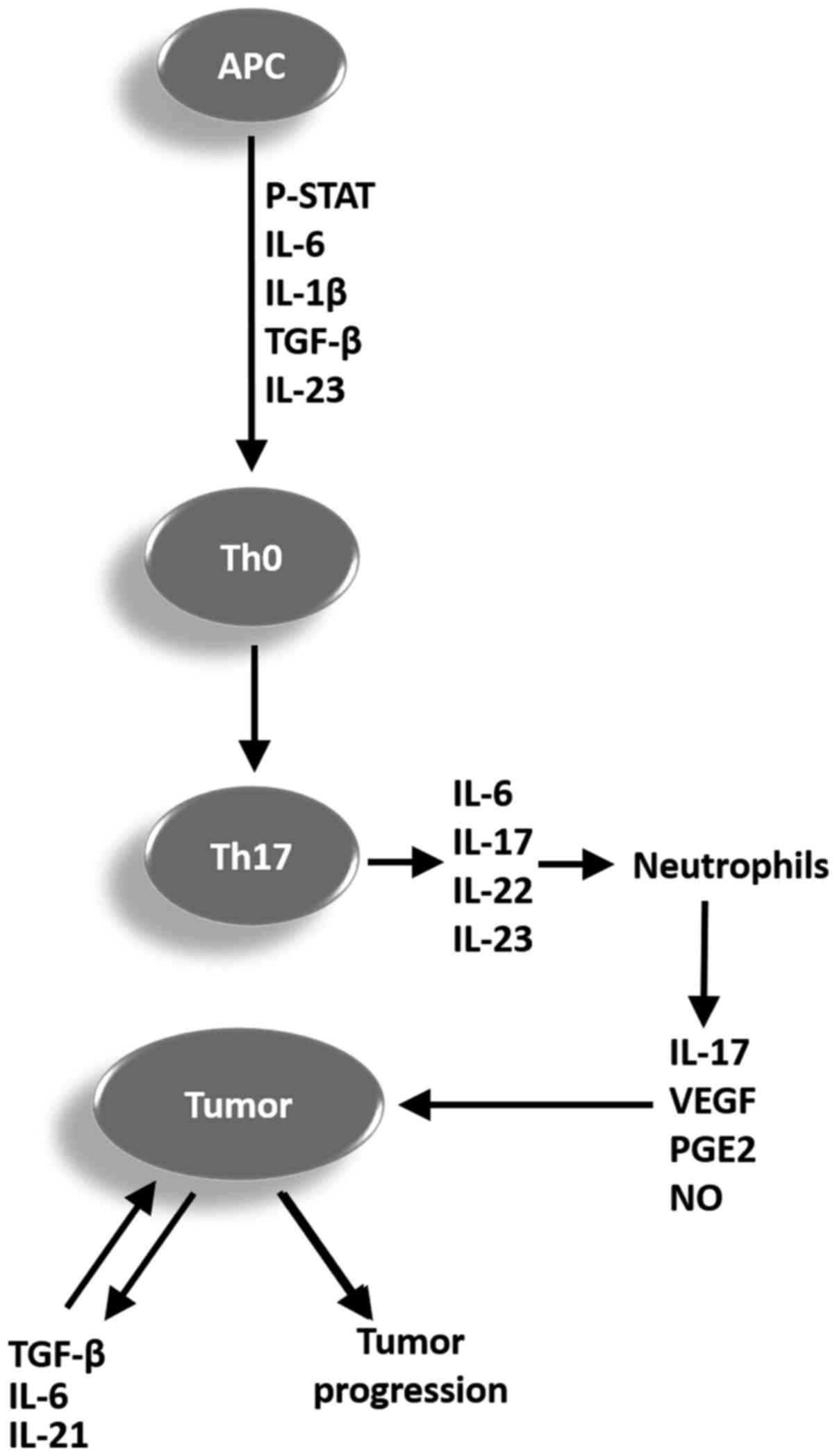
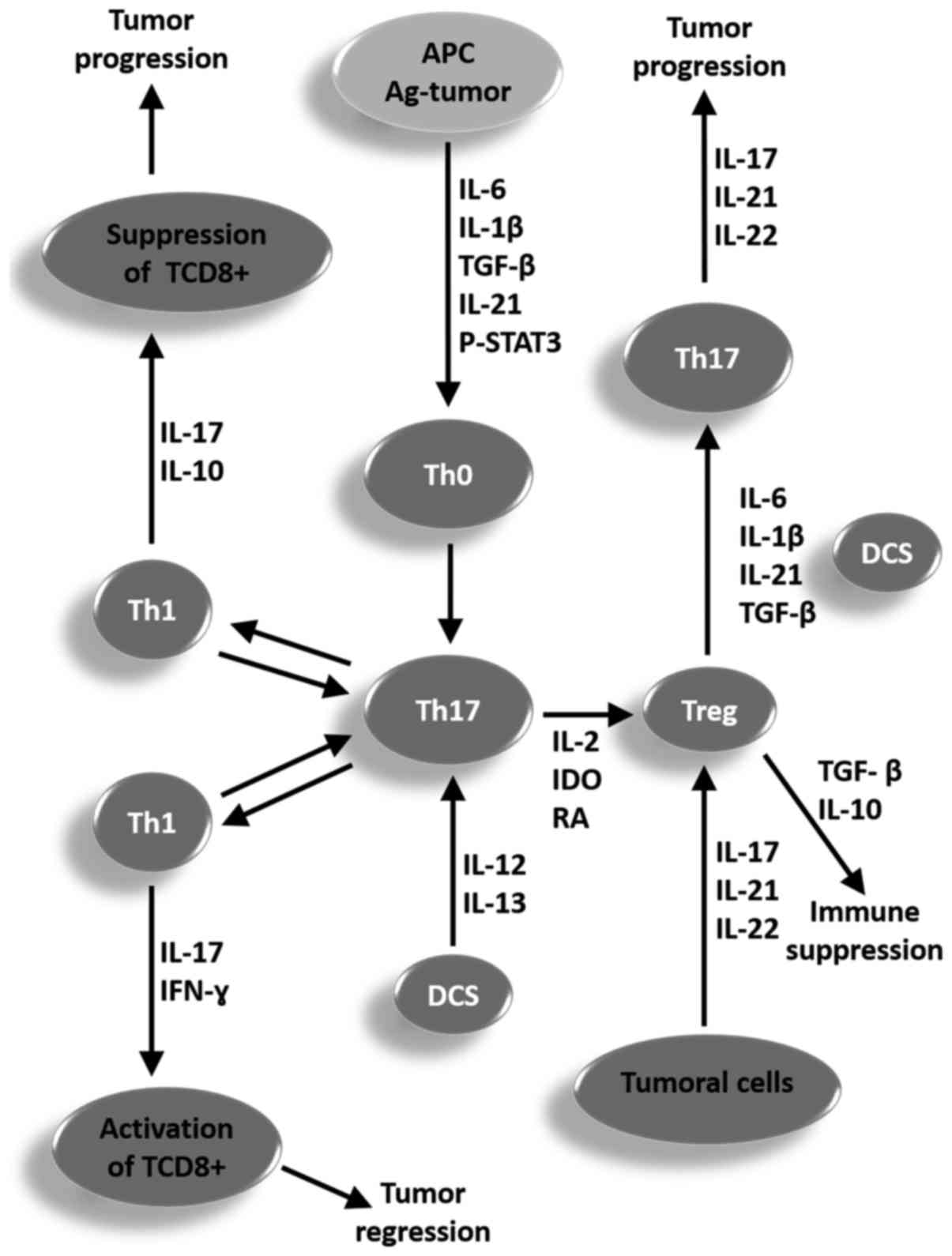
|
1
|
Catarino R, Petignat P, Dongui G and
Vassilakos P: Cervical cancer screening in developing countries at
a crossroad: Emerging technologies and policy choices. World J Clin
Oncol. 6:281–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosch FX and de Sanjosé S: The
epidemiology of human papillomavirus infection and cervical cancer.
Dis Markers. 23:213–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lima EG, de Lima DB, Miranda CA, de Sena
Pereira VS, de Azevedo JC, de Araújo JM, de Medeiros Fernandes TA,
de Azevedo PR and Fernandes JV: Knowledge about HPV and screening
of cervical cancer among women from the metropolitan region of
Natal, Brazil. ISRN Obstet Gynecol. 2013:9304792013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y and Zhan FB: Geographic variations
of racial/ethnic disparities in cervical cancer mortality in Texas.
