Introduction
Transforming growth factor (TGF)-β receptors, a
family of polypeptides, have been reported to serve a role in
regulating various biological functions, including proliferation,
migration, survival, angiogenesis, immune-surveillance, and
embryonic stem cell maintenance and differentiation (1). It has been demonstrated that a
dysregulation in the signalling of the aforementioned factors is
associated with tumour development and metastasis (2). It has been reported that the TGF-β
pathway possesses anti- and pro-tumour activities (3,4), promoting
cell cycle arrest and apoptosis at the beginning of tumorigenesis
(5–7).
In contrast, the TGF-β pathway has been reported to promote cancer
cell motility, invasion, epithelial-to-mesenchymal transition and
cell stemness following progression to more advanced tumour stages
(5–7).
Therefore, the TGF-β pathway has been demonstrated to serve an
important role in tumour progression and metastasis. The
aforementioned phenomenon is known as the first ‘TGF-β paradox’
(8). The TGF-β signalling pathway has
attracted increasing attention over the past three decades and has
become a popular drug development target for oncologists (9). As a result of the wide variety of
effects of TGF-β on tumorigenesis, blockade of TGF-β and its
signalling pathway provides multiple therapeutic opportunities.
Anti-TGF-β compounds have been developed, and through preclinical
studies and clinical trials, their efficacies have been
demonstrated (10). Therefore, it has
been suggested that TGF-β signalling inhibition may serve as a
promising strategy for regulating tumour progression, including
metastasis (10).
Materials and methods
Cell culture
HeLa cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cell lines were
cultured in Dulbecco's modified Eagle's medium-199 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing 10% (v/v) fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin-streptomycin. Cells were cultured at 37°C with 5%
CO2. HeLa cells were treated with 25, 50 and 75 µM of
LY2109761 between 0 and 72 h and subsequently analyzed and
evaluated.
TGF-β receptor inhibitor (LY2109761)
concentrations
LY2109761 (Tocris Bioscience, Bristol, UK)
concentrations used. A total of 1 mM stock solution was prepared,
and from that three different concentrations of 25, 50 and 75 µM of
LY2109761 were prepared.
Measurement of cytotoxicity
Cell index (CI)
Experiments were performed using the xCELLigence
Real-Time Cell Analysis (RTCA) DP instrument (Roche Diagnostics
GmbH, Mannheim, Germany) at 37°C with 5% CO2. In order
to measure the cytotoxic response of HeLa cells in real-time, cells
were seeded on gold microelectrodes embedded at the bottom of 16
well microplates (E-plates; Roche Diagnostics, Basel, Switzerland)
at a density of 6.0×103 cells/well for HeLa cells. The
impedance was recorded at 15 min intervals. 25, 50 and 75 µM of
LY2109761 were added to the culture 20 h subsequent to seeding. All
incubations were performed at a volume of 200 µl between 0 and 72
h. IC50 values were evaluated by the RTCA-DP software
(Roche Diagnostics GmbH) using the sigmoidal dose-response
curves.
Mitotic index (MI)
HeLa cells were plated on coverslips at a density of
2×104 cells/well and treated with Dulbecco's modified
Eagle's medium-199 medium (Sigma-Aldrich; Merck KGaA) containing
10% (v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin for the control group and with
LY2109761 for the experimental group between 0 and 72 h. The cells
were subsequently fixed using Carnoy fixative at a dilution of 3:1
ethanol and acetic acid at room temperature for 10 min. The MI was
determined using the Feulgen method, where cells were treated with
1N of HCl at room temperature for 1 min and then hydrolized with 1N
of HCl for 10.5 min at 60°C. Slides were subsequently treated with
Feulgen for 1 hour and were rinsed for 3 min in distilled water.
Cells were subsequently stained with 10% Giemsa stain solution (pH
6.8) at room temperature for 3 min and washed twice in PBS.
Finally, the MI was evaluated by counting cells with a light
microscope (magnification, ×100) in different phases of mitosis for
each tested inhibitor concentration and control, and a minimum of
3.0×103−3.5×103 cells were examined from each
slide for MI calculations. The MI percentage (%) was scored using
the following formula: (Cells in mitotic phases/total cell number)
×100.
