Introduction
Ly-6/urokinase-type plasminogen activator receptors
(uPARs) are a membrane protein superfamily characterized by the
three-finger fold structural motif and a putative
glycosylphosphatidylinositol (GPI)-anchoring site (1,2). uPARs
regulate the proteolytic degradation processes in the extracellular
matrix (ECM) by binding the extracellular protease urokinase-type
plasminogen activator, which subsequently activates plasminogen
(3). ECM molecules constitute the
cellular microenvironment and provide mechanical support for cells.
Furthermore, they also regulate intracellular signaling pathways by
interacting with transmembrane proteins (4). For example, it was reported that
ECM-integrin interaction may promote β cell proliferation by
activating the phosphoinositide 3-kinase cascade (5). uPAR-mediated modulation of the ECM is
known to alter many cellular processes, including cell adhesion,
proliferation, differentiation and migration (3,6);
therefore, uPARs have been implicated in a number of human
diseases, including cancer and other inflammatory diseases.
Previous studies have demonstrated that uPARs are generally
upregulated in cancer cells and during other inflammatory
conditions or infections, and this makes them attractive
therapeutic targets for the impairment of ECM-cell interactions and
the signaling pathways of abnormal cells (1,7–9)
Lymphocyte antigen 6 family member K (Ly6K) is a
recently discovered member of the uPAR family and was first
identified as a molecular marker for head-and-neck squamous cell
carcinoma (10). Previous studies
have demonstrated that Ly6K is upregulated in numerous types of
cancer, including esophageal squamous cell carcinoma, bladder
cancer and breast cancer, and contributes to cell growth,
migration, invasion, and immune escape (11–14). In
breast cancer, it is reported that Ly6K expression is regulated by
the transcription factor activator protein-1, and that Ly6K
promotes cell proliferation and metastasis by activating the
Raf-1/MEK/ERK signaling pathway in cancer (15). Therefore, Ly6K has been suggested as a
cancer biomarker and therapeutic target. Nevertheless, the in
vivo molecular mechanism of Ly6K remains ill-defined, and an
appropriate in vivo mouse model to study the role of Ly6K
has not yet been generated.
Lymphocytes are the blood cells responsible for
immune responses. They comprise three main types: T cells, B cells,
and natural killer cells (NK cells), which all develop from
hematopoietic stem cells (HSC) in bone marrow (16). Lymphocyte development is achieved
through several specific stages, involving coordinated regulation
of lineage-associated gene expression, which is dependent on the
type of cell (17). B cells, which
mediate the humoral immune response by producing antibodies,
undergo full development in the bone marrow via immunoglobulin gene
rearrangement, whereas T cell development occurs distal to the bone
marrow (18). HSCs migrate from the
bone marrow to the thymus and continue to proliferate to generate a
large group of immature thymocytes, which lack T-cell receptor
(TCR) expression. These cells do not express CD4 or CD8, and are
therefore termed double-negative (DN) cells (19). Following several developmental stages,
cells that achieve pre-TCR expression develop into double-positive
CD4+CD8+ cells. These cells then become
single-positive thymocytes (CD4+CD8− or
CD4−CD8+), depending on the interaction
between the TCR and the major histocompatibility complex (MHC)
ligand on the epithelial cells (20,21).
Despite the strong association between Ly6K and
cancer development, in vivo models to examine the effect of
Ly6K expression on tumors have not yet been developed. In the
present study, a transgenic mouse model overexpressing the human
LY6K (hLY6K) gene was generated to investigate the
changes resulting from LY6K overexpression in vivo.
Mouse mammary tumor virus (MMTV)/hLY6K transgenic mice
expressed LY6K mRNA strongly in the thymus and spleen. Flow
cytometric analysis demonstrated that the levels and distribution
of cytotoxic and helper T cells were decreased in the transgenic
mouse, which indicated that T cell development was defective when
LY6K was overexpressed.
Materials and methods
Generation of LY6K transgenic
mice
The construct to generate the transgenic mice was
produced by inserting human LY6K cDNA, which was previously
described (22), into the pMAMneo
vector (Clontech Laboratories, Inc., Mountainview, CA, USA). The
full-length human LY6K cDNA fragment was amplified by
polymerase chain reaction (PCR) under the following thermocycling
conditions: Initial denaturation at 98°C for 5 min, 35 cycles at
98°C for 10 sec, 50°C for 50 sec and 72°C for 20 sec; and final
extension at 72°C for 5 min. For PCR, Taq Polymerase, dNTPs and
reaction buffers (Real Biotech Corporation, Taipei, Taiwan) were
used according to the manufacturer's protocol.
Specifically-designed primers containing NheI or XhoI
restriction enzyme sites were used and the sequences were as
follows: Forward, 5′-CTAGCTAGCATGGCGCTGCT-3′; and reverse,
5′-GGCCTCGAGTCAAGACAGGC-3′. Fragments digested with NheI and
XhoI restriction enzymes (New England Biolabs, Ipswich, MA,
USA) were purified by the HiYield Gel/PCR extraction kit (Real
Biotech Corporation) according to the manufacturer's protocol, and
then inserted into the corresponding restriction sites of the
vector. The recombinant construct carrying the transgene was
microinjected into the pronuclei of fertilized eggs of B6 mice
(Animal Facility, Sookmyung Women's University, Seoul, Korea), and
the eggs were then transferred into the oviducts of pseudo-pregnant
female mice. Transgenic founder mice were bred with B6 mice to
establish transgenic lines; a total of 7 founder mice (8–9
weeks-old; average weight, ~20 g) were generated. All mice were
housed in a specific pathogen-free barrier facility with a 12 h
light/dark cycle and maintained at a humidity of 40–60% at 23°C.
Mice had ad libitum access to food and water. After 6 weeks,
mice were euthanized by cervical dislocation for further analysis.
All animal experiments were approved by the Institutional Animal
Care and Use Committee at the Sookmyung Women's University (Seoul,
South Korea).
Genomic DNA extraction and PCR
Genomic DNA was extracted from the tail biopsies of
each mouse using a Quick-DNA™ Universal Kit (Zymo
Research, Irvine, CA, USA) according to the manufacturer's
protocol. To confirm the presence of the transgene, PCR was
performed to amplify specific fragments of the hLY6K gene.
The sequences of the primers used were as follows: Forward,
5′-TGTGGTTTAGGTTGGAGTGTAGTG-3′; and reverse,
5′-CTCATCAAAAAAATCTCCCCAAC-3′. EmeraldAmp® GT PCR Master
Mix (Clontech Laboratories, Inc.) was used to amplify DNAs
according to the manufacturer's protocol. The thermocycling
conditions were as follows: Initial denaturation step for 2 min at
95°C, 30 cycles for 30 sec at 95°C, 30 sec at 58°C and 30 sec at
72°C, and the final extension step for 5 min at 72°C. The amplified
DNA samples were resolved on a 1.0% agarose gel and separated by
electrophoresis at 200 V for 40 min, which produced bands of 360
bp. To compare the amount of the transgene, standard samples
containing 1, 10 and 100 copies of the transgene were prepared and
mixed with the genomic DNA of wild-type B6 mice, then amplified and
separated on the same agarose gel.