South Med J. 107:281–288. 2014.PubMed/NCBI
|
|
7
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 30 Suppl 5:F12–F23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaccarella S, Lortet-Tieulent J, Plummer
M, Franceschi S and Bray F: Worldwide trends in cervical cancer
incidence: Impact of screening against changes in disease risk
factors. Eur J Cancer. 49:3262–3273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castellsagué X: Natural history and
epidemiology of HPV infection and cervical cancer. Gynecol Oncol.
110 3 Suppl 2:S4–S7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bodily J and Laimins LA: Persistent of
human papillomavirus infection: Keys to malignant progression.
Trends Microbiol. 19:33–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz N, Castellsagué X, de González AB
and Gissman L: Chapter 1-HPV in the etiology of human cancer.
Vaccine. 24 Suppl 3:S3/1–10. 2006. View Article : Google Scholar
|
|
12
|
Saavedra KP, Brebi PM and Roa JC:
Epigenetic alterations in preneoplastic and neoplastic lesions of
the cervix. Clin Epigenetics. 4:132012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daud II, Scott ME, Ma Y, Shiboski S,
Farhat S and Moscicki AB: Association between toll-like receptor
expression and human papillomavirus type 16 persistence. Int J
Cancer. 128:879–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mora-García ML and Monroy-García A: Immune
response in cervical cancer. Strategies for the development of
therapeutic vaccines. Rev Med Inst Mex Seguro Soc. 53 Suppl
2:S206–S211. 2015.PubMed/NCBI
|
|
15
|
Stanley MA: Immune responses to human
papilloma viruses. Indian J Med Res. 130:266–276. 2009.PubMed/NCBI
|
|
16
|
Song D, Li H, Li H and Dai J: Effect of
human papillomavirus infection on the immune system and its role in
the course of cervical cancer. Oncol Lett. 10:600–606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwasaki A: Antiviral immune responses in
the genital tract: Clues for vaccines. Nat Rev Immunol. 10:699–711.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasagawa T, Takagi H and Makinoda S:
Immune responses against human papillomavirus (HPV) infection and
evasion of host defense in cervical cancer. J Infect Chemother.
18:807–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stanley M: Immunobiology of HPV and HPV
vaccines. Gynecol Oncol. 109 Suppl 2:S15–S21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blaskewicz CD, Pudney J and Anderson DJ:
Structure and function of intercellular junctions in human cervical
and vaginal mucosal epithelia. Biol Reprod. 85:97–104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hervouet C, Luci C, Rol N, Rousseau D,
Kissenpfennig A, Malissen B, Czerkinsky C and Anjuère F: Langerhans
cells prime IL-17-producing T cells and dampen genital cytotoxic
responses following mucosal immunization. J Immunol. 184:4842–4851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kemp TJ, Hildesheim A, García-Piñeres A,
Williams MC, Shearer GM, Rodriguez AC, Schiffman M, Burk R, Freer
E, Bonilla J, et al: Elevated systemic levels of inflammatory
cytokines in older women with persistent cervical human
papillomavirus infection. Cancer Epidemiol Biomarkers Prev.
19:1954–1959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Ma D, Zhang Y, Tian Y, Wang X,
Qiao Y and Cui B: The imbalance of Th17/Treg in patients with
uterine cervical cancer. Clin Chim Acta. 412:894–900. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paradkar PH, Joshi JV, Mertia PN, Agashe
SV and Vaidya RA: Role of cytokines in genesis, progression and
prognosis of cervical cancer. Asian Pac J Cancer Prev.
15:3851–3864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cong J, Liu R, Wang X, Sheng L, Jiang H,
Wang W, Zhang Y, Yang S and Li C: Association between
interluekin-17 gene polymorphisms and the risk of cervical cancer
in a Chinese population. Int J Clin Exp Pathol. 8:9567–9573.
2015.PubMed/NCBI
|
|
26
|
Punt S, Fleuren GJ, Kritikou E, Lubberts
E, Trimbos JB, Jordanova ES and Gorter A: Angels and demons: Th17
cells represent a beneficial response, while neutrophil IL-17 is
associated with poor prognosis in squamous cervical cancer.
