Introduction
Lung cancer is a leading cause of cancer deaths
worldwide (1), while non-small cell
lung cancers (NSCLCs) accounting for >80% of all lung cancer
cases (2,3). NSCLC can be categorized into three major
histologic subtypes: Lung adenocarcinoma (LADC), lung squamous cell
carcinomas (LSCC) and large cell lung cancer (LCLC). Among them,
LADC is the most commonly occurring subtype (3). Patients with advanced NSCLC (stage IIIb
and IV) have no surgical treatment options available and will be
treated with systemic therapeutics, including radiation therapy,
chemotherapy, target therapy and immunotherapy (3). However, chemotherapy and radiation
therapy have high incidences of severe side effects. The most of
therapies targeted lung cancer mutatuion driver genes (such as
mutations in EGFR, KRAS, HER2, BRAF, RET, ROS1 and ALK) have
eventually failed due to drug resistance (4). Many clinical trials using immune
checkpoint blockade therapy have shown impressive and durable
clinical benefits in lung cancer, and in other human cancers
(5–8).
However, the majority of lung cancer patients fail to respond to
the checkpoint immunotherapy (9).
Therefore, new immunotherapeutic strategies are urgently needed for
those who fail to respond to the immune checkpoint therapy.
Recently, cancer-specific T cells have been further developed to
eradicate cancer cells (10).
Adoptive cell therapy (ACT) using cultured autologous
tumor-infiltrating lymphocytes (TILs) can induce a clinical
response in cancer patients, even in those who have previously
experienced treatment failure with other immunotherapies (11,12).
However, it remains a challenge to generate tumor-reactive TILs
from tumor tissues.
Genetic engineering enables the creation of T cells
expressing chimeric antigen receptors (CARs) that recognize tumor
membrane antigens, or T cell receptors (TCRs) recognizing tumor
membrane and intracellular antigens presented by specific major
histocompatibility complex (MHC) molecules (10,13). These
approaches redirect the antigen specificity of T cells for in
vitro expansion, and thus help overcome practical barriers that
limit the widespread use of TILs (14,15).
Notably, chimeric antigen receptor-engineered T cells (CAR-T cells)
targeting B-cell lineage differentiation antigen CD19 have acheived
impressive clinical response rates (16–18). A
great effort has been made to use CAR-T immunotherapy to treat
patients with solid cancers. However, such a CAR-T therapy has poor
clinical response in solid tumor due to the tumor microenvironment
and the lack of suitable cell-surface targets that specifically
expressed on tumor cells (19).
Cancer specific antigens/targets, which are supposed
to express in cancer cells but not in normal cells, play a vital
role in a successful cancer immunotherapy. Unfortunately, there are
few cancer specific antigens available as useful targets for
immunotherapy in solid tumor. Cancer-testis antigens are identified
as attractive immunotherapy targets in many cancers due to their
high expression in a variety of malignant neoplasms, but lack of
expression in normal adult tissues with the exception of normal
testis. However, male germ cells do not express human leukocyte
antigen (HLA) class I molecular, and thus are immunologically
protected (20–22). Moreover, expression of some
cancer-testis antigens in tumors could induce specific humoral and
cellular immune responses in cancer patients (21,23). A
recent study shows that TCR-modified CD4+ T cells
targeting cancer-testis antigen MAGE-A3 objectively respond to
metastatic cancers, including metastatic cervical cancer,
esophageal cancer, urothelial cancers and osteosarcoma (19). The cancer-testis antigen NY-ESO-1 is
one of the most promising candidate targets for immunotherapy due
to the strong associated immunogenicity (24–28). The
clinical importance of NY-ESO-1 in T cell therapy has been
supported from a case study that a patient with refractory melanoma
treated with autologous NY-ESO-1-specific CD4+ T cells
stimulated with NY-ESO-1 peptide achieved a long-term complete
remission (29). Subsequent studies
using ACT with NY-ESO-1 TCR-engineered T cells (TCR-T cells) could
effectively mediate tumor regression in melanoma and synovial cell
sarcoma, as well as multiple myeloma with well tolerance (13,14,30,31).
However, the safety and efficacy of NY-ESO-1 TCR-T cells in lung
cancer remain unknown.
NY-ESO-1 antigen is expressed in 11.8–21% of NSCLCs
(25,32,33), and
serum anti-NY-ESO-1 antibody has been detected in 13–20% patients
with lung cancers (34,35) and in 23% patients with NSCLC (35). NY-ESO-1 has already been shown as a
promising target for cancer immunotherapy with good safety and
efficiency (13,30,31).
Therefore, we choose the NY-ESO-1 as an ideal target for TCR-T
cells in our study. In the present study, four patients with NSCLC
enrolled in the clinical trial (NCT02457650) that aims at
preliminarily evaluating the safety and feasibility of NY-ESO-1
TCR-T cell therapy for HLA-A2-positive patients with NY-ESO-1
antigen-expressing malignancies revealed well tolerance. Here, we
reported that a female patient with advanced LADC revealed a
partial response (PR, 4 months) with NY-ESO-1 TCR-T cell therapy
without evident toxicity.
Patients and methods
Patients and clinical trial
design
Patients, aged one year and older, expressing HLA-A2
with NY-ESO-1 antigen-expressing solid tumors refractory to
standard treatment, were enrolled into the present clinical trial.
We recruited four subjects with NSCLC in our preliminarily study on
TCR-T cell therapy. More than 30% of cells in patients' tumor
specimen were stained with at least >1+ intensity for NY-ESO-1
antigen expression when immunohistochemical (IHC) staining was
performed using anti-NY-ESO-1 monoclonal antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA.). Staining intensity was
graded as 1+, weak staining; 2+, moderate staining; and 3+, strong
staining. A lymphodepleting chemotherapy regimen prior to adoptive
T cell infusion has been shown to dramatically enhance the
persistence of the transferred cells and improve anticancer effects
(36,37). In addition, the lymphodepleting
chemotherapy regimen may deplete Treg cells and other suppressive
cells in the circulation and the tumor micro environment, thus
enabling the survival and amplification of adoptively transferred T
cells to achieve effective killing of cancer cells (38). In the current study, a lymphodepleting
chemotherapy regimen consisting of cyclophosphamide (CTX; 30
mg/kg/d for 2 days) and fludarabine (Flud; 25 mg/m2/d
for 3 days) was purposed to be administrated to patients prior to
the NY-ESO-1 TCR-T cell infusion. A previous study reported that
the patients receiving a median of 5×1010 T cells
transduced with an anti-NY-ESO-1 TCR (range of 1.6 to
130×109) achieved objective clinical responses with good
safety in metastatic synovial cell sarcoma and melanoma (14). In the current study, total T cells at
a median of 7.16×109 cells (range of
1.67–10.60×109 cells, transfection rate between 36.6%
and 96.6%) were intravenously infused in one to three days with
interleukin (IL)-2 (Beijing Shuanglu Pharmaceutical Co Lt.,
Beijing, China) administrated subcutaneously for the following
consecutive 14 days (typically, 0.8–2.0 MIU/d) according to patient
tolerance. This clinical trial was conducted in Shenzhen Second
People's Hospital and approved by the Medical Ethics Committee of
the Institutional Review Board of the Shenzhen Second People's
Hospital. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and meets the
standards of the Declaration of Helsinki in its revised version of
1975 and its amendments of 1983, 1989, and 1996 [JAMA 1997;
277:925-926]. All patients enrolled in the trial provided written
informed consent.