Quantitative PCR (qPCR) evaluation of
transgene copy number
To examine the copy number of the transgene, a
standard curve was generated using 1, 10 and 100 copies of the
transgene diluted in the genomic DNA of wild-type mice. qPCR was
performed using SYBR-Green qPCR Master Mix (PCR Biosystems, Ltd.,
London, UK), and each reaction sample contained the following
components: 100 ng genomic DNA, 10 µM of each primer and 5 µl SYBR
Green qPCR Master Mix. The sequence of the primers used were as
follows: hLY6K forward, 5′-AGCCCATGCCCTTCTTTTACCTCA-3′; and
hLY6K reverse, 5′-CCAGCCACAGCCCACCACAG-3′; mouse
Gapdh (mGapdh) forward, 5′-GCTGAGTATGTCGTGGAGTC-3′;
and mGapdh reverse, 5′-ATGGACTGTGGTCATGAGC-3′. The
mGapdh gene was amplified as an endogenous control. qPCR
cycling conditions were as follows: A pre-incubation step of 10 min
at 95°C; followed by 45 amplification cycles of 10 sec at 95°C, 10
sec at 60°C, and 10 sec at 72°C; and a final elongation step of 1
min at 65°C. The standard curve was determined by plotting ΔCq
(ΔCq=CqhLY6K-CqGapdh) against the log of
hLY6K gene copies of the corresponding standard samples, and
the copy numbers of the transgene in transgenic mice were
determined by the 2−ΔΔCq method (23). The formula was as follows:
2−ΔΔCq=2−(ΔCq of transgenic samples-ΔCq of
wild-type samples) (24).
RNA isolation and reverse
transcription (RT)
RNAs were extracted from each tissue of 3 mice per
group (wild-type and transgenic mice) using a
NucleoSpin® DNA/RNA/Protein kit (Machery-Nagel GmbH,
Düren, Germany), according to the manufacturer's protocol. RNAs (2
µg) diluted in 12.5 µl of sterile RNase free water were reverse
transcribed to cDNAs using an M-MLV Reverse Transcription kit
(Promega Corporation, Madison, WI, USA) at 42°C for 1 h and then
inactivated at 70°C for 10 min.
RT-PCR
Following RT, as described above, cDNA samples (100
ng) were used as templates to amplify specific fragments with RBC
Taq Polymerase, and each reaction mixture contained the following:
3 µl 10X RBC Reaction Buffer, 3 µl dNTPs (2.5 mM), 10 mM of each
primer, 100 ng of cDNA, 1 µl RBC Taq Polymerase and 21.7 µl
double-distilled water. The sequences of the primers used to detect
hLY6K or mGapdh were as follows: hLY6K forward,
5′-TGCTCGCCTTGCTGCTGGTC-3′; hLY6K reverse,
5′-TCGCTGCACAACCAGCGGAG-3′; mGapdh forward,
5′-CGGTGCTGAGTATGTCGTGGAG-3′; and mGapdh reverse,
5′-TGTCATCATACTTGGCAGGTTTC-3′. Amplification condition were as
follows: Initial denaturation step for 2 min at 95°C, 30 cycles for
30 sec at 95°C, 30 sec at 58°C and 30 sec at 72°C; and the final
extension step for 5 min at 72°C. Following amplification, the PCR
products were separated on 1.0% agarose gels by electrophoresis at
200 V for 40 min.
RT-qPCR
To further compare the RNA expression level of
hLY6K in wild-type and transgenic mice, RT-qPCR was
conducted using SYBR Green qPCR Master Mix (PCR Biosystems, Ltd.).
cDNA samples (100 ng) were used as templates, and the sequences of
the primers to detect hLY6K and mouse Gapdh were same
as those used to examine the transgenic copy number. qPCR cycling
conditions were as follows: A pre-incubation step of 10 min at
95°C; 45 amplification cycles of 10 sec at 95°C, 10 sec at 60°C and
10 sec at 72°C; and a final elongation step of 1 min at 65°C. The
relative expression levels of hLY6K were calculated by the
2−ΔΔCq method, normalized against the level of
mGapdh.
Western blot analysis
To obtain proteins from the mouse tissues, tissues
was disrupted and homogenized with lysis buffer RP1 (Macherey-Nagel
GmbH & Co., Düren, Germany) containing 1% β-mercaptoethanol
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and stainless steel
beads using TissueLyser (Qiagen GmbH, Hilden, Germany) at 1500
oscillations/min for 1 min at room temperature. Protein extracts
were prepared using a NucleoSpin® DNA/RNA/Protein kit
and buffers included in kit (Macherey-Nagel GmbH & Co.),
according to the manufacturer's protocol. Protein extracts were
dissolved with Protein Solving Buffer containing the reducing agent
TCEP (Macherey-Nagel GmbH & Co.) and boiled at 98°C for 3 min.
The protein concentration was measured using a bicinchoninic acid
solution and copper (II) sulfate solution (Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany). Samples were loaded (30 µg/lane) and
separated by SDS-PAGE (10–12% gel) and transferred onto
polyvinylidene fluoride membranes. Membranes were blocked with 5%
skim milk in PBS containing 0.2% Tween-20 for 1 h at room
temperature. Immunoblotting was conducted using an anti-Ly6K
antibody (cat no. sc-87282; Santa Cruz Biotechnology, Santa Cruz,
CA, USA) for the detection of human and mouse Ly6K, and an
anti-β-actin antibody (cat no. A300-491A; Bethyl Laboratories,
Montgomery, TX, USA), which were diluted at 1:1,000 in 1% skim milk
in PBS containing the detergent 0.2% Tween-20 overnight at 4°C.
Horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat no. ADI-SAB-300-J; Enzo Life Science, Inc.,
Farmingdale, NY, USA) was used and diluted at 1:4,000 in 2% skim
milk in PBS containing the detergent 0.2% Tween-20 for 1 h at room
temperature. Immunoreactive proteins were detected using the
enhanced chemiluminescence reagent EzWestLimiplus (cat no.
WSE-7120; ATTO Corporation, Tokyo, Japan).
Flow cytometric analysis
Cell populations were isolated from the thymus or
spleen of the transgenic mouse and investigated by flow cytometry.
Cells were washed with 1X PBS and then fixed with 70% ethanol
overnight at 4°C. To examine the expression of surface markers of
thymocytes, cells were stained with specific antibodies targeting
surface markers of lymphocytes: Conjugated PerCP-Cy5.5-anti-CD44
(cat no. 45-0441-82; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), conjugated allophycocyanin (APC)-anti-CD25 and
conjugated APC-anti-B220 (cat nos. 17-0251-82 and 17-0452-82; all
from Invitrogen; Thermo Fisher Scientific, Inc.), conjugated
PE-anti-CD4 and conjugated fluorescein isothiocyanate-anti-CD8 (cat
nos. ab134354 and ab28010; all from Abcam). All antibodies were
diluted 1:200 in FACS buffer (1X PBS containing 1% BSA) and
incubated with each sample for 30 min on ice. Analysis was
conducted using a FACSCanto II flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and data were analyzed using FlowJo 7.6.5
software (Tree Star, Inc., Ashland OR, USA).