3H-thymidine labelling
index (LI) analysis
3H-thymidine labelling index analysis is
used to determine cells in the S phase. For the
3H-thymidine LI analysis, a medium containing 1 µCi/ml
3H-thymidine was applied to the control and test groups
for 20 min at room temperature. Subsequent to labelling, the cells
were fixed with Carnoy's fixative (3:1, ethanol and acetic acid) at
room temperature for 10 min and the remaining radioactive material
was washed twice with 2% perchloric acid at 4°C for 30 min.
Coverslips were coated with K.2 gel emulsion (Ilford Photo,
Cheshire, UK). Following exposure time autoradiograms were bathed
with D-19 b developer (Kodak, Rochester, NY, USA) and Fixaj B
(Kodak). The cells were subsequently stained with Giemsa at room
temperature for 3 min. Cells were considered
3H-thymidine labelled when they contained a minimum of
five discrete silver grains. Over 3×103 cells from each
coverslip were examined with light microscope (magnification,
×100). The LI percentage was scored using the following formula:
(Cells in synthesis phase/total cell number) ×100.
Apoptotic index (AI)
Cells were seeded in 6-well plates (3×104
cells/ml) and were trypsinized with 0.25% Tyripsin-EDTA (Gibco;
Thermo Fisher Scientific, Inc.) ~37°C for 3 min and were fixed at
room temperature using 1:1 methanol and PBS, respectively, and pure
methanol for 3 min. Following fixation, the nucleus of HeLa cells
was stained using 1 µg/ml of DAPI labelling solution
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature and
washed once with PBS. Cells were analysed with fluorescent
microscopy (magnification, ×1,000). The AI percentage was scored
using the following formula: (Apoptotic cell number/total cell
number) ×100.
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using SPSS statistics 17.0
software (SPSS, Inc., Chicago, IL, USA). Statistical analysis of
AI, LI and MI was performed using a two-tailed Student's t-test, in
order to determine significance. All values are expressed as the
mean ± standard deviation. Values obtained from all experimental
groups were analysed using one-way analysis of variance with
Dunnett's multiple comparison post hoc test. P<0.01 was
considered to indicate a statistically significant difference.
Results
LY2109761 affects the proliferation
and cytoskeleton of HeLa cells
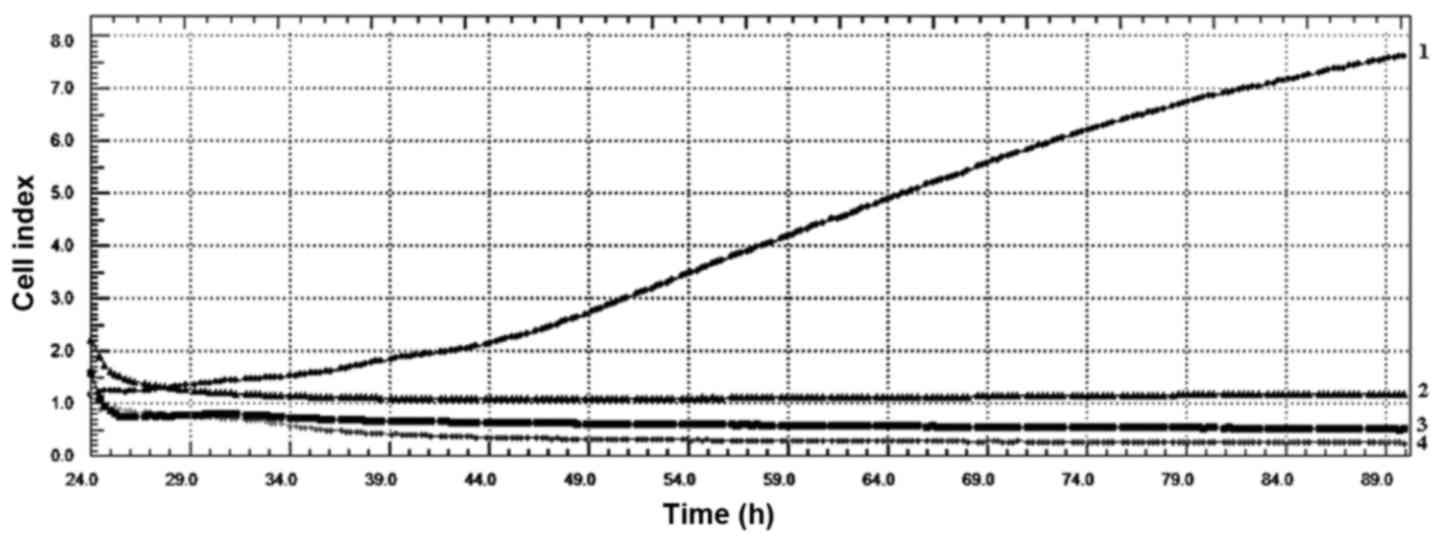
CI values obtained from xCelligence RTCA system
indicated that LY2109761 concentrations had an evident
antiproliferative effect on HeLa cell line. The values also
indicated that all LY2109761 concentrations affected the
cytoskeleton of HeLa cells (Fig. 1).
