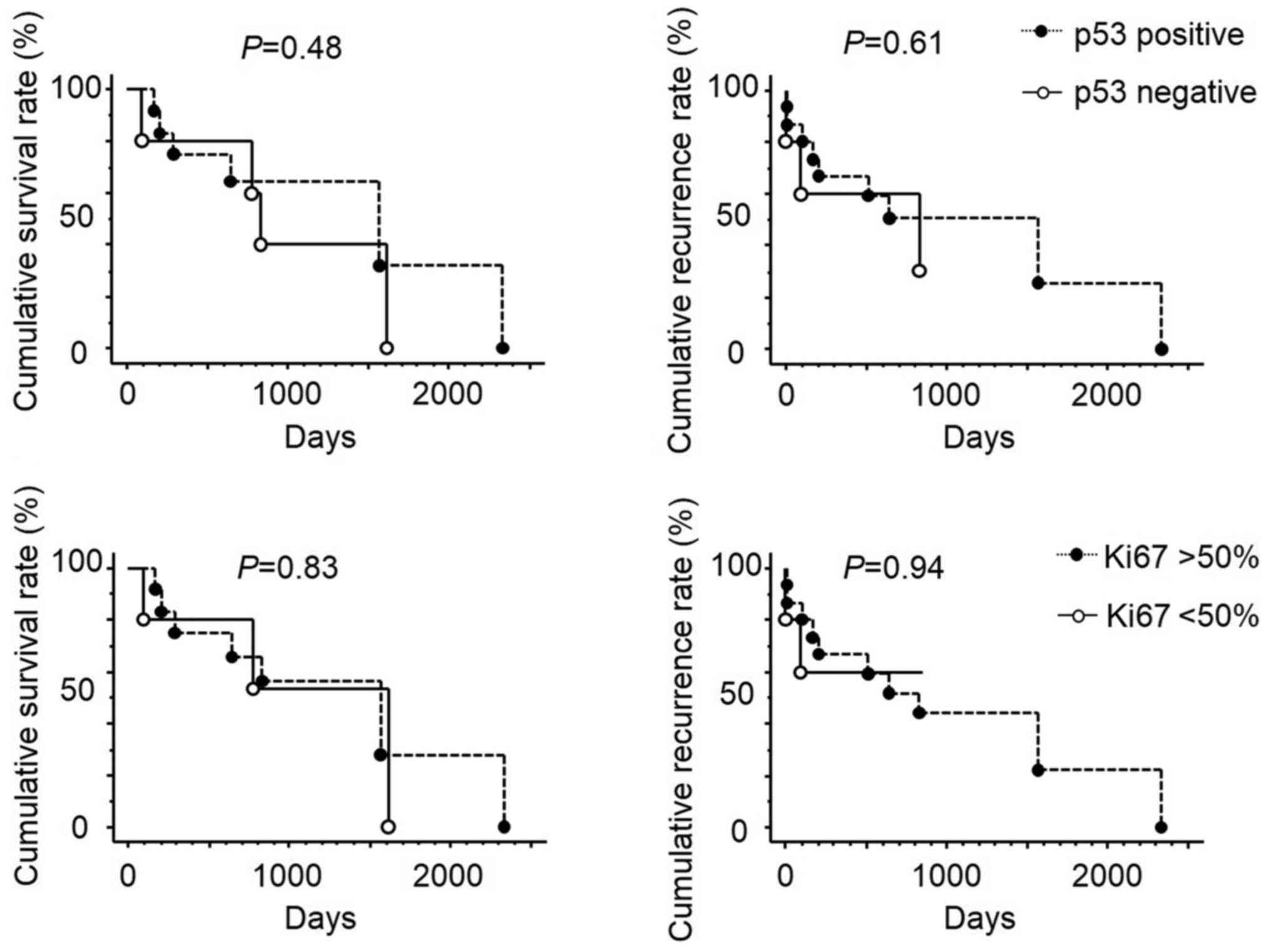
no statistical correlations between the expression levels of p53
and Ki67 and survival and recurrence were observed (Fig. 4). In the positive group, resected
margin status was the most important factor for recurrence-free
survival according to Cox-regression analysis (Table IV).
 | Table I.Clinical and pathological
characteristics of the study. |
Table I.