Statistical analysis
All experiments were repeated three times
independently. Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The
data are presented as the mean ± standard deviation. The RT-qPCR
data were analyzed by Student's t-test, and the other data were
examined by a two-way analysis of variance followed by a Bonfferoni
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
hLY6K was successfully overexpressed
in transgenic mice
To investigate the function of human LY6K on
mammary development in vivo, transgenic mice overexpressing
the human LY6K gene were established. We generated transgene
construct for the full-length human LY6K gene downstream of
the MMTV-long terminal repeat (MMTV-LTR) promoter (Fig. 1A). The transgene was injected into
fertilized mouse eggs, which were then transferred into the
oviducts of female mice. The potential founder mice were crossed
with C57BL/6 (B6) mice, and 7 transgenic mouse lines were
generated. Genomic DNAs extracted from the transgenic mice were
amplified by PCR with human LY6K-specific primers. As shown
in Fig. 1B, 4 of the 7 transgenic
mice carried the hLY6K transgene. To further examine the
copy number of the hLY6K transgene, qPCR was performed,
revealing that transgenic mouse B had the highest expression of the
transgene (Fig. 1C), and this was
used for further study.
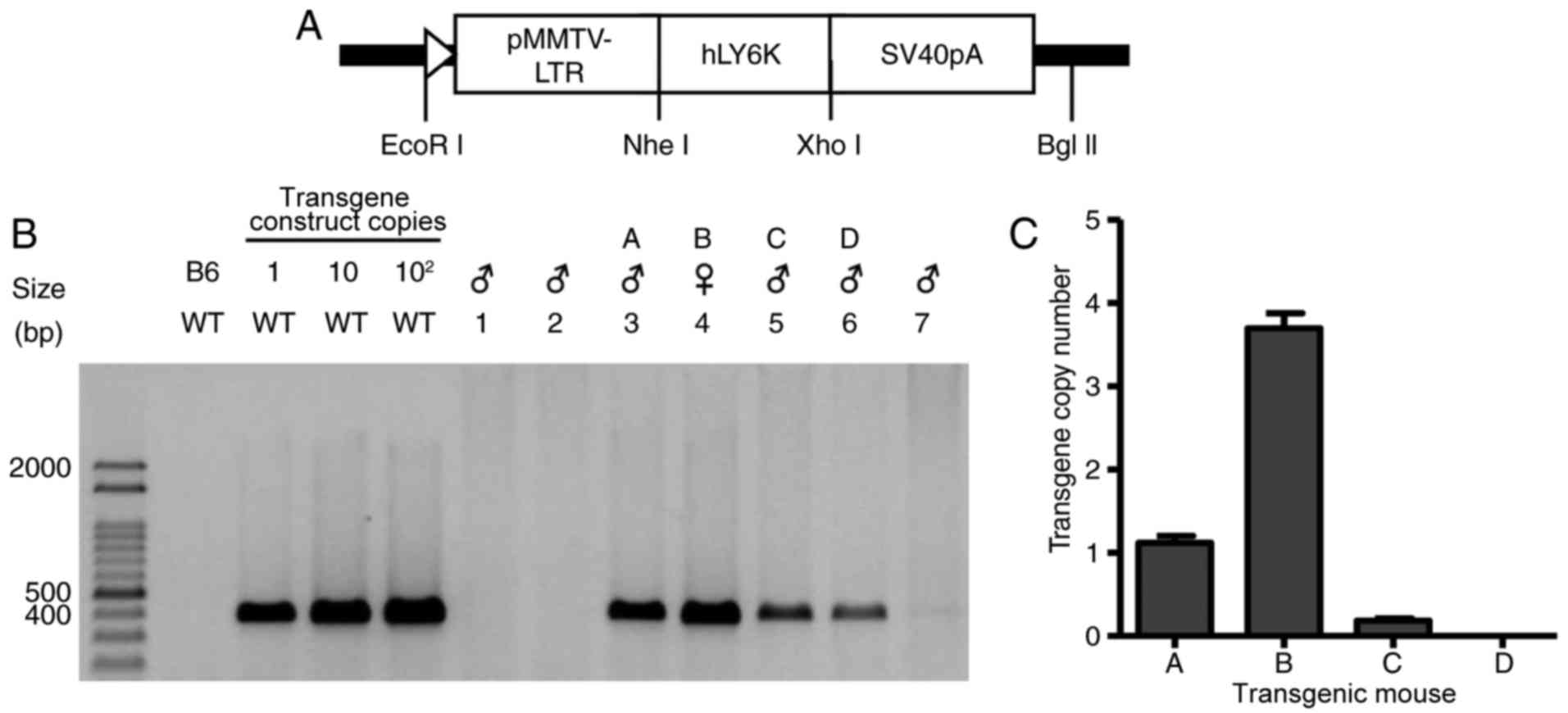 | Figure 1.Generation and identification of
transgenic mice. (A) Schematic diagram of a transgene construct for
the hLY6K gene. The full-length hLY6K cDNA was placed
under the control of the MMTV promoter. (B) PCR analysis using
hLY6K gene-specific primers. Genomic DNAs extracted from
seven transgenic mice (lanes 1–7) were amplified and size-separated
on an agarose gel. The presence of the transgene was detected in
genomic DNA from 4 of the mice, designated A, B, C, and D. PCR
bands of the transgene construct mixed with WT genomic DNA are
shown as the standard. (C) The copy number of the hLY6K
transgene in founder mice. Genomic DNA was subjected to qPCR with
primers specific to the hLY6K gene, and the signals of the
transgene were normalized to those of mouse Gapdh, an endogenous
control. Data are presented as mean ± standard deviation. hLY6K,
