Introduction
Upper urinary tract cancer is a relatively rare, but
frequently aggressive, malignancy, which is primarily derived from
urothelial cells (1,2). Owing to the lower incidence of upper
urinary tract urothelial carcinoma (UUTUC), compared with bladder
cancer, there is limited knowledge regarding specific molecular or
biological changes in UUTUC. Notably, no established prognostic
biomarkers that are beneficial for decision-making of the
management of UUTUC are available (3–5).
Forkhead box O1 (FOXO1), a forkhead transcription
factor, is known to be inactivated by phosphorylation through the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling
pathway, resulting in suppression of apoptosis and regulation of
the cell cycle, as well as control of cell invasion, in prostate
and colon cancer lines (6,7). In bladder cancer cells, microRNA-96
(8) and a derivative of a Chinese
herb isorhapontigenin (9) have been
demonstrated to mediate apoptosis and invasion, respectively, via
targeting FOXO1. Additionally, potential cross-talk between FOXO1
and nuclear receptors, particularly sex hormone receptors,
including androgen receptor (AR) and estrogen receptor-β (ERβ), has
been identified in prostate cancer cells (10,11).
Furthermore, an increasing amount of preclinical evidence has
indicated a critical role for AR and ERβ signals in the development
and progression of urothelial cancer (12–14).
Previous immunohistochemical studies in bladder
cancer specimens have indicated that loss of or decreased
expression of FOXO1, as a tumor suppressor, is associated with
poorer patient outcomes (15–17). By contrast, the expression status of
phosphorylated forms of FOXO1 and its prognostic value in
urothelial cancer, as well as the functions of FOXO1 in UUTUC,
remain poorly understood. The present study aimed to determine the
status of phospho-FOXO1 (p-FOXO1) expression in UUTUC and its
associations with clinicopathological characteristics.
Materials and methods
UUTUC tissue microarray (TMA)
A UUTUC TMA was constructed with formalin-fixed
paraffin embedded specimens [(tumor samples (n=99)] and paired
normal-appearing urothelial tissues (n=82) from patients [60 men
and 39 women with a mean/median age of 70.0/71 years (range: 48–87
years)] who underwent radical nephroureterectomy between March 1997
and September 2011, as described previously (18). All sections were reviewed by a
urologic pathologist (at The Johns Hopkins Hospital; 18) for
confirmation of the original diagnosis of urothelial carcinoma and
tumor grade/stage of each case according to the World Health
Organization histological classification (2004)/TNM classification
(American Joint Committee on Cancer 7th Edition) of tumors of the
urinary tract/renal pelvis and ureter, respectively (19). Appropriate approval from the
institutional review board at Osaka General Medical Center was
obtained prior to construction and use of the TMA.
Clinicopathological characteristics of these patients (see Table I) were obtained from medical records
and follow-up data. No patients had received neoadjuvant treatment
or other anticancer therapies, including irradiation, prior to
nephroureterectomy.
 | Table I.Association between p-FOXO1 expression
and clinicopathological profile of the patients. |
Table I.
Association between p-FOXO1 expression
and clinicopathological profile of the patients.