T cell transduction and quantitative
real-time PCR analyses
Patient peripheral blood mononuclear cells (PBMCs)
were collected via leukopheresis. Then, PBMCs were stimulated using
50 ng/ml anti-CD3 antibody (OKT3; Ortho-Biotech, Bridgewater, NJ,
USA) and 300 IU recombinant IL-2 (Peprotech, UK), followed by
transduction with retroviral vector carrying nucleic acid sequences
encoding an HLA-A2 restricted TCR recognizing NY-ESO-1:157-165
epitope (30,39,40). The
transduced cells were then expanded in vitro before being
adoptively transferred as previously described (41). Clinical grade retroviral supernatants
produced under good manufacturing practice (GMP) conditions were
obtained from the Shenzhen Institute for Innovation and
Translational Medicine (Shenzhen, China). IFN-γ released by the
TCR-T cells was measured based on recognition of tumor cell line
Mel 624 (HLA-A2+ and NY-ESO-1+), which had
been established in the Surgery Branch, National Cancer Institute
from resected tumors (42,43) as described previously (14,40).
Co-incubation of TCR-T cells with Mel 586 (HLA-A2− and
NY-ESO-1+) was used as a negative control. Mel 624 or
Mel 586 cells (5×104 cells/well) were seeded in 96-well
cell culture plates with medium, respectively, and incubated
overnight. The culture medium was replaced with fresh ones
containing TCR-T cells. After co-incubation for 18 h, IFN-γ
released in supernatant was measured by enzyme-linked immunosorbent
assay (ELISA). The persistence of NY-ESO-1 specific TCR-T cells
in vivo was evaluated by quantitative real-time PCR of
samples of whole blood at serial time-points before and after cell
infusion. Genomic DNA was isolated from whole blood samples using
QIAamp DNA blood midi kits (Qiagen, Inc., Valencia, CA, USA) and
quantified by spectrophotometer. The qPCR analyses were performed
as previously described (31,44–46) using
ABI 7900HT Real-Time PCR System (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
T cell tracking and cytokine
detection
The following antibodies (BD Biosciences, Franklin
Lakes, NJ, USA) were used to identify T cells: CD4-PE, CD8-FITC,
CD25-FITC, and CD28-PE. Flow cytometry was performed using a BD
Accuri™ C6 personal flow cytometer (BD Biosciences) and data were
analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Cytokine secretion was evaluated by V-PLEX Human Cytokine 30-Plex
kit with measurements by MESO QuickPlex SQ 120 (Meso Scale
Discovery, Rockville, MD, USA).
Results
Patients and clinical assessment
A total of four HLA-A2-positive patients with
NY-ESO-1+ metastatic NSCLC received lymphodepleting
chemotherapy and then were adoptively transferred with NY-ESO-1
TCR-T cells and coupled with systemic IL-2 administration. Clinical
symptoms experienced by the patients and administration of total T
cells were listed in Table I. It was
revealed that co-incubation of TCR-T cells with Mel 624 cells
induced the release of IFN-γ at a median of 3,409 pg/ml, compared
to <100 pg/ml in control. All patients with metastatic NSCLC
treated with given standard treatments experienced disease
progression before being enrolled in the trial. Patients 1 and 2
independently received three and two courses of infusion,
respectively, while patients 3 and 4 received just one course of
infusion. Patient 1 exhibited stable disease (SD) for nearly 3
months after adoptive transfer of NY-ESO-1 TCR-T cells based on
Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria
(Table I). Patient 2 experienced a PR
lasting 4 months after treatment. Patients (3 and 4) failed to have
an observable clinical response after infusion of TCR-T cells.
Adverse events probably related to NY-ESO-1 TCR-T cell therapy in
four patients with NSCLC were listed in Table II, and toxicities were graded
according to NCI CTCAE version 5.0 (November 27, 2017). All
patients experienced transient anemia (≤grade 2) and white blood
cell decrease (≤grade 3) which were probably induced by the
preparative lymphodepleting chemotherapy. These symptoms were
relieved after symptomatic treatment, such as granulocyte-colony
stimulating factor (G-CSF) infusion and blood transfusion. Three
patients exhibited fever (≤grade 3) after administration of IL-2
and recovered upon thermoregulation by themselves or through
appropriated treatment. Of note, patient 4 had high fever (40.1°C,
grade 3) that was resolved by oral administration of anti-fever
medicine acetaminophen and cessation of IL-2 in one hour. In
addition, half of the patients exhibited fatigue, rash, nausea,
vomiting and abdominal pain. However, it was difficult to draw
general conclusions regarding to the clinical efficacy of
anit-NY-ESO-1 TCR-T cells in NSCLC due to the small number of
patients enrolled in the present study. Therefore, further clinical
investigations with a large number of cancer patients are needed in
the future to evaluate the safety and efficacy of anit-NY-ESO-1
TCR-T cell therapy for treatment of lung cancer.
 | Table I.Clinical symptoms of the patients and
administration of anti-NY-ESO-1 TCR-T cells. |
Table I.
Clinical symptoms of the patients and
administration of anti-NY-ESO-1 TCR-T cells.
| Patient | Age/sex | Diagnosis | Metastasis | Prior
treatments | Intensity of
antigen | % of tumor cells
expressing antigen | Total cells
(×109) |
CD3+/CD4+/CD8+
(% of PBMCs, average) | Average IFN-γ
(pg/ml)a | Response
(months) |
|---|
| 1 | 27/F | LADC | Lymph nodes,
pericardium, pleura, liver, thoracic, ribs, ilium | Chemotherapy,
thoracoscope surgery | 2+ | >80 | 1.67 | – | 1377.36 | SD (3) |
| 2 | 44/F | LADC | Lymph nodes, liver,
pleura | Chemotherapy,
target therapy | 2+ | 30–40 | 7.61 | 98.5/2.2/94.8 | 1247.08 | PR (4) |
| 3 | 59/F | LADC | Sacral
vertebrae | Chemotherapy | 2+ | 30 | 10.60 | 97.88/21/79 | 7241.67 | NR |
| 4 | 62/M | LSCC | Lymph nodes | Chemotherapy,
radiofrequency ablation | 2+/3+ | 40 | 8.74 | 91.4/11.1/96.3 | 3757.10 | NR |
 | Table II.NY-ESO-1 TCR-T cell therapy-related
adverse events in four patients with NSCLC. |
Table II.