Oncoimmunology. 4:e9845392015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong C: Targeting Th17 cells in immune
diseases. Cell Res. 24:901–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitt E, Klein M and Bopp T: Th9 cells,
new players in adaptive immunity. Trends Immunol. 35:61–68. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaplan MH: Th9 cells: Differentiation and
disease. Immunol Rev. 252:104–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldszmid RS, Dzutsev A and Trinchieri G:
Host immune response to infection and cancer: Unexpected
commonalities. Cell Host Microbe. 15:295–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Damsker JM, Hansen AM and Caspi RR: Th1
and Th17 cells: Adversaries and collaborators. Ann N Y Acad Sci.
1183:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I.
Definition according to profiles of lymphokine activities and
secreted proteins. J Immunol. 136:2348–2357. 1986.PubMed/NCBI
|
|
33
|
Wilson JN, Boniface K, Chan JR, McKenzie
BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel
F, et al: Development, cytokine profile and function of human
interleukin 17-producing helper T cells. Nat Immunol. 8:950–957.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy KM and Reiner SL: The lineage
decisions of helper T cells. Nat Rev Immunol. 2:933–944. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gavin MA, Rasmussen JP, Fontenot JD, Vasta
V, Manganiello VC, Beavo JA and Rudensky AY: Foxp3-dependent
programme of regulatory T-cell differentiation. Nature.
445:771–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Josefowicz SZ, Lu LF and Rudensky AY:
Regulatory T cells: Mechanisms of differentiation and function.
Annu Rev Immunol. 30:531–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Asadzadeh Z, Mohammadi H, Safarzadeh E,
Hemmatzadeh M, Mahdian-Shakib A, Jadidi-Niaragh F, Azizi G and
Baradaran B: The paradox of Th17 cell functions in tumor immunity.
Cell Immunol. 322:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Veldhoen M, Uyttenhove C, van Snick J,
Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C and Stockinger
B: Transforming growth factor-beta ‘reprograms’ the differentiation
of T helper 2 cells and promotes an interleukin 9-producing subset.
Nat Immunol. 9:1341–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eyerich S, Eyerich K, Pennino D, Carbone
T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann
C, Behrendt H, et al: Th22 cells represent a distinct human T cell
subset involved in epidermal immunity and remodeling. J Clin
Invest. 119:3573–3585. 2009.PubMed/NCBI
|
|
40
|
Maddur MS, Miossec P, Kaveri SV and Bayry
J: Th17 cells: Biology, pathogenesis of autoimmune and inflammatory
diseases, and therapeutic strategies. Am J Pathol. 181:8–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peters A, Lee Y and Kuchroo VK: The many
faces of Th17 cells. Curr Opin Immunol. 23:702–706. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ziegler SF and Buckner JH: FOXP3 and the
regulation of Treg/Th17 differentiation. Microbes Infect.
11:594–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Hamburg JP, Mus AM, de Bruijn MJ, de
Vogel L, Boon L, Cornelissen F, Asmawidjaja P, Hendriks RW and
Lubberts E: GATA-3 protects against severe joint inflammation and
bone erosion and reduces differentiation of Th17 cells during
experimental arthritis. Arthritis Rheum. 60:750–759. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human CD4+
IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA.
105:15505–15510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nalbant A and Eskier D: Genes associated
with T helper 17 cell differentiation and function. Front Biosci
(Elite Ed). 8:427–435. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang S, Takaku M, Zou L, Gu AD, Chou WC,
Zhang G, Wu B, Kong Q, Thomas SY, Serody JS, et al: Reversing
SKI-SMAD4-mediated suppression is essential for TH17 cell
differentiation. Nature. 551:105–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim HS, Jang SW, Lee W, Kim K, Sohn H,
Hwang SS and Lee GR: PTEN drives Th17 cell differentiation by
preventing IL-2 production. J Exp Med. 214:3381–3398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao M, Tan Y, Peng Q, Huang C, Guo Y,
Liang G, Zhu B, Huang Y, Liu A, Wang Z, et al: IL-6/STAT3 pathway
induced deficiency of RFX1 contributes to Th17-dependent autoimmune
diseases via epigenetic regulation. Nat Commun. 9:5832018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Karczmarczyk A, Karp M and Giannopoulos K:
The role of Th17 cells in tumor immunity Znaczenie limfocytów Th17
w odporności przeciwnowotworowej. Acta Haematol Polonica.
45:155–160. 2014. View Article : Google Scholar
|
|
50
|
Shabgah AG, Fattahi E and Shahneh FZ:
Interleukin-17 in human inflammatory diseases. Postepy Dermatol
Alergol. 31:256–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fu B, Tian Z and Wei H: Th17 cells in
human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol.