|
| p-FOXO1
expression | P-value |
|---|
|
|
|
|
|---|
| Parameters | n | 1+ (%) | 2+ (%) | 3+ (%) | 1+ vs. 2+/3+ | 1+/2+ vs. 3+ |
|---|
| Age (mean ± SD;
years) | 99 | 67.4±10.6 | 70.8±8.4 | 69.8±9.2 | 0.384 | 0.869 |
| Sex |
|
|
|
| 0.532 | 0.680 |
| Male | 60 | 6 (10.0) | 28 (46.7) | 26 (43.3) |
|
|
|
Female | 39 | 6 (15.4) | 18 (46.2) | 15 (38.5) |
|
|
| Laterality |
|
|
|
| 0.120 | 0.220 |
|
Right | 43 | 8 (18.6) | 14 (32.6) | 21 (48.8) |
|
|
| Left | 56 | 4 (7.1) | 32 (57.1) | 20 (35.7) |
|
|
| Tumor site |
|
|
|
| 0.763c | 0.834c |
| Renal
pelvis | 45 | 5 (11.1) | 21 (46.7) | 19 (42.2) |
|
|
|
Ureter | 50 | 7 (14.0) | 24 (48.0) | 19 (38.0) |
|
|
| Both | 4 | 0 (0) | 1 (25.0) | 3 (75.0) |
|
|
| Tumor
gradea |
|
|
|
| 0.686 | 1.000 |
|
Low-grade | 15 | 1 (6.7) | 8 (53.3) | 6 (40.0) |
|
|
|
High-grade | 84 | 11 (13.1) | 38 (45.2) | 35 (41.7) |
|
|
| Pathological
stageb |
|
|
|
| 0.031d | 0.207d |
|
pTa | 19 | 3 (15.8) | 9 (47.4) | 7 (36.8) |
|
|
|
pT1 | 18 | 5 (27.8) | 8 (44.4) | 5 (27.8) |
|
|
| NMI
(pTa + pT1) | 37 | 8 (21.6) | 17 (45.9) | 12 (32.4) |
|
|
|
pT2 | 8 | 2 (25.0) | 4 (50.0) | 2 (25.0) |
|
|
|
pT3 | 48 | 2 (4.2) | 24 (50.0) | 22 (45.8) |
|
|
|
pT4 | 6 | 0 (0) | 1 (16.7) | 5 (83.3) |
|
|
| MI (pT2
+ pT3 + pT4) | 62 | 4 (6.5) | 29 (46.8) | 29 (46.8) |
|
|
| Concurrent CIS |
|
|
|
| 1.000 | 1.000 |
| No | 86 | 10 (11.6) | 40 (46.5) | 36 (41.9) |
|
|
|
Yes | 13 | 2 (15.4) | 6 (46.2) | 5 (38.5) |
|
|
| Hydronephrosis |
|
|
|
| 1.000e | 0.604e |
| No | 61 | 8 (13.1) | 28 (45.9) | 25 (41.0) |
|
|
|
Yes | 20 | 2 (10.0) | 8 (40.0) | 10 (50.0) |
|
|
|
Unknown | 18 | 2 (11.1) | 10 (55.6) | 6 (33.3) |
|
|
| Lymphovascular
invasion |
|
|
|
| 0.025 | 0.096 |
| No | 59 | 11 (18.6) | 28 (47.5) | 20 (33.9) |
|
|
|
Yes | 40 | 1 (2.5) | 18 (45.0) | 21 (52.5) |
|
|
| Lymph node
involvementb |
|
|
|
| 1.000f | 0.756f |
|
pN0 | 84 | 11 (13.1) | 38 (45.2) | 35 (41.7) |
|
|
|
pN1-3 | 12 | 1 (8.3) | 7 (58.3) | 4 (33.3) |
|
|
|
pNx | 3 | 0 (0) | 1 (33.3) | 2 (66.7) |
|
|
Immunostaining
Immunohistochemistry was performed, as described
previously (20). Briefly, the 5 µm
sections from the TMA were stained, using a primary antibody
against p-FOXO1 (Ser256; cat no SAB4300094;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 1:200; 4°C
overnight) and a broadspectrum secondary antibody (cat. no. 959643;
Histostain-SP IHC kit, DAB; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA; 10 min at room temperature plus 10 min with
enzyme conjugated solution at room temperature). All stains were
manually quantified by two pathologists who were blinded to the
identity of the samples. The German immunoreactive scores
calculated by multiplying the percentage of immunoreactive cells
(0%, 0; 1–10%, 1; 11–50%, 2; 51–80%, 3; and 81–100%, 4) by staining
intensity (negative, 0; weak, 1; moderate, 2; and strong, 3) were
grouped as negative (0; score 0–1), weakly positive (1+; score
2–4), moderately positive (2+; score 6–8) or strongly positive (3+;
score 9–12).
Statistical analyses
Data are presented as mean ± standard deviation.
Fisher's exact test and Student's t-test were used to evaluate the
association between categorized variables and those with a
continuous distribution, respectively. Correlations between
variables were determined by the Pearson's correlation coefficient
(CC). The rates of recurrence-free survival, progression-free
survival (PFS) and cancer-specific survival (CSS) were calculated
by the Kaplan-Meier method, and comparisons were analyzed using the
log-rank test. Disease progression was defined as the development
of non-bladder lesions, including recurrence at the
nephroureterectomy site and lymph node or visceral metastasis.