NY-ESO-1 TCR-T cell therapy-related
adverse events in four patients with NSCLC.
| Event | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| General disorders
and administration site conditions |
|
Chills | – | – | – | + (Grade 1) |
|
Fatigue | + (Grade 1) | – | – | + (Grade 1) |
|
Fever | + (≤38.6°C, Grade
1) | + (≤39.5°C, Grade
2) | – | + (≤40.1°C, Grade
3) |
|
Hyperhidrosis | + (Grade 1) | – | – | – |
| Skin and
subcutaneous tissue disorder |
|
|
|
|
|
Rash | – | + (Grade 2) | + (Grade 1) | – |
| Cardiac
disorders |
|
|
|
|
|
Palpitations | – | – | + (Grade 1) | – |
| Gastrointestinal
symptoms |
|
|
|
|
|
Nausea | + (Grade 1) | – | + (Grade 2) | – |
|
Vomiting | – | + (Grade 2) | + (Grade 2) | – |
|
Abdominal pain | – | – | + (Grade 1) | + (Grade 1) |
| Blood and lymphatic
system disorders |
|
|
|
|
|
Anemia | + (Grade 2) | + (Grade 2) | + (Grade 1) | + (Grade 1) |
| Investigations |
|
|
|
|
| White
blood cell decreased | + (Grade 3) | + (Grade 3) | + (Grade 3) | + (Grade 2) |
Case report of responding patient
Herein, this study focused on reporting the NY-ESO-1
TCR-T cell treatment in a recruited HLA-A2 positive 44-year-old
female patient (patient 2) with metastatic LADC carrying EGFR
mutation. Her tumor did not respond to six cycles of combination
chemotherapy (docetaxel and carboplatin) in February 2012. In July
2012, she was assessed as having progressive disease (PD) and
started to receive treatment with gefitinib for her tumor carrying
the EGFR mutation. Computed tomography (CT) scans showed
stabilization of her primary lung tumor and liver metastases.
However, in January 2015, a surveillance CT scan revealed recurrent
disease with new pleural and liver metastases. Treatment was then
switched to erlotinib. A follow-up CT scan in September 2015 showed
PD in the right pulmonary hilum, mediastinum, right pleura, right
hepatic lobe, and liver capsule. There was no central nervous
system or skeletal metastases. A bronchoscopic biopsy specimen of
the right pulmonary tumor was analyzed by immunohistochemistry and
stained strongly for NY-ESO-1 (2+ staining; Fig. 1A). In November 2015, the patient was
enrolled in a clinical trial (NCT02457650) assessing autologous
T-cell therapy for malignant tumors at the Department of Oncology
in Shenzhen Second People's Hospital and the patient provided
written informed consent.
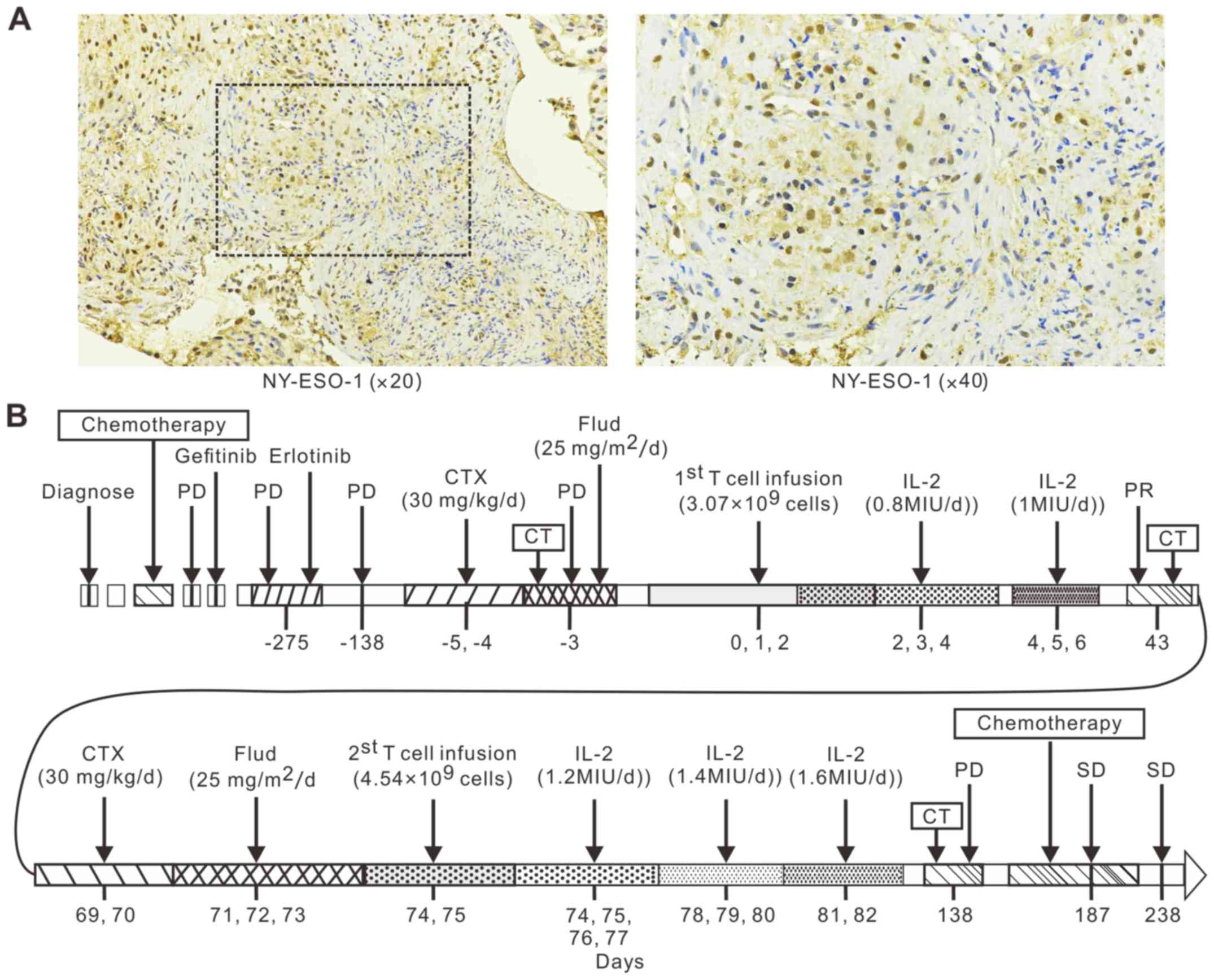 | Figure 1.NY-ESO-1 TCR-T cell therapy treatment
schedule for the patient with LADC. (A) Immunohistochemical
analysis revealed 3+ staining for NY-ESO-1. (B) The patient was
diagnosed with lung adenocarcinoma in February 2012. Her tumor did
not respond to six cycles of combination chemotherapy (docetaxel
and carboplatin), gefitinib, or erlotinib by September 2015. The
patient was then enrolled in the clinical trial (NCT02457650) and
received two separate NY-ESO-1-specific TCR-T cell infusions in
November 2015 (days 0, 1, and 2) and January 2016 (Day 74, 75). The
patient then received another six cycles of chemotherapy
(gemcitabine and cisplatin). In addition, the patient took
erlotinib throughout the entire trial period. TCR-T, T cell
receptor engineered-T cells; LADC, lung adenocarcinoma; CT,
computed tomography; PD, progressive disease; SD, stable disease;
IL, interleukin; Flud, fludarabine; CTX, cyclophosphamide; PR,
partial response. |
A lymphodepleting chemotherapy regimen consisting of
CTX (30 mg/kg/d for 2 days) and Flud (25 mg/m2/d for 3
days) was administrated on the patient 2 before the infusion of
NY-ESO-1 TCR-T cells. However, before the first infusion of TCR-T
cells, a syndrome of pain and hemoptysis aggravated after she
received Flud treatment for one day. For the safety of patient 2,
Flud treatment was discontinued for the following two days
(Fig. 1B). NY-ESO-1 TCR-T cells
(3.07×109 total T cells; 2.97×109 TCR-T cells) were then
infused over three days (day 0, 1 and 2) (Fig. 1B). Subsequently, IL-2 was
administrated according to the patient's physical condition over
six consecutive days (Fig. 1B).