human lymphocyte antigen 6 family member K; WT, wild-type; bp, base
pairs; pMMTV-LTR, mouse mammary tumor virus long terminal repeat
promoter; qPCR, quantitative polymerase chain reaction. |
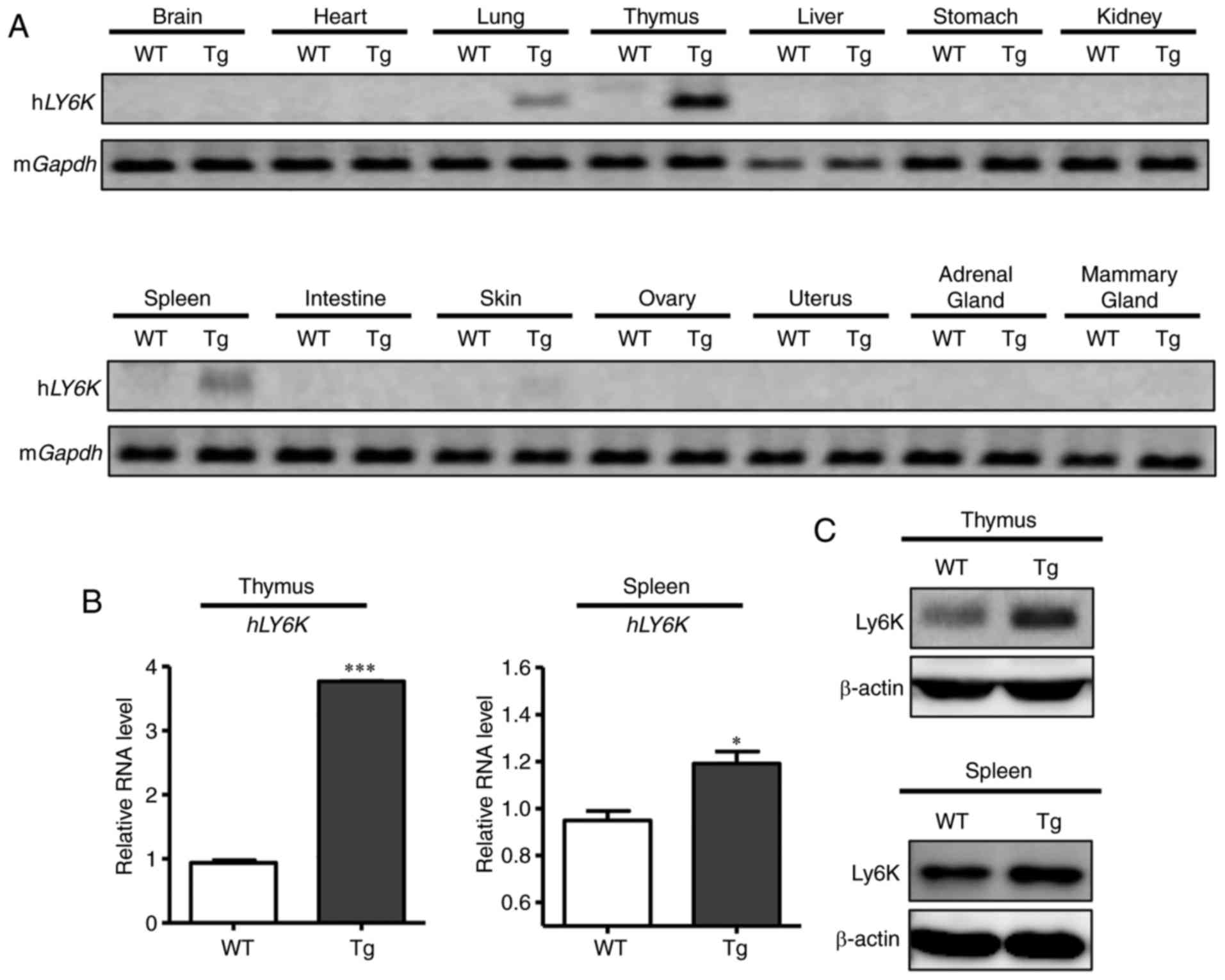
hLY6K expression in the tissues of
transgenic mice
To determine the tissue specificity of the transgene
expression, the RNA levels of hLY6K were examined in
wild-type and transgenic mice by RT-PCR (Fig. 2A). Tissues were harvested from the
thymus, spleen, mammary gland and other organs of 6-week-old virgin
mice, and RNAs were extracted from each tissue sample, which were
subsequently analyzed by RT-PCR. To avoid the detection of mouse
LY6K instead of human LY6K, their sequences were
compared using NCBI nucleotide BLAST, and there was no significant
similarity except for the Ly-6/uPAR domain [(25), https://blast.ncbi.nlm.nih.gov/Blast.cgi; human LY6K
gene accession number, NM_017527; mouse LY6K gene accession number,
NM_029627]. Thus, PCR primers were designed to recognize only human
LY6K mRNA but not the endogenous mouse LY6K, and it
was confirmed that hLY6K-specific primers only amplified
LY6K mRNAs extracted from human cell lines but not those
from mouse cells (data not shown). Amplified products were
separated on the agarose gel. As Fig.
2A illustrates, the mRNA expression of hLY6K was
detected strongly in the thymus and weakly in the spleen and lung,
while other tissues, including the mammary gland, displayed no
expression of the transgene. For further examination, RT-qPCR was
performed to quantitatively compare the RNA level of hLY6K
in the wild-type and transgenic mice. RNAs extracted from the
thymus and spleen were examined and, as indicated in Fig. 2B, the relative RNA expression of
hLY6K was markedly increased in the thymus and spleen of the
transgenic mouse compared with those of the wild-type mice.
Furthermore, western blot analysis revealed that the transgenic
mouse had markedly higher protein levels of Ly6K in the thymus and
spleen compared with those of wild-type mice (Fig. 2C). These results indicated that the
hLY6K transgene with the MMTV promoter was expressed
specifically in the thymus and spleen, rather than in other
tissues, including the mammary gland.