Metachronous or synchronous recurrence in the lower urinary tract
was not considered as tumor progression. Cox proportional hazards
model was used to determine the statistical significance of
prognostic indicators in a multivariate setting. These statistical
analyses were performed using SPSS (version 22; IBM Corp., Armonk,
NY, USA) or GraphPad Prism 5 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of p-FOXO1 in tumor and
corresponding non-neoplastic tissues
In the present study, 99 UUTUC specimens and 82
matched normal-appearing urothelial tissues were
immunohistochemically stained for an inactivated form of FOXO1
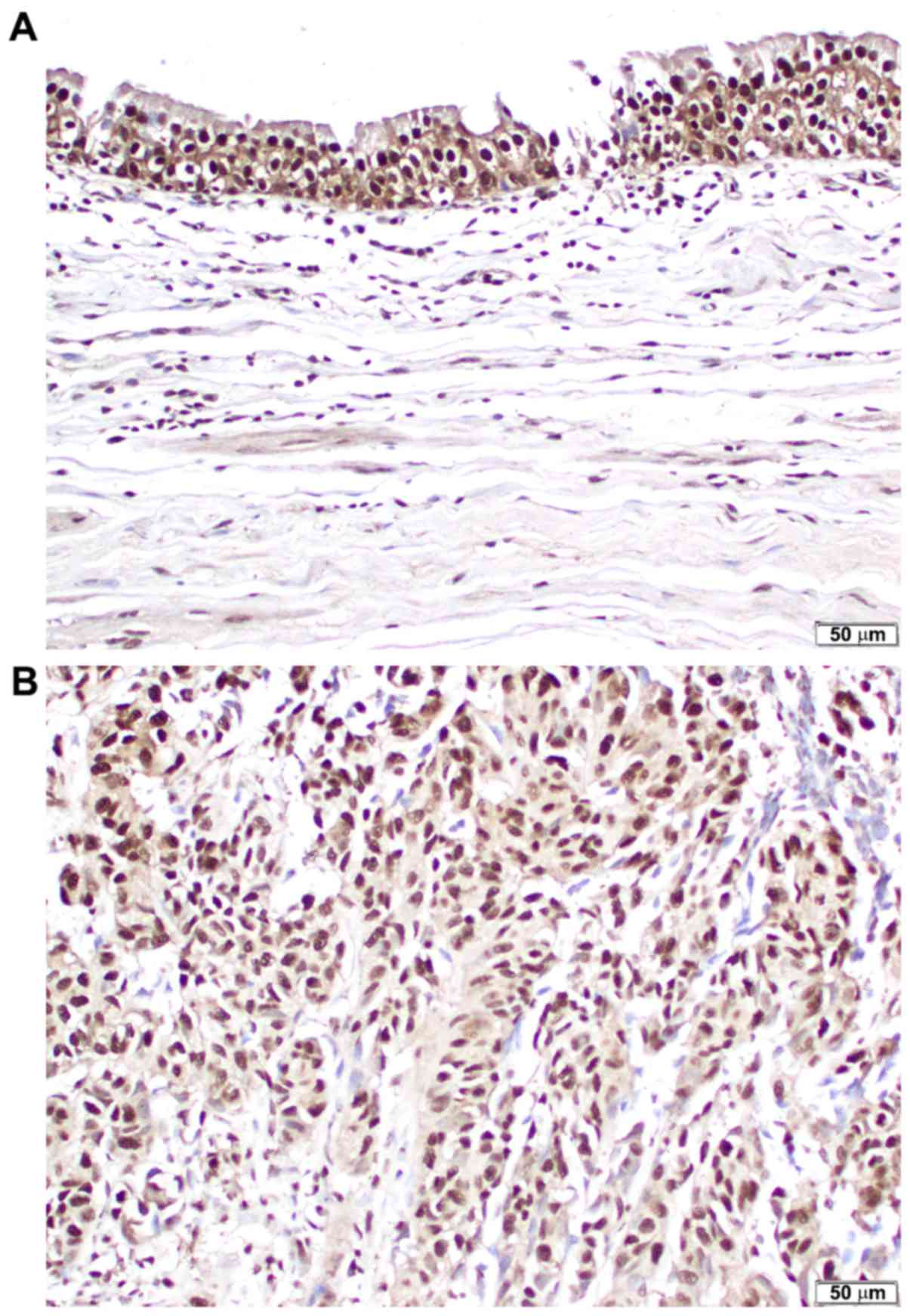
(p-FOXO1). Positive signals for p-FOXO1 were primarily detected in
the nuclei of non-neoplastic (Fig.