A CT scan obtained in day 43 after the first T cell
infusion revealed regression of the primary lung tumor and liver
metastases, absorption of hydrothorax and pulmonary re-expansion
(Fig. 2A). The primary pulmonary
lesion size had reduced from 95×86×54 mm to 64×44×54 mm. The
metastatic liver lesion had also reduced from 19.8×19.6×20 mm to
10×10×10 mm. The therapeutic effect was assessed as PR according to
RECIST 1.1. To further improve treatment efficacy, about one month
later, patient 2 received lymphodepleting chemotherapy of CTX (30
mg/kg/d for 2 days) and Flud (25 mg/m2/d for 3 days), a
second TCR-T cell infusion (4.54×109 total T cells;
2.87×109 TCR-T cells) within two days (days 74 and 75), and then
IL-2 for eight consecutive days (Fig.
1B). However, the patient's disease had progressed when
assessed about two months (day 138) after the second infusion
(Fig. 1B). CT scans showed the lung
tumor (94×88×56 mm) and liver metastases (17.3×16.2×20 mm) had
progressed, and the hydrothorax recurred (Fig. 2A). In addition, emission computed
tomography (ECT) revealed bone metastases. The efficacy evaluation
was PD.
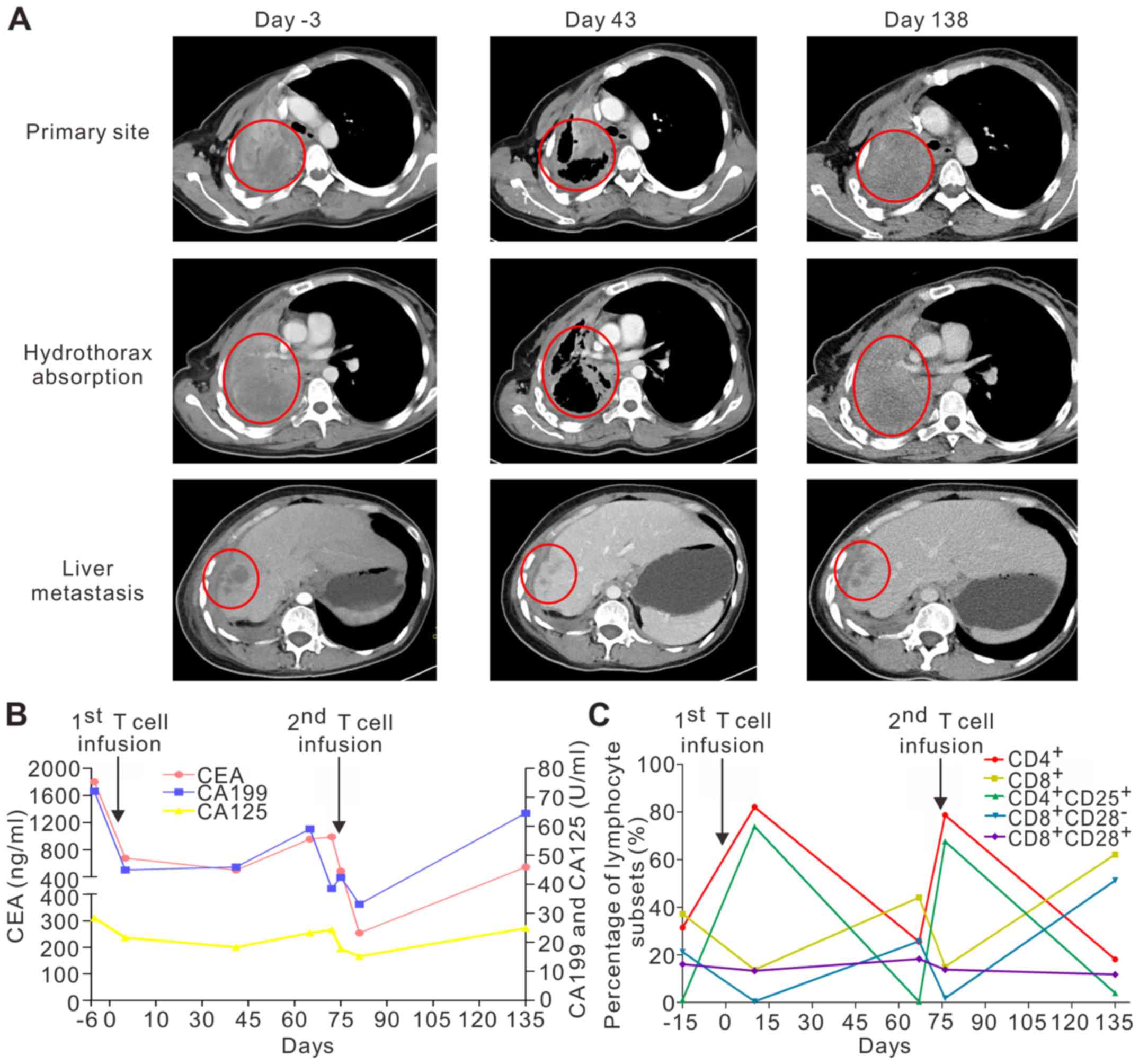 | Figure 2.Clinical examination. (A) CT scans
revealed a primary tumor located in the right pulmonary hilum with
metastases to the mediastinum, right pleura, right hepatic lobe,
and liver capsule prior to T cell infusions. In January 2016 (day
43), a CT scan obtained 2 months after the first T-cell infusion
showed objective regression of the primary lung tumor and liver
metastases, as well as hydrothorax absorption and pulmonary
re-expansion. In March 2016 (Day 138), a surveillance CT scan
detected growth of the primary lung tumor and liver metastases with
re-establishment of the hydrothorax. (B) Levels of the tumor
biomarkers (CEA, CA125, and CA199) were reduced 2 weeks post
infusion, but then increased 4 weeks after the initial infusion of
TCR-T cells. A similar pattern was observed after the second
infusion of the NY-ESO-1 TCR engineered T-cells. (C) Proportions of
T-cell subsets: Percentages of CD4+ and CD8+
T cells in the peripheral blood of the patient were increased and
decreased, respectively, by day 10 after the T cell infusion. The
CD8+CD28− and CD4+CD25+
T cell subgroups were smaller and larger, respectively, 10 days
after the T-cell infusion. There were no obvious changes in the
quantity of CD8+CD28+ T cells. CT, computer
tomography; TCR-T, T cell receptor engineered-T cells. |
Levels of tumor biomarkers (CEA, CA125, and CA199)
were decreased after the initial infusion of TCR-T cells, but later
increased. A similar pattern was seen after the second infusion of
TCR-T cells targeting NY-ESO-1 (Fig.