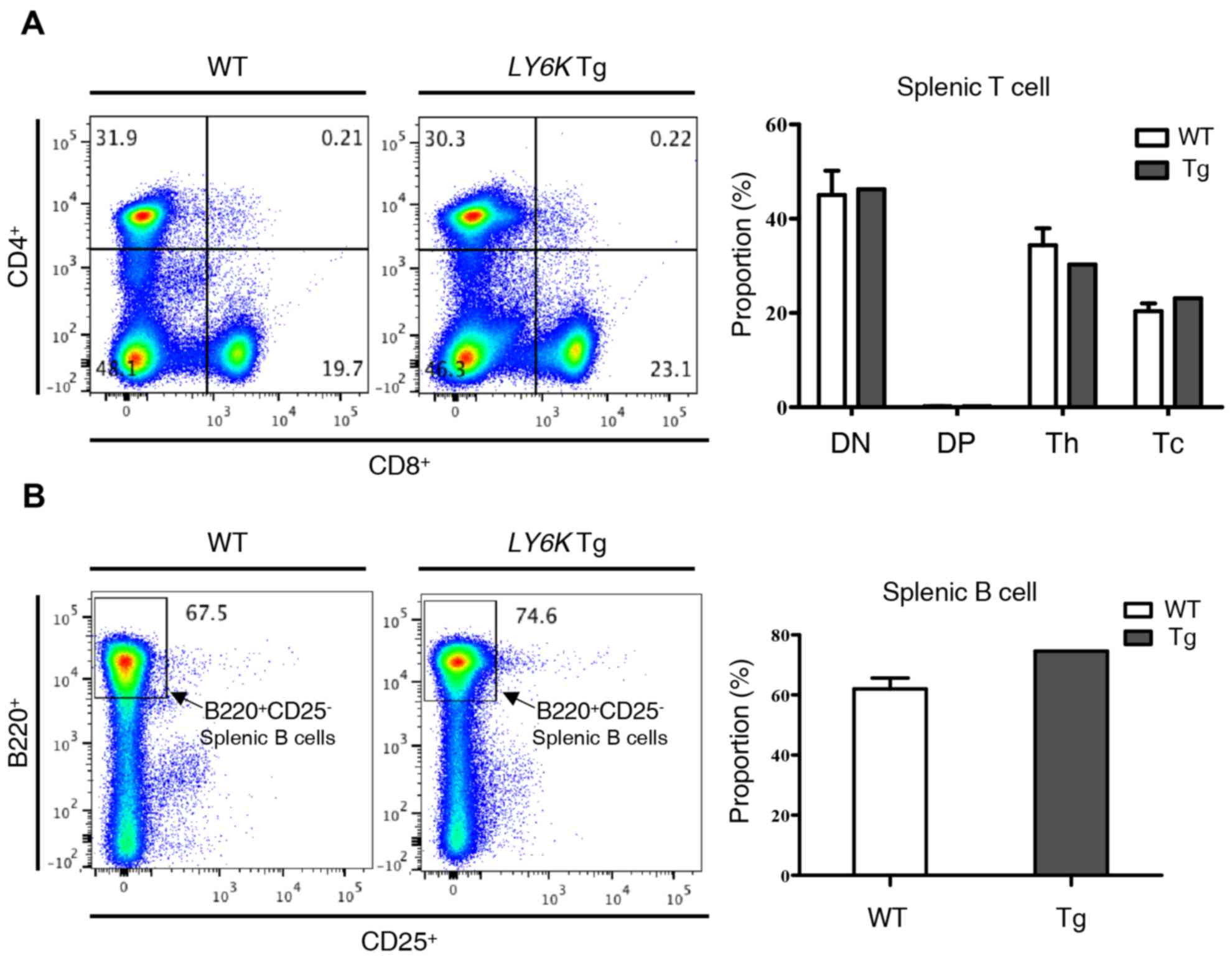
Effect of hLY6K overexpression on
lymphocyte development in the spleen
As previously stated, the hLY6K transgene was
significantly expressed in the spleen of the transgenic mouse
examined. The spleen filters and generates blood cells; however, it
is also involved in lymphocyte proliferation and activation as one
of the major sites where blood-circulating antigens are presented
to lymphocytes in order to generate an immune response (26). Therefore, the present study
investigated whether the overexpression of hLY6K caused any
changes to lymphocytes in the spleen. First, the distribution of T
cells was verified according to the expression of CD4 and CD8, the
surface markers of mature T cells. As shown in Fig. 3A, including helper T cells
(CD8−CD4+) and cytotoxic T cells
(CD8+CD4−), the proportion of each cell type
did not exhibit any significant change in the spleen compared with
the levels in wild-type mice. B cell populations in the spleen were
then investigated by analyzing the expression of B220, a murine B
cell marker, and CD25, the α chain of the IL-2 receptor (27) (Fig. 3B).
Normally, CD25 expression is characteristic of
CD4+FoxP3+ regulatory T cells in mice and
only 2% of B cells express CD25 in the spleen (28). As shown in Fig. 3B, there was no significant change in
the proportion of B220+ and CD25− B cells
between the wild-type and transgenic mice. These findings implied
that the overexpression of hLY6K did not have a significant
effect on lymphocyte development in the spleen.
Effect of LY6K overexpression on T
cell development in the thymus
The expression of hLY6K was detected in the
spleen and in the thymus (Fig. 2).
The thymus is a specialized lymphoid organ where T cell development
occurs. Therefore, the present study investigated whether
hLY6K overexpression influenced T cell development in the
transgenic mouse. DN naïve T cells, which are negative for CD4 and
CD8 expression, can be subdivided into four subgroups (DN1-DN4)
depending on their expression of CD44 and CD25 (29). Flow cytometric analysis was conducted
to examine the expression of CD44 and CD25 in thymocytes isolated
from wild-type and transgenic mice, and cells were categorized
depending on the expression of those markers. Fig. 4A demonstrates the distribution of
early naïve T cells in the thymus of wild-type and transgenic mice.
The present study identified that the proportion of the DN2
(CD25+CD44+) subgroup was decreased markedly
in the transgenic mouse compared with wild-type mice, whereas the
DN1 (CD25−CD44+) and DN3
(CD25+CD44−) populations exhibited no
significant differences. Notably, the DN4
(CD25−CD44−) population was markedly
increased in the transgenic mouse, suggesting that more naïve T
cells had completed TCR rearrangement and had begun to express CD4
and CD8 molecules for the next stage of development. Therefore, the
levels of CD4 and CD8 (surface markers of mature T cells) were
examined in the thymic T cells of each type of mouse to compare
further developmental steps (Fig.
4B). The results revealed that the double positive
(CD4+CD8+) subgroup was increased in the
transgenic mouse, as hypothesized. However, the proportions of
cytotoxic T cells (CD8+CD4−) and helper T
cells (CD8−CD4+) were decreased in the
transgenic mouse. This implied that fewer T cells were fully
differentiated in the transgenic mouse, when hLY6K was
overexpressed, compared with the wild-type mice, despite an
increased number of lymphocytes undergoing TCR rearrangement.