1A) and neoplastic (Fig. 1B)
urothelial cells. p-FOXO1 was expressed in 77/82 (93.9%) benign
urothelial tissues [18 (22.0%) 1+, 41 (50.0%) 2+ and 18 (22.0%) 3+]
and all 99 (100%) urothelial neoplasms [12 (12.1%) 1+, 46 (46.5%)
2+ and 41 (41.4%) 3+]. Thus, the levels of p-FOXO1 expression were
significantly increased in tumor samples, compared with benign
tissues (0 vs. 1+/2+/3+, P=0.018; 0/1+ vs. 2+/3+, P=0.008; and
0/1+/2+ vs. 3+, P=0.007).
Association of p-FOXO1 expression with
clinicopathological features of UUTUC samples
Table I displays the
levels of p-FOXO1 expression in UUTUC samples on the basis of their
clinicopathological characteristics. The expression of p-FOXO1 was
significantly (1+ vs. 2+/3+: P=0.031) increased in muscle-invasive
(≥pT2) tumor samples, compared with non-muscle-invasive (≤pT1)
tumor samples, whereas it was not statistically different between
low-grade and high-grade carcinoma samples. Additionally, the rate
of lymphovascular invasion was significantly (P=0.025) increased in
p-FOXO1(2+/3+) tumor samples [39/87 (44.8%)], compared with
p-FOXO1(1+) tumor samples [1/12 (8.3%)]. There were no significant
associations between the levels of p-FOXO1 expression and other
characteristics, including patient age or sex, tumor laterality or
site, and presence of concomitant carcinoma in situ,
hydronephrosis or lymph node metastasis.
Subsequently, the associations between the
expression of p-FOXO1 and steroid hormone receptors including AR,
ERα, ERβ, glucocorticoid receptor (GR) and progesterone receptor
(PR), were assessed. In the same cohort of 99 patients with UUTUC,
it was demonstrated previously that AR, ERα, ERβ, GR and PR were
positive in 20 (20.2%), 18 (18.2%), 62 (62.6%), 62 (62.6%) and 16
(16.2%) UUTUC samples, respectively (20). A significant (P<0.05) weak positive
(CC=0.2–0.4) correlation between p-FOXO1 and ERβ expression
(CC=0.244; P=0.015), particularly in tumor samples from male
patients (CC=0.330; P=0.010), was observed (Table II). Specifically, of 58
p-FOXO1(1+/2+) vs. 41 p-FOXO1(3+) tumor samples, 29 (50.0%) vs. 33
(80.5%) were immunoreactive for ERβ (P=0.003), respectively.
Similarly, of 34 p-FOXO1(1+/2+) vs. 26 p-FOXO1(3+) tumor samples
from male patients, 14 (41.2%) vs. 21 (80.8%) were immunoreactive
for ERβ (P=0.003), respectively. However, p-FOXO1 levels were not
significantly associated with the positivity of AR, ERα, GR or PR
in all 99, 60 male or 39 female tumor samples.
 | Table II.Correlation between p-FOXO1 and
AR/ERα/ERβ/GR/PR expression. |
Table II.
Correlation between p-FOXO1 and
AR/ERα/ERβ/GR/PR expression.
|
|
| AR | ERα | ERβ | GR | PR |
|---|
|
|
|
|
|
|
|
|
|---|
| Patients | n | CC | P-value | CC | P-value | CC | P-value | CC | P-value | CC | P-value |
|---|
| All cases | 99 | 0.155 | 0.125 | −0.011 | 0.917 | 0.244 | 0.015 | 0.088 | 0.384 | −0.069 | 0.497 |
| Males | 60 | 0.213 | 0.103 | 0.184 | 0.160 | 0.330 | 0.010 | 0.036 | 0.783 | 0.085 | 0.516 |
| Females | 39 | 0.009 | 0.955 | −0.259 | 0.111 | 0.141 | 0.392 | 0.143 | 0.386 | −0.195 | 0.235 |
Association of p-FOXO1 expression with
patient outcomes
Kaplan-Meier analysis coupled with the log-rank test
was performed to assess the prognostic values of p-FOXO1 expression
in 95 UUTUC samples with no distant metastasis at the time of
nephroureterectomy. There were no significant associations between
p-FOXO1 levels and tumor recurrence in the bladder (1+ vs. 2+ vs.
3+, P=0.705; 1+ vs. 2+/3+, P=0.406; 1+/2+ vs. 3+, P=0.852).