11:564–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arnold CE, Gordon P, Barker RN and Wilson
HM: The activation status of human macrophages presenting antigen
determines the efficiency of Th17 responses. Immunobiology.
220:10–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guéry L and Hugues S: Th17 cell plasticity
and functions in cancer immunity. Biomed Res Int. 2015:3146202015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Komatsu N, Okamoto K, Sawa S, Nakashima T,
Oh-hora M, Kodama T, Tanaka S, Bluestone JA and Takayanagi H:
Pathogenic conversion of Foxp3+ T cells into TH17 cells in
autoimmune arthritis. Nat Med. 20:62–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gagliani N, Vesely Amezcua MC, Iseppon A,
Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS,
Ching T, et al: Th17 cells transdifferentiate into regulatory T
cells during resolution of inflammation. Nature. 523:221–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eyerich S, Eyerich K, Cavani A and
Schmidt-Weber C: IL-17 and IL-22: Siblings, not twins. Trends
Immunol. 31:354–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ye J, Livergood RS and Peng G: The role
and regulation of human Th17 cells in tumor immunity. Am J Pathol.
182:10–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gálvez J: Role of Th17 cells in the
pathogenesis of human IBD. ISRN Inflamm. 2014:9284612014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Annunziato F, Cosmi L, Liotta F, Maggi E
and Romagnani S: Human T helper type 1 dichotomy: Origin, phenotype
and biological activities. Immunology. 144:343–351. 2015.
View Article : Google Scholar
|
|
60
|
Barnes MJ and Powrie F: Regulatory T cells
reinforce intestinal homeostasis. Immunity. 31:401–411. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Davidson MG, Alonso MN, Yuan R, Axtell RC,
Kenkel JA, Suhoski MM, González JC, Steinman L and Engleman EG:
Th17 cells induce Th1-polarizing monocyte-derived dendritic cells.
J Immunol. 191:1175–1187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Morrison PJ, Ballantyne SJ and Kullberg
MC: Interleukin-23 and T helper 17-type responses in intestinal
inflammation: From cytokines to T-cell plasticity. Immunology.
133:397–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei L, Wang H, Yang F, Ding Q and Zhao J:
Interleukin-17 potently increases non-small cell lung cancer
growth. Mol Med Rep. 13:1673–1680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Young MR, Levingston CA and Johnson SD:
Treatment to sustain a Th17-type phenotype to prevent skewing
toward Treg and to limit premalignant lesion progression to cancer.
Int J Cancer. 138:2487–2498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu Q, Lou XM and He Y: Preferential
recruitment of Th17 cells to cervical cancer via CCR6-CCL20
pathway. PLoS One. 10:e01208552015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu MY, Kuo TY and Ho HN:
Tumor-infiltrating lymphocytes contain a higher proportion of
FOXP3(+) T lymphocytes in cervical cancer. J Formos Med Assoc.
110:580–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang
T, Kong B and Cui B: Distribution of Th17 cells and
Foxp3-expressing T cells in tumor-infiltrating lymphocytes in
patients with uterine cervical cancer. Clin Chim Acta.
413:1848–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen Z, Ding J, Pang N, Du R, Meng W, Zhu
Y, Zhang Y, Ma C and Ding Y: The Th17/Treg balance and the
expression of related cytokines in Uygur cervical cancer patients.
Diagn Pathol. 8:612013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hou F, Ma D and Cui B: Treg cells in
different forms of uterine cancer. Clin Chim Acta. 415:337–340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gosmann C, Mattarollo SR, Bridge JA,
Frazer IH and Blumenthal A: IL-17 suppresses immune effector
functions in human papillomavirus-associated epithelial
hyperplasia. J Immunol. 193:2248–2257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shukla S, Mahata S, Shishodia G, Pandey A,
Tyagi A, Vishnoi K, Basir SF, Das BC and Bharti AC: Functional
regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis.
PLoS One. 8:e678492013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Backert I, Koralov SB, Wirtz S, Kitowski
V, Billmeier U, Martini E, Hofmann K, Hildner K, Wittkopf N, Brecht
K, et al: STAT3 activation in Th17 and Th22 cells controls
IL-22-mediated epithelial host defense during infectious colitis. J
Immunol. 193:3779–3791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gagliani N, Hu B, Huber S, Elinav E and
Flavell RA: The fire within: Microbes inflame tumors. Cell.