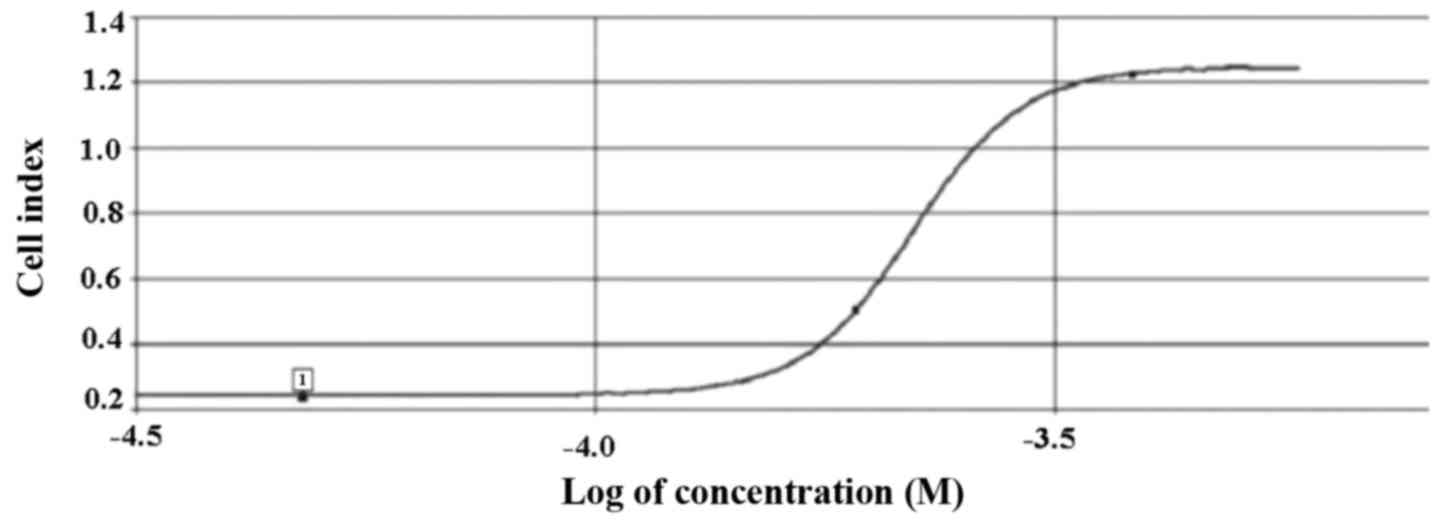
As indicated in Fig. 2, the
IC50 value for HeLa cells was determined as 39 µM
LY2109761, according to the data analysis performed using
xCelligence DP software. Therefore, 39 µM LY2109761 was used in the
subsequent experiments performed in the present study.