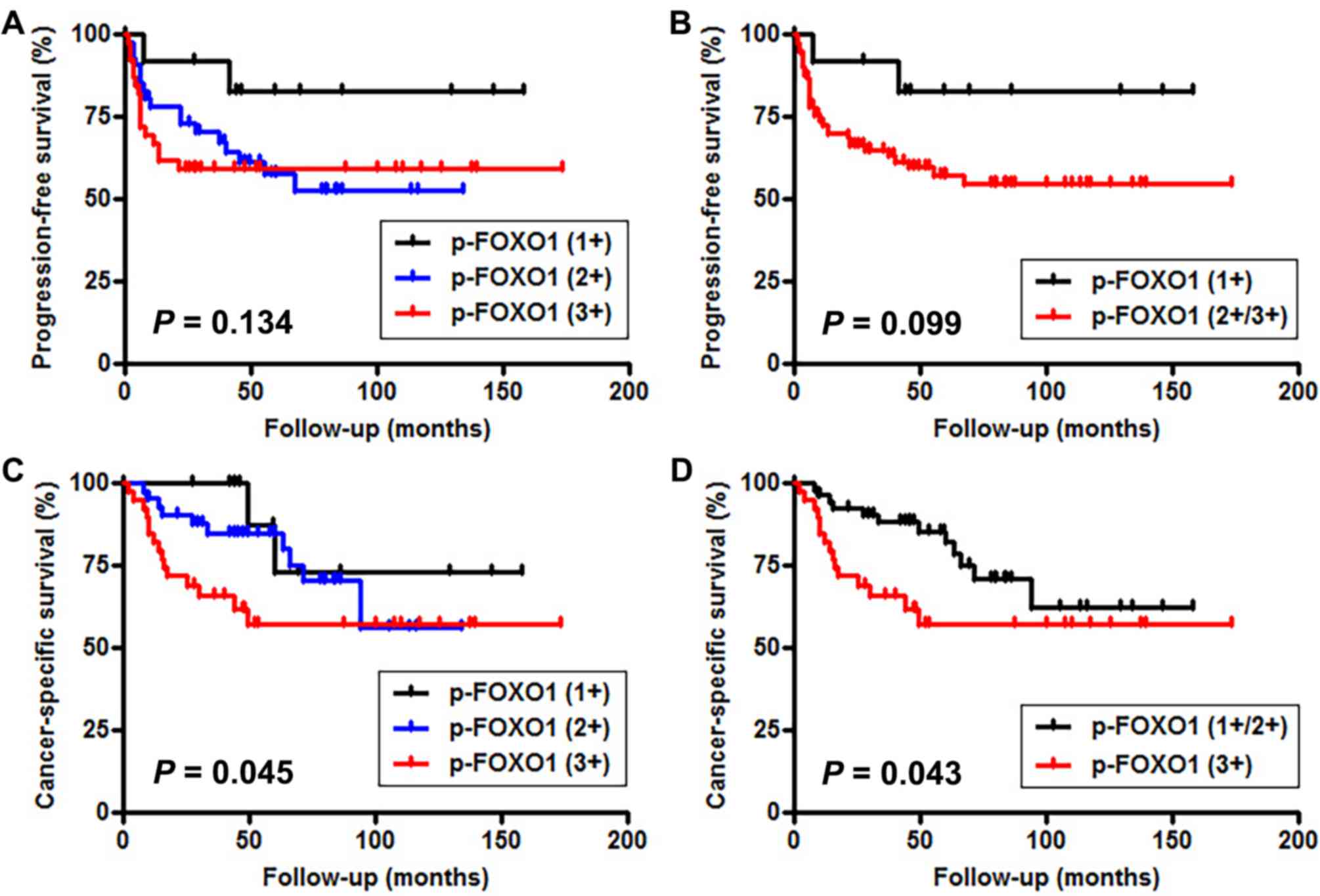
However, the increased expression of p-FOXO1 was insignificantly
and significantly associated with decreased PFS [(1+ vs. 2+ vs. 3+,
P=0.134 (Fig. 2A); 1+ vs. 2+/3+,
P=0.099 (Fig. 2B); 1+/2+ vs. 3+,
P=0.341) and CSS (1+ vs. 2+ vs. 3+, P=0.045 (Fig. 2C); 1+ vs. 2+/3+, P=0.249; 1+/2+ vs.