157:776–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li YX, Zhang L, Simayi D, Zhang N, Tao L,
Yang L, Zhao J, Chen YZ, Li F and Zhang WJ: Human papillomavirus
infection correlates with inflammatory Stat3 signaling activity and
IL-17 level in patients with colorectal cancer. PLoS One.
10:e01183912015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Vidal AC, Skaar D, Maguire R, Dodor S,
Musselwhite LW, Bartlett JA, Oneko O, Obure J, Mlay P, Murphy SK
and Hoyo C: IL-10, IL-15, IL-17, and GMCSF levels in cervical
cancer tissue of Tanzanian women infected with HPV16/18 vs.
non-HPV16/18 genotypes. Infect Agent Cancer. 10:102015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liang W and Ferrara N: The complex role of
neutrophils in tumor angiogenesis and metastasis. Cancer Immunol
Res. 4:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gaffen SL: An overview of IL-17 function
and signaling. Cytokine. 43:402–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bettelli E, Korn T, Oukka M and Kuchroo
VK: Induction and effector functions of T(H)17 cells. Nature.
453:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cua DJ and Tato CM: Innate IL-17-producin
cells: The sentinels of the immune system. Nat Rev Immunol.
10:479–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pappu R, Ramirez-Carrozzi V and Sambandam
A: The interleukin-17 cytokine family: Critical players in host
defence and inflammatory diseases. Immunology. 134:8–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bie Q, Jin C, Zhang B and Dong H: IL-17B:
A new area of study in the IL-17 family. Mol Immunol. 90:50–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mills KH: Induction, function and
regulation of IL-17-producing T cells. Eur J Immunol. 38:2636–2649.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Huber M, Heink S, Grothe H, Guralnik A,
Reinhard K, Elflein K, Hünig T, Mittrücker HW, Brüstle A, Kamradt T
and Lohoff M: A Th17-like developmental process leads to CD8(+)
Tc17 cells with reduced cytotoxic activity. Eur J Immunol.
39:1716–1725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tajima M, Wakita D, Satoh T, Kitamura H
and Nishimura T: IL-17/IFN-γ double producing CD8+ T (Tc17/IFN-γ)
cells: A novel cytotoxic T-cell subset converted from Tc17 cells by
IL-12. Int Immunol. 23:751–759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang Y, Hou F, Liu X, Ma D, Zhang Y, Kong
B and Cui B: Tc17 cells in patients with uterine cervical cancer.
PLoS One. 9:e868122014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hu Y, Shen F, Crellin NK and Ouyang W: The
IL-17 pathway as a major therapeutic target in autoimmune diseases.
Ann N Y Acad Sci. 1217:60–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liang SC, Long AJ, Bennett F, Whitters MJ,
Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams
CM, Wright JF and Fouser LA: An IL-17F/A heterodimer protein is
produced by mouse Th17 cells and induces airway neutrophil
recruitment. J Immunol. 179:7791–7799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wright JF, Guo Y, Quazi A, Luxenberg DP,
Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN,
Dunussi-Joannopoulos K, et al: Identification of an interleukin
17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem.
282:13447–13455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ron D, Fuchs Y and Chorev DS: Know thy
Sef: A novel class of feedback antagonists of receptor tyrosine
kinase signaling. Int J Biochem Cell Biol. 40:2040–2052. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mellett M, Atzei P, Horgan A, Hams E,
Floss T, Wurst W, Fallon PG and Moynagh PN: Orphan receptor IL-17RD
tunes IL-17A signalling and is required for neutrophilia. Nat
Commun. 3:11192012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Trajkovic V, Stosic-Grujicic S, Samardzic
T, Markovic M, Miljkovic D, Ramic Z and Stojkovic Mostarica M:
Interleukin-17 stimulates inducible nitric oxide synthase
activation in rodent astrocytes. J Neuroimmunol. 119:183–191. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ruddy MJ, Wong GC, Liu XK, Yamamoto H,
Kasayama S, Kirkwood KL and Gaffen SL: Functional cooperation
between interleukin-17 and tumor necrosis factor-alpha is mediated
by CCAAT/enhancer-binding protein family members. J Biol Chem.
279:2559–2567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhu S and Qian Y: IL-17/IL-17 receptor
system in autoimmune disease: Mechanisms and therapeutic potential.