Clinical and pathological
characteristics of the study.
|
Characteristics | n |
|---|
| Age (years) | 69.0 |
| Sex |
|
|
Male/female | 61/32 |
| Disease |
|
|
CBD/HCCA/GB | 47/25/21 |
| Ductal margin |
|
|
Positive/negative | 20/73 |
| pT |
|
|
1/2/3/4 | 11/21/31/30 |
| pN |
|
|
0/1/2/3 | 50/27/13/3 |
| pM |
|
|
0/1 | 92/1 |
| v |
|
|
0/1/2/3 | 37/21/30/5 |
| ly |
|
|
0/1/2/3 | 17/31/31/14 |
| ne |
|
|
0/1/2/3 | 29/11/19/34 |
| Hinf |
|
|
0/1/2/3 | 61/16/6/10 |
| Du |
|
|
0/1/2/3 | 81/1/8/3 |
| Panc |
|
|
0/1/2/3 | 66/14/7/6 |
| PA |
|
|
0/1/2/3 | 89/3/1/0 |
| PV |
|
|
0/1/2/3 | 84/4/2/3 |
| pEM |
|
|
0/1/2 | 66/14/13 |
| pHM |
|
|
0/1/2 | 79/5/9 |
| pDM |
|
|
0/1/2 | 77/8/8 |
| Cur |
|
|
A/B/C | 42/25/26 |
| pStage |
|
|
I/II/III/IVa/IVb | 6/18/28/34/7 |
 | Table II.Clinicopathological findings in the
positive and negative groups. |
Table II.
Clinicopathological findings in the
positive and negative groups.
| Variable | Negative
(n=73) | Positive
(n=20) | P-value |
|---|
| Age (years) | 68.9 | 69.1 | 0.9416 |
| Sex |
|
|
|
|
Male/female | 45/28 | 16/4 | 0.1258 |
| Disease |
|
|
|
|
CBD/HCCA/GB | 35/19/19 | 12/5/3 | 0.7102 |
| pT |
|
|
|
|
1/2/3/4 | 6/16/24/27 | 5/5/7/3 | 0.1033 |
| pN |
|
|
|
|
0/1/2/3 | 40/18/12/3 | 10/5/5/0 | 0.6260 |
| pM |
|
|
|
|
0/1 | 73/0 | 19/1 | 0.0548 |
| v |
|
|
|
|
0/1/2/3 | 25/16/23/4 | 5/5/7/1 | 0.9096 |
| ly |
|
|
|
|
0/1/2/3 | 12/22/25/12 | 2/9/6/2 | 0.5706 |
| ne |
|
|
|
|
0/1/2/3 | 19/9/14/25 | 3/2/5/9 | 0.6623 |
| Hinf |
|
|
|
|
0/1/2/3 | 44/12/5/8 | 9/4/1/2 | 0.9090 |
| Du |
|
|
|
|
0/1/2/3 | 43/0/7/3 | 13/1/1/0 | 0.1775 |
| Panc |
|
|
|
|
0/1/2/3 | 30/10/7/6 | 10/4/0/0 | 0.2269 |
| PA |
|
|
|
|
0/1/2/3 | 65/1/0/0 | 12/5/1/0 | 0.0218 |
| PV |
|
|
|
|
0/1/2/3 | 59/2/2/2 | 15/2/0/1 | 0.4286 |
| pEM |
|
|
|
|
0/1/2 | 51/8/9 | 9/6/4 | 0.0530 |
| pStage |
|
|
|
|
I/II/III/IVa/IVb | 4/13/22/28/6 | 2/5/6/6/1 | 0.8305 |
 | Table III.No statistical correlation between
the clinicopathological findings and expression of p53 and Ki67 was
observed in the CIS and invasive groups. |
Table III.
No statistical correlation between
the clinicopathological findings and expression of p53 and Ki67 was
observed in the CIS and invasive groups.