3+, P=0.043 (Fig. 2D)],
respectively.
To determine whether the status of p-FOXO1
expression was an independent indicator of prognosis in the 95
patients with UUTUC, multivariate analysis, including the factors
demonstrating statistical significance in univariate analysis, was
performed with the Cox model (Table
III). Although tumor grade, pT stage and pN stage were not
significantly associated with CSS, lymphovascular invasion was
identified to be an independent factor for CSS [hazard ratio
(HR)=3.222; P=0.028]. Additionally, there was a notable, albeit not
significant, association between p-FOXO1 expression and CSS
(HR=2.204; P=0.053).
 | Table III.Univariate and multivariate analysis
of cancer-specific survival in 95 patients with upper urinary tract
urothelial carcinoma. |
Table III.
Univariate and multivariate analysis
of cancer-specific survival in 95 patients with upper urinary tract
urothelial carcinoma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor grade | 6.411 | 0.868–47.372 | 0.036 | 4.770 | 0.626–36.331 | 0.131 |
| pT
stagea | 3.416 | 1.598–7.305 | 0.002 | 2.047 | 0.712–5.883 | 0.184 |
| LVI | 6.712 | 2.827–15.934 | <0.001 | 3.222 | 1.132–9.168 | 0.028 |
| pN stage | 4.379 | 1.762–10.884 | 0.001 | 2.348 | 0.815–6.761 | 0.114 |
|
p-FOXO1b | 2.262 | 1.028–4.975 | 0.043 | 2.204 | 0.989–4.910 | 0.053 |
Discussion
There is limited knowledge regarding the function of
the potential tumor suppressor FOXO1 in urothelial carcinogenesis
and tumor growth. Furthermore, to the best of our knowledge, the
status of FOXO1 or p-FOXO1 expression in UUTUC has not previously
been investigated. Using immunohistochemistry, the levels of
p-FOXO1 expression were compared in UUTUC samples and corresponding
adjacent normal-appearing tissues in the upper urinary tract and it
was demonstrated that they were significantly increased in tumor
cells, compared with the non-neoplastic urothelial cells.
Consistent with the present data, a recent study demonstrated
downregulation of FOXO1 expression in bladder cancer, compared with
non-cancerous bladder mucosa (17).
These observations may indicate that FOXO1 contributes to the
prevention of urothelial tumorigenesis. Relatively high levels of
p-FOXO1 expression in normal-appearing urothelial tissues in the
TMA used in the present study may be due to the cancer field effect
(21) that potentially affected the
immunoreactivity.
FOXO1 has also been implicated in the regulation of
cell proliferation, apoptosis and cell cycle control, as well as
cell migration and invasion, via its phosphorylation/inactivation
through the PI3K/Akt signaling pathway (6,7). In the
present study, p-FOXO1 overexpression was identified to be
associated with muscle invasion and lymphovascular invasion in
UUTUC. Univariate and multivariate analyses further demonstrated
that p-FOXO1 overexpression was significantly and insignificantly,
respectively, associated with cancer-specific mortality in patients
with UUTUC. Additionally, a few immunohistochemical studies in
bladder cancer tissue samples have indicated the prognostic
significance of FOXO1 expression (15–17). Thus,
FOXO1 activity is indicated to predict the prognosis of UUTUC.
However, although FOXO1 expression in superficial bladder tumor
samples was demonstrated to predict the risk of their recurrence
(15), the present study did not
indicate a significant association between p-FOXO1 overexpression
in UUTUC samples and its recurrence in the bladder.
The functional interplay between FOXO1 and steroid
hormone receptors, particularly AR and ERβ, has been demonstrated
previously. Specifically, androgen and estrogen were able to reduce
the expression or activity of FOXO1 in the presence of AR and ERβ,
respectively, in prostate cancer cells (10,11).
Estrogen-mediated ER (ERα, ERβ or both) signals were also
demonstrated to induce phosphorylation of FOXO1 in breast cancer
cells (22). In bladder cancer cell
lines, it was identified that androgens and estrogens could
inactivate FOXO1 via the AR and ERβ pathways, respectively (Ide
et al, unpublished data). In accordance with these
observations, the present study indicated that p-FOXO1 expression
was significantly correlated with ERβ expression in UUTUC samples.
However, the levels of p-FOXO1 expression were not significantly
correlated with the expression of other steroid hormone receptors,
including AR, ERα, GR and PR.