2B). The percentage of CD4+ T cells in the
peripheral blood of the patient had increased by day 10 after the
TCR-T cell infusion, while the percentage of CD8+ T
cells was decreased. Furthermore, there was a reduction in the
CD8+CD28− subgroup in the blood samples,
whereas the percentage of CD4+CD25+ in the
peripheral blood had increased by 10 days after the T-cell
infusion. However, there were no obvious fluctuations in the
proportion of CD8+CD28+cell (Fig. 2C). Of note, the patient had
improvement in Karnofsky performance status (KPS) with a score from
50 to 90 post infusion, and resolution of hemoptysis and chest
pain. These results indicate that TCR-T cell treatment has improved
the patient's clinical symptoms.
Laboratory assays were conducted to track the
persistence of TCR-T cells and examine related immunologic response
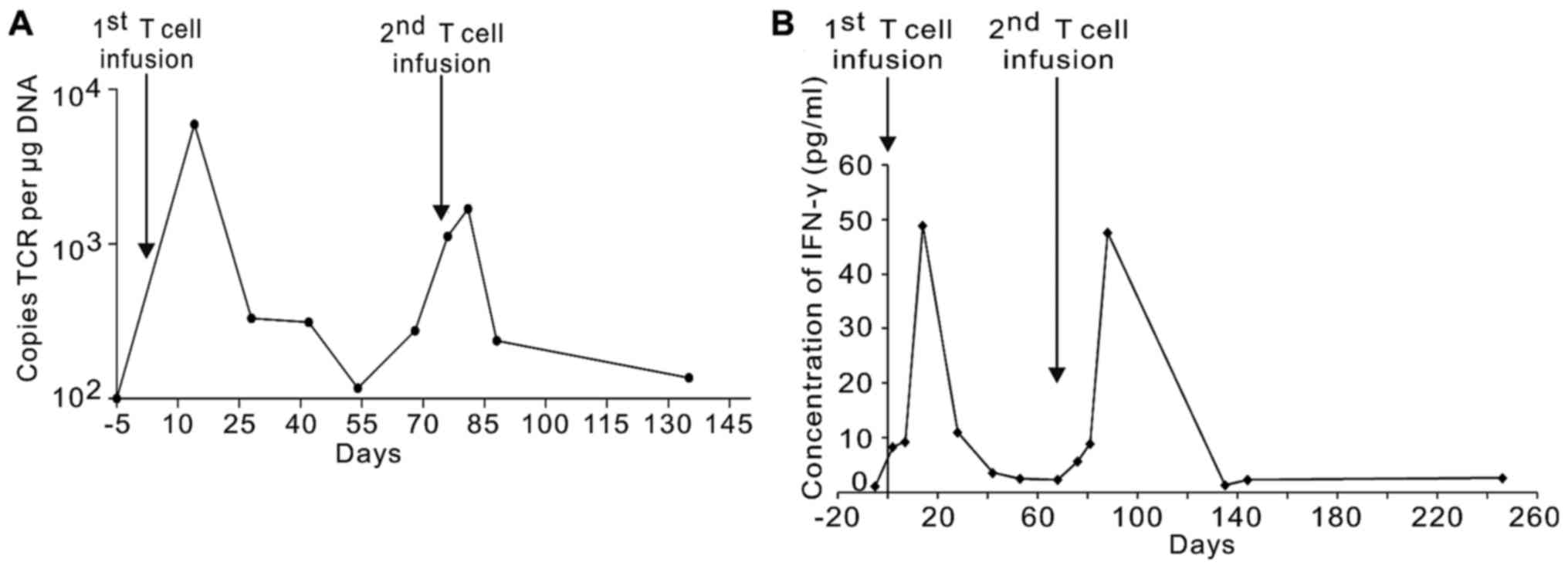
in vivo. As shown in Fig. 3A,
after the first infusion (peak value, 6784.48 copies/µg DNA), there
was a rapid rise in the quantity of TCR DNA copies in the whole
blood samples over 2 weeks after the first infusion. This was
followed by a rapid decline. Transduced DNA copies of the NY-ESO-1
specific TCR fluctuated between 330.1 and 166.8 copies per µg DNA
at 4 weeks. Similar to the first infusion, the DNA copies of
NY-ESO-1-specific TCR reached high levels over the second week
(peak value, 2362.02 copies/µg DNA) and then quickly declined over
the fourth week after the second TCR-T cell infusion.
Furthermore, patient serum cytokine levels were
measured at serial timepoints before and after the cell infusion.
IFN-γ levels peaked at the second week after cell infusion (48.92
pg/ml post the first infusion, 47.63 pg/ml post the second
infusion) and then gradually degraded to low levels (Fig. 3B). Serum cytokine concentrations of
IL-6, IL-10, and granulocyte-macrophage CSF (GM-CSF) displayed
little changes (data not shown).
ACT of TCR-T cells was well-tolerated by patient 2
and did not induce clinically apparent cytokine release syndrome
(CRS). Although patient 2 had a fever (maximum temperature 39.5°C,
Grade 2) during and after the infusion, it was successfully
resolved within three days. In general, there was no clinical or
laboratory evidences revealing SAEs during TCR-T cell therapy for
this patient. However, patient 2 suffered a relapse after the TCR-T
cell therapy and thus received six cycles of combination
chemotherapy (docetaxel and carboplatin). Notably, she kept taking
erlotinib before, during and after the clinical trial. As of
October 10, 2017, the patient was still alive with SD.