|
| CIS (n=8) | Invasive
(n=12) | P-value |
|---|
| Age (years) | 65.6 | 71.4 | 0.1462 |
| Sex |
|
|
|
|
Male/female | 5/3 | 11/1 | 0.1101 |
| Disease |
|
|
|
|
CBD/HCCA/GB | 5/2/1 | 6/4/2 | 0.8594 |
| pT |
|
|
|
|
1/2/3/4 | 2/1/3/2 | 3/4/4/1 | 0.6276 |
| pN |
|
|
|
|
0/1/2/3 | 6/1/1/0 | 4/4/4/0 | 0.1889 |
| pM |
|
|
|
|
0/1 | 7/1 | 12/0 | 0.2089 |
| v |
|
|
|
|
0/1/2/3 | 2/2/4/0 | 5/3/2/1 | 0.5924 |
| ly |
|
|
|
|
0/1/2/3 | 1/3/3/1 | 2/6/3/1 | 0.9065 |
| ne |
|
|
|
|
0/1/2/3 | 2/2/1/3 | 2/0/4/6 | 0.2440 |
| Hinf |
|
|
|
|
0/1/2/3 | 5/1/1/1 | 8/3/0/1 | 0.5784 |
| Du |
|
|
|
|
0/1/2/3 | 8/0/0/0 | 11/0/1/0 | 0.4022 |
| Panc |
|
|
|
|
0/1/2/3 | 6/2/0/0 | 10/2/0/0 | 0.6481 |
| PA |
|
|
|
|
0/1/2/3 | 6/2/0/0 | 11/0/1/0 | 0.1478 |
| PV |
|
|
|
|
0/1/2/3 | 5/2/0/1 | 11/0/0/0 | 0.0709 |
| pEM |
|
|
|
|
0/1/2 | 5/2/1 | 5/4/3 | 0.6360 |
| pStage |
|
|
|
|
I/II/III/IVa/IVb | 0/2/2/3/1 | 2/3/4/3/0 | 0.5112 |
| p53 |
|
|
|
|
Negative/positive | 4/4 | 1/11 | 0.0350 |
| Ki67 |
|
|
|
|
<50%/>50% | 4/4 | 1/11 | 0.0350 |
 | Table IV.Cox-regression analysis of
recurrence-free survival in the positive group. |
Table IV.
Cox-regression analysis of
recurrence-free survival in the positive group.
| Recurrence-free
survival | P-value | Hazard ratio | 95% CI |
|---|
| Ductal margin | 0.0229 | 15.8745 | 1.468–171.7 |
| p53 | 0.0778 | 0.0662 | 0.003–1.354 |
| Ki67 | 0.4885 | 2.6464 | 0.1686–41.55 |
Discussion
Although histologically negative margins have been
recognized as the most important independent determinant of
survival, the additional resection of a diagnosed positive proximal
bile duct is still controversial (4).
Many studies have reported positive surgical margins as an
important predictor of poor prognosis (8–11).
According to several reports, however, the prognosis for patients
with positive bile duct stumps does not differ significantly from
that of carcinoma-negative patients. Moreover, some studies have
even described long-term survival for patients with a positive
surgical bile duct stump (12,13).
Histologically positive surgical bile duct stumps can be further
subclassified into two subtypes: Invasive carcinoma and CIS
(4). Some reports have shown the
survival rate of CIS groups to be no less than that of margin
negative groups and higher than that of invasive groups (4). In this study, bile duct stumps were also
divided into the same two groups. The recurrence and survival rates
in the CIS group were not lower than those in the negative group
(data not shown). The recurrence and survival rates were
significantly lower and higher in the CIS group than in the
Invasive group. The biological nature of main tumours with
extensive superficial spread, which is likely to be responsible for
remnant CIS, tends to be less malignant than that of conventional
cholangiocarcinoma (4). Main tumours
of extrahepatic bile duct carcinoma with extensive superficial
spreading tend to have shallower invasion, more localized type
gross appearance, and a more defined histological differentiation
than conventional cholangiocarcinoma (14–16).
Despite the usefulness of intraoperative histological examination
in assessing tumour involvement in the bile duct, there are
limitations to this method (17,18). A
common feature of tumour spread is a submucosal pattern extending
from 1 to 2 cm beyond the abnormal lesions, as observed in an
imaging study (19). Intraoperative
examination of margin status can be difficult due to the biological
characteristics of this tumour, which involve the proximal
microscopic spread of the disease along the bile duct, extending
beyond the palpable macroscopic boundaries (17,20).