Using the identical UUTUC TMA, we previously
determined the expression status of various transcription factors
(20,23–26).
Notably, the positive rates of 6 transcription factors, including
AR (20), ERβ (20), GATA-binding protein 3 (GATA3)
(23), zinc finger with KRAB and SCAN
domains 3 (ZKSCAN3) (24), nuclear
factor of activated T-cells 1 (NFATc1) (25) and a phosphorylated form of ELK1
(p-ELK1) (26), were significantly
(P<0.05; GATA3, ZKSCAN3) or insignificantly (0.05≤P<0.1; AR,
ERβ, NFATc1, p-ELK1) different between renal pelvic and ureteral
tumor samples, although the underlying reasons remain unclear.
However, similar to other transcription factors previously
examined, including ERα (20), GR
(20) and PR (20), no significant change (P≥0.1) in the
levels of p-FOXO1 expression at different sites of UUTUC was
identified in the present study.
The function of the potential tumor suppressor FOXO1
in the development and progression of UUTUC remains poorly
understood. In the present study, the expression status of p-FOXO1
in UUTUC specimens and its prognostic significance were
immunohistochemically determined. The levels of p-FOXO1 expression
were compared in tumor samples and adjacent normal tissues in the
upper urinary tract, and it was identified that p-FOXO1 expression
was significantly upregulated in UUTUC, compared with
non-neoplastic urothelium. A recent study also demonstrated
downregulation of FOXO1 expression in bladder cancer, compared with
non-cancerous bladder mucosa (17).
These results indicate that FOXO1 may contribute to the prevention
of urothelial tumorigenesis.
In conclusion, a significant increase in the
expression of p-FOXO1 in UUTUC samples, compared with corresponding
normal-appearing urothelial tissues, was demonstrated, implying the
involvement of FOXO1, as a tumor suppressor, in the outgrowth of
UUTUC. The results of the present study further indicate that
p-FOXO1 overexpression serves as a predictor of poor prognosis in
patients with UUTUC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used or analyzed used during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HI and HM conceived and designed the study. HI, GJ
and SI performed the experiments. HI, GJ, TM, KF, SY, HF and NN
analyzed the data. KF, SY, HF and NN contributed reagents,
materials and analysis tools. HI drafted the manuscript and HM
edited it. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present research study was conducted with the
approval from the Institutional Review Board at Osaka General
Medical Center (Osaka, Japan; IRB no. 25-2014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Oya M and Kikuchi E; Committee for
Establishment of Clinical Practice Guideline for Management of
Upper Tract Urothelial Carcinoma and Japanese Urological
Association, . Evidenced-based clinical practice guideline for
upper tract urothelial carcinoma (summary-Japanese Urological
Association, 2014 edition). Int J Urol. 22:3–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chromecki TF, Bensalah K, Remzi M,
Verhoest G, Cha EK, Scherr DS, Novara G, Karakiewicz PI and Shariat
SF: Prognostic factors for upper urinary tract urothelial
carcinoma. Nat Rev Urol. 8:440–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lughezzani G, Burger M, Margulis V, Matin
SF, Novara G, Roupret M, Shariat SF, Wood CG and Zigeuner R:
Prognostic factors in upper urinary tract urothelial carcinomas: A
comprehensive review of the current literature. Eur Urol.
62:100–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krabbe LM, Heitplatz B, Preuss S,
Hutchinson RC, Woldu SL, Singla N, Boegemann M, Wood CG, Karam JA,
Weizer AZ, et al: Prognostic value of PD-1 and PD-L1 expression in
patients with high grade upper tract urothelial carcinoma. J Urol.
198:1253–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura N, Ramaswamy S, Vazquez F,
Signoretti S, Loda M and Sellers WR: Forkhead transcription factors
are critical effectors of cell death and cell cycle arrest
downstream of PTEN. Mol Cell Biol. 20:8969–8982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coomans de Brachène A and Demoulin JB:
FOXO transcription factors in cancer development and therapy. Cell
Mol Life Sci. 73:1159–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Y, Liu H, Zhang H, Shang C and Song Y:
miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer
cells. Oncol Lett. 4:561–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang G, Wu AD, Huang C, Gu J, Zhang L,
Huang H, Liao X, Li J, Zhang D, Zheng X, et al: Isorhapontigenin
(ISO) inhibits invasive bladder cancer formation in vivo and human
bladder cancer in vitro by targeting STAT1/FOXO1 axis. Cancer Prev
Res. 9:567–580. 2016. View Article : Google Scholar
|
|
10
|
Huang H, Muddiman DC and Tindall DJ:
Androgens negatively regulate forkhead transcription factor FKHR
(FOXO1) through a proteolytic mechanism in prostate cancer cells. J
Biol Chem. 279:13866–13877. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakajima Y, Akaogi K, Suzuki T, Osakabe A,
Yamaguchi C, Sunahara N, Ishida J, Kato K, Ogawa S, Fujimura T, et
al: Estrogen regulates tumor growth through a nonclassical pathway
that includes the transcription factors ERβ and KLF5. Sci Signal.