Clin Sci (Lond). 122:487–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang F, Kao CY, Wachi S, Thai P, Ryu J
and Wu R: Requirement for both JAK-mediated PI3K signaling and
ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in
enhancing cytokine expression in human airway epithelial cells. J
Immunol. 179:6504–6513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Saleh A, Shan L, Halayko AJ, Kung S and
Gounni AS: Critical role for STAT3 in IL-17A-mediated CCL11
expression in human airway smooth muscle cells. J Immunol.
182:3357–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hartupee J, Liu C, Novotny M, Sun D, Li X
and Hamilton TA: IL-17 signaling for mRNA stabilization does not
require TNF receptor-associated factor 6. J Immunol. 182:1660–1666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sun D, Novotny M, Bulek K, Liu C, Li X and
Hamilton TA: Treatment with IL-17 prolongs the half-life of
chemokine CXCL1 mRNA via the adaptor TRAF5 and the
splicing-regulatory factor SF2 (ASF). Nature Immunol. 12:853–860.
2011. View Article : Google Scholar
|
|
99
|
Lv Q, Zhu D, Zhang J, Yi Y, Yang S and
Zhang W: Association between six genetic variants of IL-17A and
IL-17F and cervical cancer risk: A case-control study. Tumour Biol.
36:3979–3984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tartour E, Fossiez F, Joyeux I, Galinha A,
Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V,
et al: Interleukin 17, a T-cell-derived cytokine, promotes
tumorigenicity of human cervical tumors in nude mice. Cancer Res.
59:3698–3704. 1999.PubMed/NCBI
|
|
101
|
Feng M, Wang Y, Chen K, Bian Z, Jinfang Wu
and Gao Q: IL-17A promotes the migration and invasiveness of
cervical cancer cells by coordinately activating MMPs expression
via the p38/NF-κB signal pathway. PLoS One. 9:e1085022014.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Walch-Rückheim B, Mavrova R, Henning M,
Vicinus B, Kim YJ, Bohle RM, Juhasz-Böss I, Solomayer EF and Smola
S: Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support
the recruitment of Th17 cells during cervical cancer progression.
Cancer Res. 75:5248–5259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xue J, Wang Y, Chen C, Zhu X, Zhu H and Hu
Y: Effects of Th17 cells and IL-17 in the progression of cervical
carcinogenesis with high-risk human papillomavirus infection.
Cancer Med. 7:297–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Quan Y, Zhou B, Wang Y, Duan R, Wang K,
Gao Q, Shi S, Song Y, Zhang L and Xi M: Association between IL17
polymorphisms and risk of cervical cancer in Chinese women. Clin
Dev Immunol. 2012:2582932012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li L, Tian YL, Lv XM, Yu HF, Xie YY, Wang
JD and Shi W: Association analysis of IL-17A and IL-17F
polymorphisms in Chinese women with cervical cancer. Genet Mol Res.
14:12178–12183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sun LX, Wang XB and Huang XJ: Association
analysis of rs2275913G>A and rs763780T>C interleukin 17
polymorphisms in Chinese women with cervical cancer. Genet Mol Res.
14:13612–13617. 2015.PubMed/NCBI
|
|
107
|
Hardikar S, Johnson LG, Malkki M,
Petersdorf EW, Galloway DA, Schwartz SM and Madeleine MM: A
population-based case-control study of genetic variation in
cytokine genes associated with risk of cervical and vulvar cancers.
Gynecol Oncol. 139:90–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Huang Q, Duan L, Qian X, Fan J, Lv Z,
Zhang X, Han J, Wu F, Guo M, Hu G, et al: IL-17 promotes angiogenic
factors IL-6, IL-8, and Vegf production via Stat1 in lung
adenocarcinoma. Sci Rep. 6:365512016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mandic A, Knezevic Usaj S and Ivkovic
Kapicl T: Tissue expression of VEGF in cervical intraepithelial
neoplasia and cervical cancer. J BUON. 19:958–964. 2014.PubMed/NCBI
|
|
110
|
Zhang J, Liu J, Zhu C, He J, Chen J, Liang
Y, Yang F, Wu X and Ma X: Prognostic role of vascular endothelial
growth factor in cervical cancer: A meta-analysis. Oncotarget.
8:24797–24803. 2017.PubMed/NCBI
|