Discussion
Cancer-testis antigens, such as MAGE-A3 and
NY-ESO-1, are promising candidate targets for cell transfer-based
immunotherapies due to their specific expression patterns and
strong immunogenicity (19,30,31).
MAGE-A3 antigen was previously thought to be a preferred target for
immunotherapy of cancers, since its expression had been frequently
detected in multiple types of tumors but limited in normal somatic
cells (47,48). Nevertheless, adoptive transfer of
TCR-T cells targeting MAGE-A3 antigen lead to deaths of two
patients due to severe neurological toxicity in recent clinical
trials (48). This may due to
cross-reactivity of TCR-T cells with MAGE-A12, which is expressed
at the low level in the brain tissue (48). In addition, engineered T cells
expressing affinity-enhanced TCRs targeting MAGE-A3 resulted in
deaths of the first two patients in another preliminary clinical
trial on melanoma and myeloma due to severe cardiac toxicity, which
was confirmed by histopathological analysis of the T cell
infiltration (47). The following
in-depth investigation in vitro shows that the off-target
and off-tumor reactivities maybe due to the cross-reactivity of
MAGE-A3 TCR-T cells with the human protein titin, which is highly
expressed in cardiac tissue (47,49,50).
NY-ESO-1 is one of the best cancer-testis antigens
for immunotherapy due to its strong immunogenicity and specific
expression pattern. Early study of adoptive transfer of autologous
CD4+ T cells sensitized to NY-ESO-1 peptide in
vitro induced tumor regression of metastatic melanoma in 1 of 9
patients (29). Moreover, in a
clinical trial conducted by Robbins et al, 11 of 18
HLA-A*0201-positive patients with NY-ESO-1+ synovial
cell sarcomas, and 11 of 20 HLA-A*0201-positive patients with
NY-ESO-1+ melanoma achieved objective clinical responses
following adoptive transfer of NY-ESO-1 TCR-T cells (30). However, one patient with synovial cell
sarcomas died three days following the adoptive transfer of
NY-ESO-1 TCR-T cells due to septic shock caused by Escherichia
coli bacterial infection (30).
In another study conducted by Rapoport et al, 16 of 20
patients with myeloma revealed sustained clinical responses
following NY-ESO-1 TCR-T cell therapy (31). It was noted that SAEs likely related
to treatment, including hypoxia, neutropenia, hyponatremia,
hypotension, graft vs. host disease, pancytopenia, and dehydration,
were resolved and no treatment related fatalities occurred
(31). Meanwhile, no clinically
apparent CRS occurred, with the exception of high IL-6 levels
(31). By contrast, CRS, which could
be potentially life-threatening, was frequently occurred (93%) in
94 patients with refractory large B-Cell lymphoma when treated with
CAR-T cells targeting CD19 antigen (51). In the present study, although adverse
events likely associated with the TCR-T cell treatment also
occurred, including anemia, white blood cell decrease, fever,
nausea, fatigue and abdominal pain, they were then resolved by
symptomatic treatment. Clinically apparent CRS was not observed,
despite transiently high IFN-γ levels. Meanwhile, there were no
treatment-related deaths in patients with NSCLC. Taken together,
our results show no off-target/off-tumor toxicity and infection. It
suggested that NY-ESO-1 TCR-T cell therapy seems to be relatively
safe and well-tolerated. Nevertheless, further clinical studies
using NY-ESO-1 TCR-T cells in lung cancer and other types of solid
tumors are needed in a large number of patients to assess the
safety and clinical efficacy of this new treatment.
We showed that treatment with NY-ESO-1 TCR-T cells
mediated tumor regression in a patient (1/4) with metastatic NSCLC.
Although patient 2 continued to take erlotinib throughout NY-ESO-1
TCR-T cell treatment for her tumor carrying EGFR mutation, it was
unlikely that erlotinib played the main part in tumor size
reduction, since the patient did not respond to erlotinib alone
prior to infusion. Previous study indicated that lymphodepleting
chemotherapy regimen and IL-2 administrated to all of the patients
may have contributed to the PR in melanoma and/or synovial cell
sarcoma patients (30). Nevertheless,
we considered that NY-ESO-1 TCR-T cells played a vital role in
tumor regression after the first infusion of the TCR-T cells in
this case. Firstly, the expression levels of tumor biomarkers (CEA,
CA125, and CA199) showed an inverse association with TCR-T cell
persistence in the peripheral blood of the patient. This was
indicative of the relationship between the curative effect and
NY-ESO-1 TCR-T cells. Secondly, IFN-γ secretion by CD4+
T cells has been shown to be a potential mechanism underlying the
therapeutic effect of tumor-specific CD4+ T cells in a
mouse model bearing B16 melanoma (52,53). The
proportion of CD4+ T cells and levels of IFN-γ in the
peripheral blood were increased after TCR-T cell infusion and were
positively correlated with NY-ESO-1 TCR-T cells.
Although patient 2 initially responded well to the
ACT with TCR-T cells and achieved PR for nearly 4 months, tumor
relapse eventually occurred after the second infusion of TCR-T
cells. According to previous studies, several factors may
contribute to tumor recurrence after TCR-T cell therapy. Firstly, a
loss of persistence and function of genetically-modified T cells
may be associated with tumor relapse (30,31). The
persistence of peptide-reactive and tumor-reactive T cells with
MART-1- and gp100-recognizing TCRs was positively associated with
clinical response (41), while
another study showed that relapse was related to the loss of TCR-T
cells (31). Therefore, the
approaches to sustain the long-term persistence and function of
engineered T cells in vivo may benefit the durability of the
treatment efficacy (31).
Moreover, the antigen expression pattern, such as
expression uniformity at diagnosis, loss of target antigen
expression, and/or growth of tumor variants lacking expression of
the target antigen post-infusion, was one of principal factors that
may influence outcome and tumor relapse following treatment with
engineered T cells. Compared to the unsatisfying efficacy of TCR-T
cells, clinical trials with CAR-T cells targeting B-cell lineage
CD19-differentiation antigen demonstrated remarkable clinical
efficacy in the induction of long-term stable remission for B-cell
malignancies (17,54,55).
Notably, unlike CD19 antigen, which is highly and uniformly
expressed on B cells, IHC staining of cancer tissues from the
patients in studies revealed heterogeneous expression of
cancer-testis antigens, such as NY-ESO-1 in the current study.
Furthermore, tumor cells display very strong
plasticity, where therapeutic failure and drug resistance may be
due to intratumor heterogeneity, which is featured as dynamic
genetic diversity and epigenetic plasticity (56). Patients with metastatic melanoma that
underwent adoptive transfer of melanocyte antigen-specific
CD8+ T cells displayed post-infusion relapse, where
residual nodules revealed selective loss of targeted antigens
(gp100, tyrosinase, and MART1) in three of the five patients
(57). Other studies have shown
antigen escape was associated with PD after treatment with NY-ESO-1
and MAGE-A3 TCR-T cells (19,31). Meanwhile, immunotherapy with
antigen-specific T cells has resulted in the outgrowth of
antigen-loss tumor variants in some studies (57,58).