Intraoperative histological examination may inaccurately
differentiate between invasive carcinoma from epithelial atypia or
dysplasia, especially in cases of inflammation of the ducts due to
obstruction or a biliary drainage procedure (21). These factors lead to inaccurate
diagnosis. Moreover, in our study, major vessel invasion was
significantly more frequent in the carcinoma-positive group than in
the carcinoma-negative group. When a positive surgical margin is
identified, the state of the positive surgical margin must be
examined more closely. The bile duct stump was also examined using
immunohistochemical staining targeting Ki67 and p53. Ki67 is a
prognostic marker of tumour proliferation that has been extensively
researched in retrospective studies (22). Its cellular location is strongly cell
cycle dependent (23,24). Ki67 levels are low during G1 and early
S phase and peak during mitosis (22). p53 functions as a transcription factor
involved in cell-cycle control, DNA repair, apoptosis and cellular
stress responses (25). The tumour
suppressor gene p53 was the first identified cancer gene (26). During cell cycle arrest, p53 functions
by upregulating cyclin-dependent kinase (cdk) inhibitor p21, which
can be detected as an immunohistochemical overexpression (27–29).
Inactivation of p53 caused by missense mutations or interaction
with oncogenic viral proteins results in a selective growth
advantage for cancer cells (30,31). Most
p53 gene mutations stop tumour suppressor activity (32). The frequency of p53 overexpression in
cholangiocarcinoma is reported to vary from 19 to 58% (32–37). In
this study, immunohistochemical staining targeting Ki67 and p53 in
surgical bile duct stumps did not show a correlation with overall
survival or recurrence-free survival. The histopathological
findings of surgical bile duct stumps, whether positive with CIS or
positive with invasive carcinoma, were more important than
immunohistochemical staining findings.
In this study, we did not perform the experiment
using the cell lines. Further studies were recommended to verify
our results.
Some report showed the radiotherapy for biliary
tract cancer (38). However, the
radiotherapy for biliary tract cancer is not established. Some
report showed the benefit of disease-free survival (39), but the evidence is insufficient due to
the small number (<10) of 5-year survivors and retrospective
study (38). Moreover, some reports
have revealed the effectiveness of chemotherapy for biliary tract
cancer (40). However, adjuvant
chemotherapy for biliary tract cancer has not been investigated.
Some reports evaluating the impact of adjuvant treatment with
systemic chemotherapy suggested a benefit for high-risk patients
with positive lymph nodes or positive resection margins (41,42).
Prospective studies are needed. A large multi-national study
(ACTICCA-1) is evaluating the combination of gemcitabine plus
cisplatin for adjuvant chemotherapy with results expected within
the next years (40,43).
Intraoperative histological examination of the
surgical margin of the bile duct is essential during biliary tract
cancer surgery. The status of the resected margin in the positive
group was the most important factor for postoperative survival and
recurrence in biliary tract cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JU, HY and EU designed the study, performed the
experiments and wrote the manuscript. YMa, NT, MY, AH, YK, YMi, TS,
TK, HT and RK performed the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of Nippon Medical School, Tamanagayama Hospital (approval
no. 407) and written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castro FA, Koshiol J, Hsing AW and Devesa
SS: Biliary tract cancer incidence in the United States-demographic
and temporal variations by anatomic site. Int J Cancer.
133:1664–1671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konishi M, Ochiai A, Ojima H, Hasebe T,
Mano M, Ohta T, Ito I, Sasaki K, Yasukawa S, Shimada K, et al: A
new histological classification for intra-operative histological
examination of the ductal resection margin in cholangiocarcinoma.