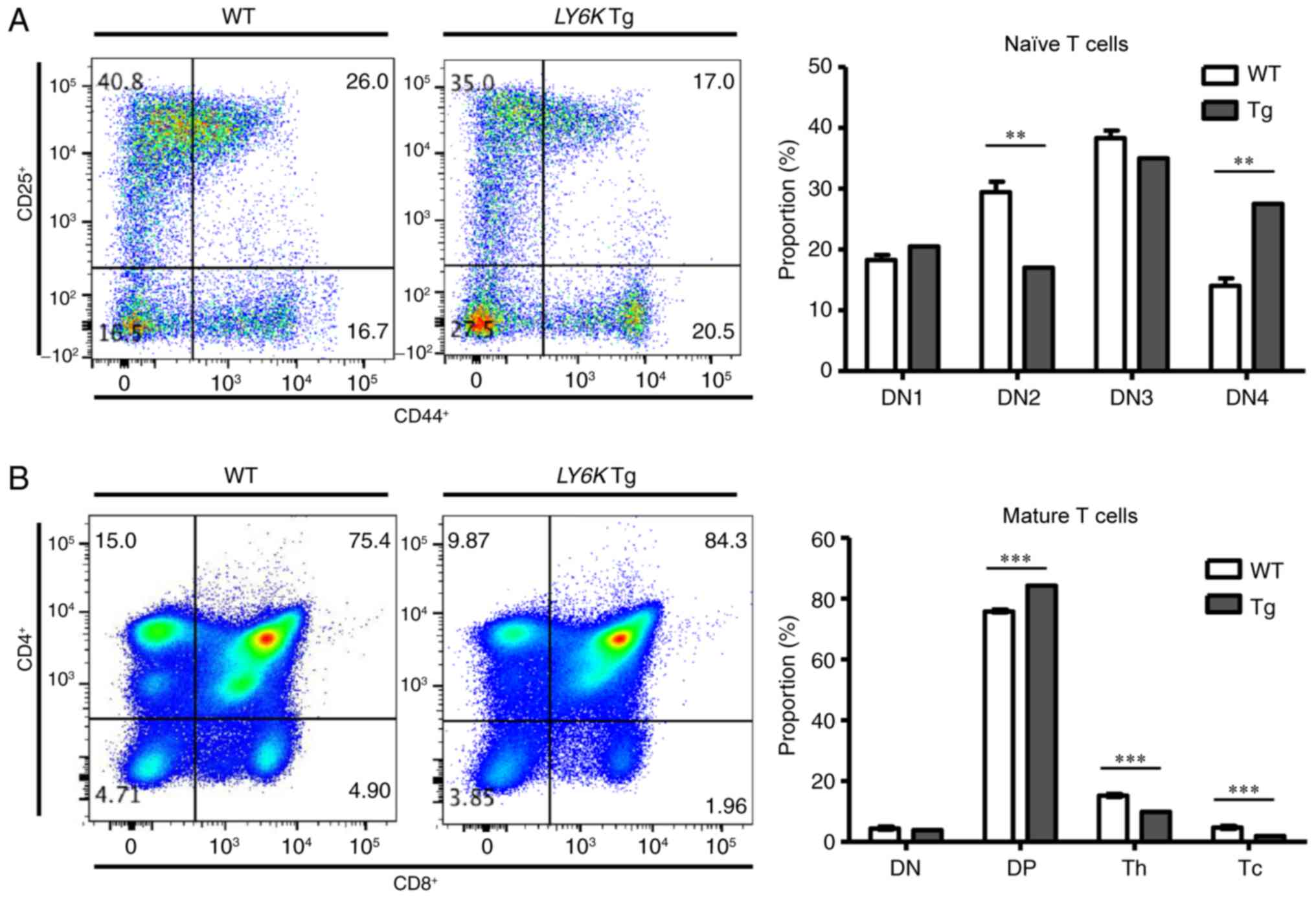 | Figure 4.Distribution of T cells in the thymus
of the WT and Tg mice. The distribution of T cells was confirmed by
flow cytometric analysis. (A) The distribution of naïve T cells in
WT and Tg mice was examined using antibodies specific for CD44 or
CD25. (B) The proportion of each type of mature T cell was analyzed
using antibodies specific for CD4 or CD8. Data are presented as the
mean ± SD; ***P<0.001, and **P<0.01 (t-test). WT, wild-type;
Tg, transgenic; LY6K, lymphocyte antigen 6 family member K; DN1,
CD44+CD25−; DN2,
CD44+CD25+; DN3,
CD44−CD25+; DN4,
CD44−CD25−; DN, double-negative
(CD4−CD8−); DP, double-positive
(CD4+CD8+); Th, helper T cells
(CD4+CD8−); Tc, cytotoxic T cells
(CD4−CD8+). |
Discussion
Ly6K is known to be upregulated in numerous types of
cancer and its increased expression is associated with a poor
outcome in patients with cancer (30). In our previous study, Ly6K expression
was identified to be inversely associated with estrogen receptor α
(ERα) levels, suggesting that high expression of Ly6K may result in
tamoxifen resistance of cancer cells (31). Furthermore, it was recently reported
that Ly6K/E signaling involving transforming growth factor β
promotes cancer progression and drug resistance in breast cancer
(14). Therefore, Ly6K is considered
to be both a prognostic marker and a therapeutic target in cancer,
particularly in breast cancer (14),
and a number of studies have been conducted on the molecular
mechanism of LY6K in vitro. However, the in vivo
mechanism of LY6K has received little attention. Therefore, in the
present study, a transgenic mouse model overexpressing the
hLY6K gene was constructed to investigate the effects of
hLY6K overexpression in vivo.
hLY6K cDNA was inserted downstream of the
MMTV-LTR promoter, which was subsequently injected into the mouse
embryo to generate hLY6K transgenic C57BL/6 mice. The
C57BL/6 mouse is one of the most commonly used animal models for
human diseases (32). Since they have
a relatively long life-span, are easy to breed, and are permissive
for maximal expression of the majority of mutations, they are
widely used to generate transgenic mice (33). MMTV is a milk-transmitted infectious
agent involved in mammary epithelial cell tumors in mice (34). It was originally identified as an
endogenous murine retrovirus, and has been used to generate animal
models for the study of human cancer due to its LTR sequence
flanking the MMTV genome and its ability to direct the expression
of downstream genes (35,36). In particular, it can induce gene
expression in mammary epithelial cells; therefore, MMTV-LTR has
been widely used to generate transgenic mice overexpressing an
exogenous gene in the mammary gland for the study of human breast
cancer (37). Furthermore, as MMTV is
expressed in other organs, including the salivary glands, lungs,
spleen, and thymus, a transgene under the control of the MMTV-LTR
promoter can be expressed in organs other than mammary glands
(38,39).
In the present study, the human LY6K gene was
inserted downstream of MMTV-LTR to generate transgenic mice, which
resulted in the overexpression of hLY6K in the thymus and
the spleen, but not in the mammary glands. Notably, it has been
reported that MMTV-LTR activity is regulated by hormones of
pregnancy, such as glucocorticoids and prolactin, and increases
during pregnancy and lactation (40).
It was also demonstrated that the MMTV promoter did not facilitate
transgene expression in the virgin mammary glands, and the gene
only began to be expressed during pregnancy and lactation in the
examined mice (40,41). Considering that the transgenic mouse
used in the present study was a 6-week-old virgin mouse, the lack
of transgene expression in the mammary glands was attributed to the
lack of pregnancy hormones responsible for the activity of the MMTV
promoters. However, high expression of hLY6K gene was
validated by qPCR analysis in the thymus and spleen of the
transgenic mouse using hLY6K-specific primers.
The thymus and the spleen are the principal sites
for lymphocyte development; therefore, the present study
investigated whether the overexpression of hLY6K induced any
defects in lymphocyte generation. Although hLY6K
overexpression did not cause any significant changes in the
distribution of lymphocytes in the spleen, the proportions of
various types of functional mature T cells were decreased markedly
in the thymus of the transgenic mouse. The increase in the DN4
(CD25−CD44−) population in the transgenic
mouse, which implied that more premature T cells had undergone TCR
rearrangement, suggested that T cell maturation was defective when
hLY6K was overexpressed.