Suppressive tumor microenvironments expressing inhibitory molecules
and receptors, such as PD-1/PD-L1, also contributed to tumor
recurrence (59). In the current
study, we could not obtain tumor tissue samples for further
evaluation after recurrence in the patient 2 due to proximity of
the tumor to her right hilus pulmonis. Interestingly, co-treatment
with chemotherapy (docetaxel and carboplatin) and erlotinib after
treatment with TCR-T cells have controlled disease progression and
resulted in SD (as of October 10, 2017).
In summary, immunotherapy with NY-ESO-1 TCR-T cells
in four HLA-A2-positive patients with NSCLC is well tolerated
without evident severe toxicities. Among the treated patients, the
one with advanced LADC revealed a short-term PR (4 months).
Although there are some obstacles that need to be overcome for
TCR-T cell therapy in solid tumors, such as identification of
suitable target antigens, maintenance of persistence and activity
of TCR-T cells, enhancement of TCR-T cell trafficking and function,
and improvement of tumor microenvironment with immune suppression
(59–61), this and other clinical studies in
solid tumors strongly suggest that NY-ESO-1 TCR-T cell
immunotherapy is relatively safe and well-tolerated. However,
further clinical studies are still warranted in a large number of
lung cancer patients.
Acknowledgements
The authors would like to thank Dr Wenlan Liu (The
First Affiliated Hospital of Shenzhen University) for support with
laboratory instruments.
Funding
The present study was funded by Shenzhen Peacock
Plan (grant no. KQTD20130416114522736); the National Basic Research
Program of China (grant no. 2014CB745203), Guangdong Innovative
Research Team Program (grant no. 201001Y0104687244), Shenzhen
Technology Research Program (grant no. JSGG20160301161836370),
Special funds for Dapeng New district industry development (grant
nos. KY20150116 and KY20160111) and Natural Science Foundation of
Guangdong Province (grant no. 2016A030313238).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to technology secrecy
but are available from the corresponding author on reasonable
request.
Authors' contributions
YX and XT wrote the manuscript, and were responsible
for data acquisition, analysis and interpretation; JW, DQ, DB, JC,
JY performed TCR-T cell preparation and experimental studies; XL,
LX, WL performed clinical studies; MW, GT and RW supervised the
entire study, designed the design and approved the protocol. All
authors contributed to and approved the final manuscript.
Ethics approval and consent to
participate
The clinical trial (NCT02457650) is registered on
ClinicalTrial.gov (https://clinicaltrials.gov/). It was approved by the
Medical Ethics Committee of the Institutional Review Board,
Shenzhen Second People's Hospital. All procedures performed in
studies involving human subjects were in accordance with the
ethical standards of the institutional and/or national research
committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. All of the patients
enrolled in the trail provided with written informed consent.
Patient consent for publication
The patient, or parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5 (Suppl
4):S389–S396. 2013.PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito M, Suzuki H, Kono K, Takenoshita S
and Kohno T: Treatment of lung adenocarcinoma by molecular-targeted
therapy and immunotherapy. Surg Today. 48:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined Nivolumab and Ipilimumab or
Monotherapy in Untreated Melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman HL, Russell J, Hamid O, Bhatia S,
Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M,
et al: Avelumab in patients with chemotherapy-refractory metastatic
Merkel cell carcinoma: A multicentre, single-group, open-label,
phase 2 trial. Lancet Oncol. 17:1374–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer JR: Immune checkpoint blockade:
The hope for immunotherapy as a treatment of lung cancer? Semin
Oncol. 41:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevanovic S, Draper LM, Langhan MM,
Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry
RM, Kammula US, et al: Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted
tumor-infiltrating T cells. J Clin Oncol. 33:1543–1550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robbins PF, Lu YC, El-Gamil M, Li YF,
Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al:
Mining exomic sequencing data to identify mutated antigens
recognized by adoptively transferred tumor-reactive T cells. Nat
Med. 19:747–752. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
San Miguel JF, Paiva B and Lasarte JJ:
Engineering anti-myeloma responses using affinity-enhanced
TCR-engineered T cells. Cancer Cell. 28:281–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh H, Huls H, Kebriaei P and Cooper LJ:
A new approach to gene therapy using Sleeping Beauty to genetically
modify clinical-grade T cells to target CD19. Immunol Rev.
257:181–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu YC, Parker LL, Lu T, Zheng Z, Toomey
MA, White DE, Yao X, Li YF, Robbins PF, Feldman SA, et al:
Treatment of patients with metastatic cancer using a major
histocompatibility complex class II-restricted T-cell receptor
targeting the cancer germline antigen MAGE-A3. J Clin Oncol.
35:3322–3329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boon T and Old LJ: Cancer tumor antigens.
Curr Opin Immunol. 9:681–683. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salmaninejad A, Zamani MR, Pourvahedi M,
Golchehre Z, Hosseini Bereshneh A and Rezaei N: Cancer/testis
antigens: Expression, regulation, tumor invasion, and use in
immunotherapy of cancers. Immunol Invest. 45:619–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stockert E, Jäger E, Chen YT, Scanlan MJ,
Gout I, Karbach J, Arand M, Knuth A and Old LJ: A survey of the
humoral immune response of cancer patients to a panel of human
tumor antigens. J Exp Med. 187:1349–1354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maio M, Coral S, Sigalotti L, Elisei R,
Romei C, Rossi G, Cortini E, Colizzi F, Fenzi G, Altomonte M, et
al: Analysis of cancer/testis antigens in sporadic medullary
thyroid carcinoma: Expression and humoral response to NY-ESO-1. J
Clin Endocrinol Metab. 88:748–754. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scanlan MJ, Altorki NK, Gure AO,
Williamson B, Jungbluth A, Chen YT and Old LJ: Expression of
cancer-testis antigens in lung cancer: Definition of bromodomain
testis-specific gene (BRDT) as a new CT gene, CT9. Cancer Lett.
150:155–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scanlan MJ, Gout I, Gordon CM, Williamson
B, Stockert E, Gure AO, Jäger D, Chen YT, Mackay A, O'Hare MJ and
Old LJ: Humoral immunity to human breast cancer: antigen definition
and quantitative analysis of mRNA expression. Cancer Immun.
1:42001.PubMed/NCBI
|
|
27
|
Chen YT: The journey from autologous
typing to SEREX, NY-ESO-1 and cancer/testis antigens. Cancer Immun.