Cancer Sci. 100:255–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konishi M, Iwasaki M, Ochiai A, Hasebe T,
Ojima H and Yanagisawa A: Clinical impact of intraoperative
histological examination of the ductal resection margin in
extrahepatic cholangiocarcinoma. Br J Surg. 97:1363–1368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakanishi Y, Kondo S, Zen Y, Yonemori A,
Kubota K, Kawakami H, Tanaka E, Hirano S, Itoh T and Nakanuma Y:
Impact of residual in situ carcinoma on postoperative survival in
125 patients with extrahepatic bile duct carcinoma. J Hepatobiliary
Pancreat Sci. 17:166–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ribero D, Amisano M, Lo Tesoriere R, Rosso
S, Ferrero A and Capussotti L: Additional resection of an
intraoperative margin-positive proximal bile duct improves survival
in patients with hilar cholangiocarcinoma. Ann Surg. 254:776–783.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuda Y, Yamamoto T, Kudo M, Kawahara K,
Kawamoto M, Nakajima Y, Koizumi K, Nakazawa N, Ishiwata T and Naito
Z: Expression and roles of lumican in lung adenocarcinoma and
squamous cell carcinoma. Int J Oncol. 33:1177–1185. 2008.PubMed/NCBI
|
|
7
|
Miyazaki M, Ohtsuka M, Miyakawa S, Nagino
M, Yamamoto M, Kokudo N, Sano K, Endo I, Unno M, Chijiiwa K, et al:
Classification of biliary tract cancers established by the Japanese
Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English
edition. J Hepatobiliary Pancreat Sci. 22:181–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kayahara M, Nagakawa T, Ohta T, Kitagawa
H, Tajima H and Miwa K: Role of nodal involvement and the
periductal soft-tissue margin in middle and distal bile duct
cancer. Ann Surg. 229:76–83. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burke EC, Jarnagin WR, Hochwald SN,
Pisters PW, Fong Y and Blumgart LH: Hilar Cholangiocarcinoma:
Patterns of spread, the importance of hepatic resection for
curative operation and a presurgical clinical staging system. Ann
Surg. 228:385–394. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma: Thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondo S, Takada T, Miyazaki M, Miyakawa S,
Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M,
et al: Guidelines for the management of biliary tract and ampullary
carcinomas: Surgical treatment. J Hepatobiliary Pancreat Surg.
15:41–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhuiya MR, Nimura Y, Kamiya J, Kondo S,
Nagino M and Hayakawa N: Clinicopathologic factors influencing
survival of patients with bile duct carcinoma: Multivariate
statistical analysis. World J Surg. 17:653–657. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M,
Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D and Blumgart
LH: Staging, resectability, and outcome in 225 patients with hilar
cholangiocarcinoma. Ann Surg. 234:507–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Igami T, Nagino M, Oda K, Nishio H, Ebata
T, Yokoyama Y and Shimoyama Y: Clinicopathologic study of
cholangiocarcinoma with superficial spread. Ann Surg. 249:296–302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakanishi Y, Zen Y, Kawakami H, Kubota K,
Itoh T, Hirano S, Tanaka E, Nakanuma Y and Kondo S: Extrahepatic
bile duct carcinoma with extensive intraepithelial spread: A
clinicopathological study of 21 cases. Mod Pathol. 21:807–816.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ojima H, Kanai Y, Iwasaki M, Hiraoka N,
Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Sakamoto M and
Hirohashi S: Intraductal carcinoma component as a favorable
prognostic factor in biliary tract carcinoma. Cancer Sci.
100:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Endo I, House MG, Klimstra DS, Gonen M,
D'Angelica M, Dematteo RP, Fong Y, Blumgart LH and Jarnagin WR:
Clinical significance of intraoperative bile duct margin assessment
for hilar cholangiocarcinoma. Ann Surg Oncol. 15:2104–2112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okazaki Y, Horimi T, Kotaka M, Morita S
and Takasaki M: Study of the intrahepatic surgical margin of hilar
bile duct carcinoma. Hepatogastroenterology. 49:625–627.
2002.PubMed/NCBI
|
|
19
|
Baton O, Azoulay D, Adam DV and Castaing
D: Major hepatectomy for hilar cholangiocarcinoma type 3 and 4:
Prognostic factors and longterm outcomes. J Am Coll Surg.
204:250–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakamoto E, Nimura Y, Hayakawa N, Kamiya
J, Kondo S, Nagino M, Kanai M, Miyachi M and Uesaka K: The pattern
of infiltration at the proximal border of hilar bile duct
carcinoma: A histologic analysis of 62 resected cases. Ann Surg.
227:405–411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Hwang DW, Lee SY, Park KM and Lee
YJ: The proximal margin of resected hilar cholangiocarcinoma: The
effect of microscopic positive margin on long-term survival. Am
Surg. 78:471–477. 2012.PubMed/NCBI
|
|
22
|
Konstantinos K, Marios S, Anna M, Nikolaos
K, Efstratios P and Paulina A: Expression of Ki-67 as proliferation
biomarker in imprint smears of endometrial carcinoma. Diagn
Cytopathol. 41:212–217. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isola J, Helin H and Kallioniemi OP:
Immunoelectron-microscopic localization of a
proliferation-associated antigen Ki-67 in MCF-7 cells. Histochem J.