4:ra222011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyamoto H, Zheng Y and Izumi K: Nuclear
hormone receptor signals as new therapeutic targets for urothelial
carcinoma. Curr Cancer Drug Tar. 12:14–22. 2012. View Article : Google Scholar
|
|
13
|
Hsu I, Vitkus S, Da J and Yeh S: Role of
oestrogen receptors in bladder cancer development. Nat Rev Urol.
10:317–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inoue S, Mizushima T and Miyamoto H: Role
of the androgen receptor in urothelial cancer. Mol Cell Endocrinol.
465:73–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim
WJ and Yun SJ: Forkhead box O-class 1 and forkhead box G1 as
prognostic markers for bladder cancer. J Korean Med Sci.
24:468–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lloreta J, Font-Tello A, Juanpere N,
Frances A, Lorenzo M, Nonell L, de Muga S, Vázquez I, Cecchini L
and Hernández-Llodrà S: FOXO1 down-regulation is associated with
worse outcome in bladder cancer and adds significant prognostic
information to p53 overexpression. Hum Pathol. 62:222–231. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Jia L, Zhang Y, Ji W and Li H:
Higher expression of FOXOs correlates to better prognosis of
bladder cancer. Oncotarget. 8:96313–96322. 2017.PubMed/NCBI
|
|
18
|
Munari E, Fujita K, Faraj S, Chaux A,
Gonzalez-Roibon N, Hicks J, Meeker A, Nonomura N and Netto GJ:
Dysregulation of mammalian target of rapamycin pathway in upper
tract urothelial carcinoma. Hum Pathol. 44:2668–2676. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization Classification of Tumours.
Pathology and Genetics of Tumours of the Urinary System and Male
Genital Organs. IARC Press; Lyon: 2004
|
|
20
|
Kashiwagi E, Fujita K, Yamaguchi S,
Fushimi H, Ide H, Inoue S, Mizushima T, Reis LO, Sharma R, Netto
GJ, et al: Expression of steroid hormone receptors and its
prognostic significance in urothelial carcinoma of the upper
urinary tract. Cancer Biol Ther. 17:1188–1196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai H and Brown RE: Field effect in
cancer-An update. Ann Clin Lab Sci. 39:331–337. 2009.PubMed/NCBI
|
|
22
|
Mazumdar A and Kumar R: Estrogen
regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS
Lett. 535:6–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue S, Mizushima T, Fujita K, Meliti A,
Ide H, Yamaguchi S, Fushimi H, Netto GJ, Nonomura N and Miyamoto H:
GATA3 immunohistochemistry in urothelial carcinoma of the upper
urinary tract as a urothelial marker as well as a prognosticator.
Hum Pathol. 64:83–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jalalizadeh M, Inoue S, Fujita K, Ide H,
Mizushima T, Yamaguchi S, Fushimi H, Nonomura N and Miyamoto H:
ZKSCAN3 expression in urothelial carcinoma of the upper urinary
tract and its impact on patient outcomes. Integr Cancer Sci Ther.
4:10002412017.
|
|
25
|
Kawahara T, Inoue S, Fujita K, Mizushima
T, Ide H, Yamaguchi S, Fushimi H, Nonomura N and Miyamoto H: NFATc1
expression as a prognosticator in urothelial carcinoma of the upper
urinary tract. Transl Oncol. 10:318–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue S, Ide H, Fujita K, Mizushima T,
Jiang G, Kawahara T, Yamaguchi S, Fushimi H, Nonomura N and
Miyamoto H: Expression of phospho-ELK1 and its prognostic
significance in urothelial carcinoma of the upper urinary tract.
Int J Mol Sci. 19:e7772018. View Article : Google Scholar : PubMed/NCBI
|