12:82012.PubMed/NCBI
|
|
28
|
Jager E, Chen YT, Drijfhout JW, Karbach J,
Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, et
al: Simultaneous humoral and cellular immune response against
cancer-testis antigen NY-ESO-1: Definition of human
histocompatibility leukocyte antigen (HLA)-A2-binding peptide
epitopes. J Exp Med. 187:265–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hunder NN, Wallen H, Cao J, Hendricks DW,
Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA and Yee
C: Treatment of metastatic melanoma with autologous CD4+ T cells
against NY-ESO-1. N Engl J Med. 358:2698–2703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robbins PF, Kassim SH, Tran TL, Crystal
JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR,
Sherry RM, et al: A pilot trial using lymphocytes genetically
engineered with an NY-ESO-1-reactive T-cell receptor: Long-term
follow-up and correlates with response. Clin Cancer Res.
21:1019–1027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rapoport AP, Stadtmauer EA, Binder-Scholl
GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B,
Finklestein J, et al: NY-ESO-1-specific TCR-engineered T cells
mediate sustained antigen-specific antitumor effects in myeloma.
Nat Med. 21:914–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SH, Lee S, Lee CH, Lee MK, Kim YD,
Shin DH, Choi KU, Kim JY, Park DY and Sol MY: Expression of
cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell
lung carcinomas and their relationship with immune cell
infiltration. Lung. 187:401–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gjerstorff MF, Pøhl M, Olsen KE and Ditzel
HJ: Analysis of GAGE NY-ESO-1 and SP17 cancer/testis antigen
expression in early stage non-small cell lung carcinoma. BMC
Cancer. 13:4662013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oshima Y, Shimada H, Yajima S, Nanami T,
Matsushita K, Nomura F, Kainuma O, Takiguchi N, Soda H, Ueda T, et
al: NY-ESO-1 autoantibody as a tumor-specific biomarker for
esophageal cancer: Screening in 1969 patients with various cancers.
J Gastroenterol. 51:30–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tureci O, Mack U, Luxemburger U, Heinen H,
Krummenauer F, Sester M, Sester U, Sybrecht GW and Sahin U: Humoral
immune responses of lung cancer patients against tumor antigen
NY-ESO-1. Cancer Lett. 236:64–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laport GG, Levine BL, Stadtmauer EA,
Schuster SJ, Luger SM, Grupp S, Bunin N, Strobl FJ, Cotte J, Zheng
Z, et al: Adoptive transfer of costimulated T cells induces
lymphocytosis in patients with relapsed/refractory non-Hodgkin
lymphoma following CD34+-selected hematopoietic cell
transplantation. Blood. 102:2004–2013. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rapoport AP, Stadtmauer EA, Aqui N, Badros
A, Cotte J, Chrisley L, Veloso E, Zheng Z, Westphal S, Mair R, et
al: Restoration of immunity in lymphopenic individuals with cancer
by vaccination and adoptive T-cell transfer. Nat Med. 11:1230–1237.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klebanoff CA, Khong HT, Antony PA, Palmer
DC and Restifo NP: Sinks, suppressors and antigen presenters: How
lymphodepletion enhances T cell-mediated tumor immunotherapy.
Trends Immunol. 26:111–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robson NC, McAlpine T, Knights AJ, Schnurr
M, Shin A, Chen W, Maraskovsky E and Cebon J: Processing and
cross-presentation of individual HLA-A, -B, or -C epitopes from
NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the
mode of antigen delivery. Blood. 116:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robbins PF, Li YF, El-Gamil M, Zhao Y,
Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, et al:
Single and dual amino acid substitutions in TCR CDRs can enhance
antigen-specific T cell functions. J Immunol. 180:6116–6131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson LA, Morgan RA, Dudley ME, Cassard
L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich
JR, et al: Gene therapy with human and mouse T-cell receptors
mediates cancer regression and targets normal tissues expressing
cognate antigen. Blood. 114:535–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hughes MS, Yu YY, Dudley ME, Zheng Z,
Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA
and Morgan RA: Transfer of a TCR gene derived from a patient with a
marked antitumor response conveys highly active T-cell effector
functions. Hum Gene Ther. 16:457–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Topalian SL, Solomon D and Rosenberg SA:
Tumor-specific cytolysis by lymphocytes infiltrating human
melanomas. J Immunol. 142:3714–3725. 1989.PubMed/NCBI
|
|
44
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Linette GP, Stadtmauer EA, Maus MV,
Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM,
Cimino PJ, et al: Cardiovascular toxicity and titin
cross-reactivity of affinity-enhanced T cells in myeloma and
melanoma. Blood. 122:863–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morgan RA, Chinnasamy N, Abate-Daga D,
Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry
RM, et al: Cancer regression and neurological toxicity following
anti-MAGE-A3 TCR gene therapy. J Immunother. 36:133–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
LeWinter MM and Granzier H: Cardiac titin:
A multifunctional giant. Circulation. 121:2137–2145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Herman DS, Lam L, Taylor MR, Wang L,
Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B,
Sparks E, et al: Truncations of titin causing dilated
cardiomyopathy. N Engl J Med. 366:619–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Quezada SA, Simpson TR, Peggs KS, Merghoub
T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et
al: Tumor-reactive CD4(+) T cells develop cytotoxic activity and
eradicate large established melanoma after transfer into
lymphopenic hosts. J Exp Med. 207:637–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wei G, Ding L, Wang J, Hu Y and Huang H:
Advances of CD19-directed chimeric antigen receptor-modified T
cells in refractory/relapsed acute lymphoblastic leukemia. Exp
Hematol Oncol. 6:102017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tasian SK and Gardner RA: CD19-redirected
chimeric antigen receptor-modified T cells: A promising
immunotherapy for children and adults with B-cell acute
lymphoblastic leukemia (ALL). Ther Adv Hematol. 6:228–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Greaves M: Evolutionary determinants of
cancer. Cancer Discov. 5:806–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yee C, Thompson JA, Byrd D, Riddell SR,
Roche P, Celis E and Greenberg PD: Adoptive T cell therapy using
antigen-specific CD8+ T cell clones for the treatment of patients
with metastatic melanoma: In vivo persistence, migration and
antitumor effect of transferred T cells. Proc Natl Acad Sci USA.
99:16168–16173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mackensen A, Meidenbauer N, Vogl S, Laumer
M, Berger J and Andreesen R: Phase I study of adoptive T-cell
therapy using antigen-specific CD8+ T cells for the treatment of
patients with metastatic melanoma. J Clin Oncol. 24:5060–5069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hay KA and Turtle CJ: Chimeric antigen
receptor (CAR) T cells: Lessons learned from targeting of CD19 in
B-cell malignancies. Drugs. 77:237–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Irving M, Vuillefroy de Silly R, Scholten
K, Dilek N and Coukos G: Engineering chimeric antigen receptor
T-cells for racing in solid tumors: Don't forget the fuel. Front
Immunol. 8:2672017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Frigault MJ and Maus MV: Chimeric antigen
receptor-modified T cells strike back. Int Immunol. 28:355–363.
2016. View Article : Google Scholar : PubMed/NCBI
|