22:498–506. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verheijen R, Kuijpers HJ, Schlingemann RO,
Boehmer AL, van Driel R, Brakenhoff GJ and Ramaekers FC: Ki-67
detects a nuclear matrix-associated proliferation-related antigen.
I. Intracellular localization during interphase. J Cell Sci.
92:123–130. 1989.PubMed/NCBI
|
|
25
|
Rufini A, Tucci P, Celardo I and Melino G:
Senescence and aging: The critical roles of p53. Oncogene.
32:5129–5143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greenblatt MS, Bennett WP, Hollstein M and
Harris CC: Mutations in the p53 tumor suppressor gene: Clues to
cancer etiology and molecular pathogenesis. Cancer Res.
54:4855–4878. 1994.PubMed/NCBI
|
|
28
|
Hussain SP and Harris CC: Molecular
epidemiology of human cancer: Contribution of mutation spectra
studies of tumor suppressor genes. Cancer Res. 58:4023–4037.
1998.PubMed/NCBI
|
|
29
|
Kang YK, Kim WH and Jang JJ: Expression of
G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in
intrahepatic cholangiocarcinoma. Hum Pathol. 33:877–883. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harris CC: Structure and function of the
p53 tumor suppressor gene: Clues for rational cancer therapeutic
strategies. J Natl Cancer Inst. 88:1442–1455. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine AJ: The p53 tumor-suppressor gene.
N Engl J Med. 326:1350–1352. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diamantis I, Karamitopoulou E, Perentes E
and Zimmermann A: p53 protein immunoreactivity in extrahepatic bile
duct and gallbladder cancer: Correlation with tumor grade and
survival. Hepatology. 22:774–779. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jan YY, Yeh TS, Yeh JN, Yang HR and Chen
MF: Expression of epidermal growth factor receptor, apomucins,
matrix metalloproteinases, and p53 in rat and human
cholangiocarcinoma: Appraisal of an animal model of
cholangiocarcinoma. Ann Surg. 240:89–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohashi K, Nakajima Y, Kanehiro H, Tsutsumi
M, Taki J, Aomatsu Y, Yoshimura A, Ko S, Kin T, Yagura K, et al:
Ki-ras mutations and p53 protein expressions in intrahepatic
cholangiocarcinomas: relation to gross tumor morphology.
Gastroenterology. 109:1612–1617. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tannapfel A, Engeland K, Weinans L,
Katalinic A, Hauss J, Mössner J and Wittekind C: Expression of p73,
a novel protein related to the p53 tumour suppressor p53, and
apoptosis in cholangiocellular carcinoma of the liver. Br J Cancer.
80:1069–1074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teh M, Wee A and Raju GC: An
immunohistochemical study of p53 protein in gallbladder and
extrahepatic bile duct/ampullary carcinomas. Cancer. 74:1542–1545.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Washington K and Gottfried MR: Expression
of p53 in adenocarcinoma of the gallbladder and bile ducts. Liver.
16:99–104. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Labib PL, Davidson BR, Sharma RA and
Pereira SP: Locoregional therapies in cholangiocarcinoma. Hepat
Oncol. 4:99–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shinohara E, Mitra N, Guo M and Metz J:
Radiation therapy is associated with improved survival in the
adjuvant and definitive treatment of intrahepatic
cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 72:1495–1501.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siebenhüner AR, Seifert H, Bachmann H,
Seifert B, Winder T, Feilchenfeldt J, Breitenstein S, Clavien PA,
Stupp R, Knuth A, et al: Adjuvant treatment of resectable biliary
tract cancer with cisplatin plus gemcitabine: A prospective single
center phase II study. BMC Cancer. 18:722018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: A
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McNamara MG, Walter T, Horgan AM, Amir E,
Cleary S, McKeever EL, Min T, Wallace E, Hedley D, Krzyzanowska M,
et al: Outcome of adjuvant therapy in biliary tract cancers. Am J
Clin Oncol. 38:382–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stein A, Arnold D, Bridgewater J,
Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J,
Schrum S, et al: Adjuvant chemotherapy with gemcitabine and
cisplatin compared to observation after curative intent resection
of cholangiocarcinoma and muscle invasive gallbladder carcinoma
(ACTICCA-1 trial)-a randomized, multidisciplinary, multinational
phase III trial. BMC Cancer. 15:5642015. View Article : Google Scholar : PubMed/NCBI
|