CD8+ cytotoxic T cells and CD4+ helper T cells are
known to perform antitumor functions in numerous types of cancer
(42,43). Cytotoxic T cells can specifically
eliminate infected cells that present antigens bound to MHC class I
molecules, which are recognized by cytotoxic T cells (44). Notably, the majority of cancer cells
express MHC class I molecules; therefore, tumor antigen-specific
cytotoxic T cells can infiltrate tumor sites and attack tumor cells
specifically by recognizing the antigens linked with MHC molecules
(45,46). In addition, CD4+ helper T
cells, which aid the adaptive immune response of other immune cells
by releasing cytokines, are reported to be required for effective
antitumor immunity (47,48). In addition to their ability to
maintain immune responses to tumor cells by helping cytotoxic T
cells and other immune cells, helper T cells are also able to kill
cancer cells directly, as mutated or fusion proteins in cancer
cells generate MHC class II molecules that are recognized by helper
T cells (49,50). Therefore, the immune responses
mediated by the different types of T cells execute protective
mechanisms against cancer.
The experimental results demonstrating the decrease
of the functional T cell population under Ly6K overexpression in
the thymus indicated that Ly6K may negatively regulate normal T
cell differentiation, which in turn may lead to the suppression of
the antitumor effect of immune cells.
As previously stated, a number of studies have
demonstrated that Ly6K is upregulated in numerous types of cancer
and positively regulates tumor progression. However, the expression
patterns and function of Ly6K in the thymus of patients with cancer
have not yet been investigated. Considering the potential negative
effect of Ly6K on antitumor immunity identified in the present
study, further studies are required to investigate whether Ly6K
expression is upregulated in the thymus of patients with cancer and
whether Ly6K overexpression suppresses the generation of functional
lymphocytes.
Notably, it has been reported that uPAR expression
is increased to a greater extent in regional lymph node metastasis
than in the primary intraprostatic tumor mass (51). Given that thymic metastasis occurs in
several types of cancer, including breast cancer (52–54), the
expression of Ly6K in the thymus may be altered by the metastasis
from the primary tumor mass in these cancer patients. Together with
its own oncogenic effect, the potential role of Ly6K in T cell
development and anticancer immunity makes it a promising
therapeutic target in human cancer.
In conclusion, the present in vivo study
demonstrated that Ly6K has the potential to suppress the immune
response against tumor cells by inhibiting T cell development.
However, the present study did not reveal the molecular mechanisms
of Ly6K in T cell development. Therefore, further investigation on
Ly6K at the molecular level is required in order to demonstrate the
involvement of the Ly6K pathway in lymphocyte development.
Additionally, the effect of Ly6K on the antitumor immune response
in patients with cancer should be studied to fully understand the
role of Ly6K in human cancer. Eventually, Ly6K might provide an
effective therapeutic target to treat cancer.
Acknowledgements
The authors would like to thank Dr. Je Yeong Ko and
Bo Hye Kim (Sookmyung Women's University, Seoul, South Korea) for
productive discussions.
Funding
The present study was supported by the Sookmyung
Women's University BK21 Plus Scholarship and by the National
Research Foundation of Korea grant funded by the Korean government
(grant no. 2016R1A2A1A05005295).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS served a major role in the experiments and
prepared the manuscript draft; HKK was involved in these
experiments and prepared the manuscript draft; YK was involved in
these experiments; MJS was involved in producing the mouse model;
HPL was involved in producing the mouse model; HWL served a major
role in producing the mouse model; and JHP designed the experiments
and prepared the manuscript draft.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee at the Sookmyung
Women's University (Seoul, South Korea).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loughner CL, Bruford EA, McAndrews MS,
Delp EE, Swamynathan S and Swamynathan SK: Organization, evolution
and functions of the human and mouse Ly6/uPAR family genes. Hum
Genomics. 10:102016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blasi F and Sidenius N: The urokinase
receptor: Focused cell surface proteolysis, cell adhesion and
signaling. FEBS Lett. 584:1923–1930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: The dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parnaud G, Hammar E, Ribaux P, Donath MY,
Berney T and Halban PA: Signaling pathways implicated in the
stimulation of beta-cell proliferation by extracellular matrix. Mol
Endocrinol. 23:1264–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blasi F and Carmeliet P: uPAR: A versatile
signalling orchestrator. Nat Rev Mol Cell Biol. 3:932–943. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huber MC, Mall R, Braselmann H,
Feuchtinger A, Molatore S, Lindner K, Walch A, Gross E, Schmitt M,
Falkenberg N and Aubele M: uPAR enhances malignant potential of
triple-negative breast cancer by directly interacting with uPA and
IGF1R. BMC Cancer. 16:6152016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobsen B and Ploug M: The urokinase
receptor and its structural homologue C4.4A in human cancer:
Expression, prognosis and pharmacological inhibition. Curr Med
Chem. 15:2559–2573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bené MC, Castoldi G, Knapp W, Rigolin GM,
Escribano L, Lemez P, Ludwig WD, Matutes E, Orfao A, Lanza F, et
al: CD87 (urokinase-type plasminogen activator receptor), function
and pathology in hematological disorders: A review. Leukemia.
18:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Nooij-van Dalen AG, van Dongen GA,
Smeets SJ, Nieuwenhuis EJ, Stigter-van Walsum M, Snow GB and
Brakenhoff RH: Characterization of the human Ly-6 antigens, the
newly annotated member Ly-6K included, as molecular markers for
head-and-neck squamous cell carcinoma. Int J Cancer. 103:768–774.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda R, Enokida H, Chiyomaru T, Kikkawa
N, Sugimoto T, Kawakami K, Tatarano S, Yoshino H, Toki K, Uchida Y,
et al: LY6K is a novel molecular target in bladder cancer on basis
of integrate genome-wide profiling. Br J Cancer. 104:376–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Zhang Z, Zhang X, Gao X,
Kernstine KH and Zhong L: Serological antibodies against LY6K as a
diagnostic biomarker in esophageal squamous cell carcinoma.
Biomarkers. 17:372–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
AlHossiny M, Luo L, Frazier WR, Steiner N,
Gusev Y, Kallakury B, Glasgow E, Creswell K, Madhavan S, Kumar R
and Upadhyay G: Ly6E/K Signaling to TGFβ promotes breast cancer
progression, immune escape, and drug resistance. Cancer Res.
76:3376–3386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong HK, Park SJ, Kim YS, Kim KM, Lee HW,
Kang HG, Woo YM, Park EY, Ko JY, Suzuki H, et al: Epigenetic
activation of LY6K predicts the presence of metastasis and poor
prognosis in breast carcinoma. Oncotarget. 7:55677–55689. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong HK, Yoon S and Park JH: The
regulatory mechanism of the LY6K gene expression in human breast
cancer cells. J Biol Chem. 287:38889–38900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balakrishnan K and Adams LE: The role of
the lymphocyte in an immune response. Immunol Invest. 24:233–244.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roth PE and DeFranco AL: Lymphocyte
development: Intrinsic checkpoints for lineage progression. Curr
Biol. 5:349–352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagasawa T: Microenvironmental niches in
the bone marrow required for B-cell development. Nat Rev Immunol.
6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai AY and Kondo M: T and B lymphocyte
differentiation from hematopoietic stem cell. Semin Immunol.
20:207–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zúñiga-Pflücker JC: T-cell development
made simple. Nat Rev Immunol. 4:67–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Germain RN: T-cell development and the
CD4-CD8 lineage decision. Nat Rev Immunol. 2:309–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi SH, Kong HK, Park SY and Park JH:
Metastatic effect of LY-6K gene in breast cancer cells. Int J
Oncol. 35:601–607. 2009.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin J, Zhang Q, Zhu LQ, Yu QH and Yang Q:
The copy number and integration site analysis of IGF-1 transgenic
goat. Int J Mol Med. 34:900–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altschul SF, Gish W, Miller W, Myers EW
and Lipman DJ: Basic local alignment search tool. J Mol Biol.
215:403–410. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batista FD and Harwood NE: The who, how
and where of antigen presentation to B cells. Nat Rev Immunol.
9:15–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu D, Liu H, Komai-Koma M, Campbell C,
McSharry C, Alexander J and Liew FY: CD4+CD25+ regulatory T cells
suppress differentiation and functions of Th1 and Th2 cells,
Leishmania major infection, and colitis in mice. J Immunol.
170:394–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amu S, Gjertsson I, Tarkowski A and
Brisslert M: B-cell CD25 expression in murine primary and secondary
lymphoid tissue. Scand J Immunol. 64:482–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hager-Theodorides AL, Rowbotham NJ, Outram
SV, Dessens JT and Crompton T: Beta-selection: Abundance of
TCRbeta-/gammadelta-CD44-CD25-(DN4) cells in the foetal thymus. Eur
J Immunol. 37:487–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo L, McGarvey P, Madhavan S, Kumar R,
Gusev Y and Upadhyay G: Distinct lymphocyte antigens 6 (Ly6) family
members Ly6D, Ly6E, Ly6K and Ly6H drive tumorigenesis and clinical
outcome. Oncotarget. 7:11165–11193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YS, Park SJ, Lee YS, Kong HK and Park
JH: miRNAs involved in LY6K and estrogen receptor α contribute to
tamoxifen-susceptibility in breast cancer. Oncotarget.
7:42261–42273. 2016.PubMed/NCBI
|
|
32
|
Bryant CD, Zhang NN, Sokoloff G, Fanselow
MS, Ennes HS, Palmer AA and McRoberts JA: Behavioral differences
among C57BL/6 substrains: Implications for transgenic and knockout
studies. J Neurogenet. 22:315–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon MM, Greenaway S, White JK, Fuchs H,
Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, et
al: A comparative phenotypic and genomic analysis of C57BL/6J and
C57BL/6N mouse strains. Genome Biol. 14:R822013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dudley JP, Golovkina TV and Ross SR:
Lessons learned from mouse mammary tumor virus in animal models.
ILAR J. 57:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ross SR: Mouse mammary tumor virus
molecular biology and oncogenesis. Viruses. 2:2000–2012. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taneja P, Frazier DP, Kendig RD, Maglic D,
Sugiyama T, Kai F, Taneja NK and Inoue K: MMTV mouse models and the
diagnostic values of MMTV-like sequences in human breast cancer.
Expert Rev Mol Diagn. 9:423–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fantozzi A and Christofori G: Mouse models
of breast cancer metastasis. Breast Cancer Res. 8:2122006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang G, Park S, Cao G, Goltsov A, Ren C,
Truong LD, Demayo F and Thompson TC: MMTV promoter-regulated
caveolin-1 overexpression yields defective parenchymal epithelia in
multiple exocrine organs of transgenic mice. Exp Mol Pathol.
89:9–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bérard J, Gaboury L, Landers M, De
Repentigny Y, Houle B, Kothary R and Bradley WE: Hyperplasia and
tumours in lung, breast and other tissues in mice carrying a RAR
beta 4-like transgene. EMBO J. 13:5570–5580. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davies BR, Platt-Higgins AM, Schmidt G and
Rudland PS: Development of hyperplasias, preneoplasias, and mammary
tumors in MMTV-c-erbB-2 and MMTV-TGFα transgenic rats. Am J Pathol.
155:303–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haraguchi S, Good RA, Engelman RW and Day
NK: Human prolactin regulates transfected MMTV LTR-directed gene
expression in a human breast-carcinoma cell line through
synergistic interaction with steroid hormones. Int J Cancer.
52:928–933. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kennedy R and Celis E: Multiple roles for
CD4+ T cells in anti-tumor immune responses. Immunol Rev.
222:129–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pluhar GE, Pennell CA and Olin MR:
CD8+ T Cell-independent immune-mediated mechanisms of
anti-tumor activity. Crit Rev Immunol. 35:153–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huppa JB and Davis MM: T-cell-antigen
recognition and the immunological synapse. Nat Rev Immunol.
3:973–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pardoll DM and Topalian SL: The role of
CD4+ T cell responses in antitumor immunity. Curr
Opinion Immunol. 10:588–594. 1998. View Article : Google Scholar
|
|
46
|
Kast WM, Offringa R, Peters PJ, Arie CV,
Rob HM, Alex JV and Cornelis JMM: Eradication of adenovirus
E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell.
59:603–614. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haabeth OA, Tveita AA, Fauskanger M,
Schjesvold F, Lorvik KB, Hofgaard PO, Omholt H, Munthe LA, Dembic
Z, Corthay A and Bogen B: How Do CD4(+) T cells detect and
eliminate tumor cells that either lack or express MHC Class II
molecules? Front Immunol. 5:1742014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao FG, Khammanivong V, Liu WJ, Leggatt
GR, Frazer IH and Fernando GJ: Antigen-specific CD4+ T-cell help is
required to activate a memory CD8+ T cell to a fully functional
tumor killer cell. Cancer Res. 62:6438–6441. 2002.PubMed/NCBI
|
|
49
|
Zanetti M: Tapping CD4 T cells for cancer
immunotherapy: The choice of personalized genomics. J Immunol.
194:2049–2056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang RF: The role of MHC class
II-restricted tumor antigens and CD4+ T cells in
antitumor immunity. Trends Immunol. 22:269–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sehgal I, Foster TP and Francis J:
Prostate cancer cells show elevated urokinase receptor in a mouse
model of metastasis. Cancer Cell Int. 6:212006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park SB, Kim HH, Shin HJ, Paik MH, Kim DB
and Gong G: Thymic metastasis in breast cancer: A case report.
Korean J Radiol. 8:360–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Peters HC, Liu X, Iqbal A, Cunningham LA
and Tan SA: Colorectal cancer metastasis to the thymus gland: Rare
presentation of colorectal cancer as anterior mediastinal mass.
Case Rep Surg. 2017:65819652017.PubMed/NCBI
|
|
54
|
Hayashi S, Hamanaka Y, Sueda T, Yonehara S
and Matsuura Y: Thymic metastasis from prostatic carcinoma: Report
of a case. Surg Today. 23:632–634. 1993. View Article : Google Scholar : PubMed/NCBI
|