LY2109761 decreases the rate of
mitotic cells in HeLa cell culture
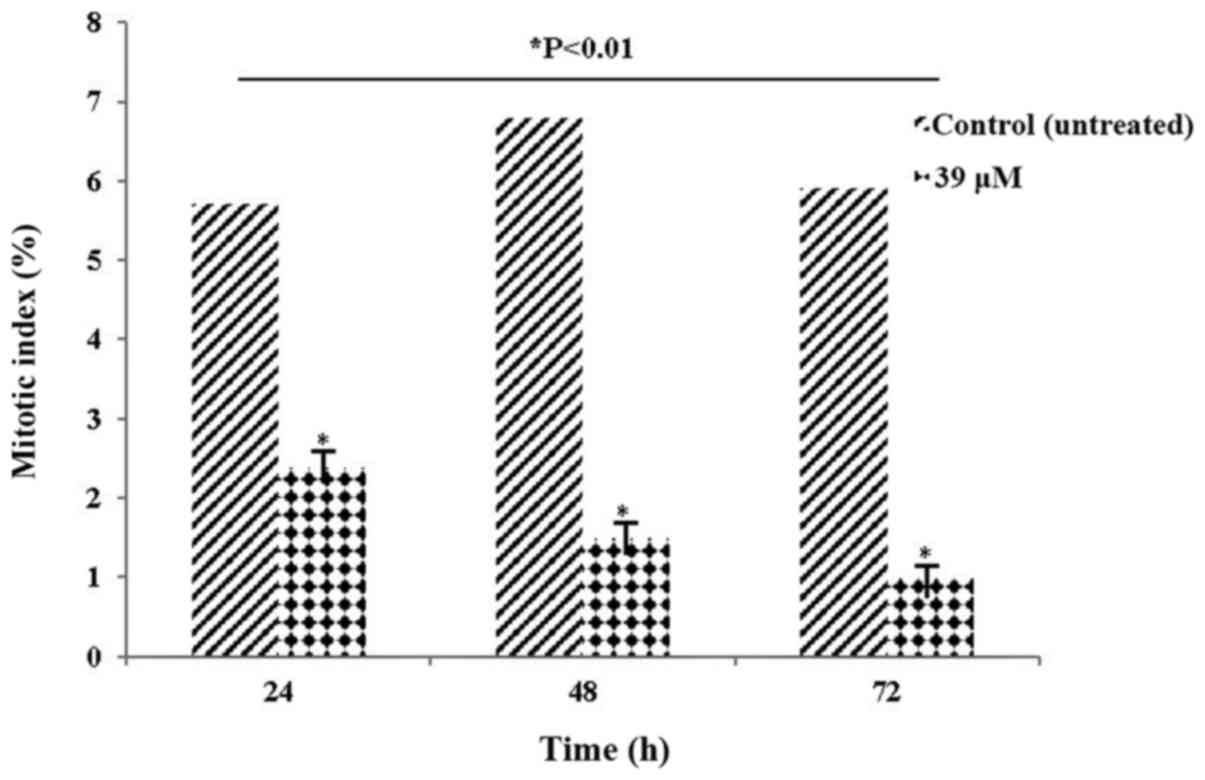
The MI values at a LY2109761 concentration of 39 µM
are presented in Table I and Fig. 3. According to the presented data, the
MI values of HeLa cells treated with LY2109761 started to exhibit a
significant decline at 24 h (P<0.01), and continued to
significantly decline (P<0.01) for up to 72 h compared with the
control. The decrease in the mitosis parameter at these rates is of
considerable value in terms of cancer prognosis. A decrease in
mitotic rate of cells may assist to prevent the progression of the
cancer, allowing the treatment to be carried out successfully.
 | Table I.Mitotic index (%) values of HeLa cells
treated with 39 µM of LY2109761 for 24, 48 and 72 h. |
Table I.
Mitotic index (%) values of HeLa cells
treated with 39 µM of LY2109761 for 24, 48 and 72 h.
|
| Mitotic index
(%) |
|---|
|
|
|
|---|
| Time (h) | Control | 39 µM |
|---|
| 24 | 5.7±0.03 |
2.39±0.04a |
| 48 | 6.8±0.02 |
1.49±0.02a |
| 72 | 5.9±0.04 |
0.98±0.01a |
LY2109761 reduces the proportion of
cells in the synthesis phase in HeLa cell cultures
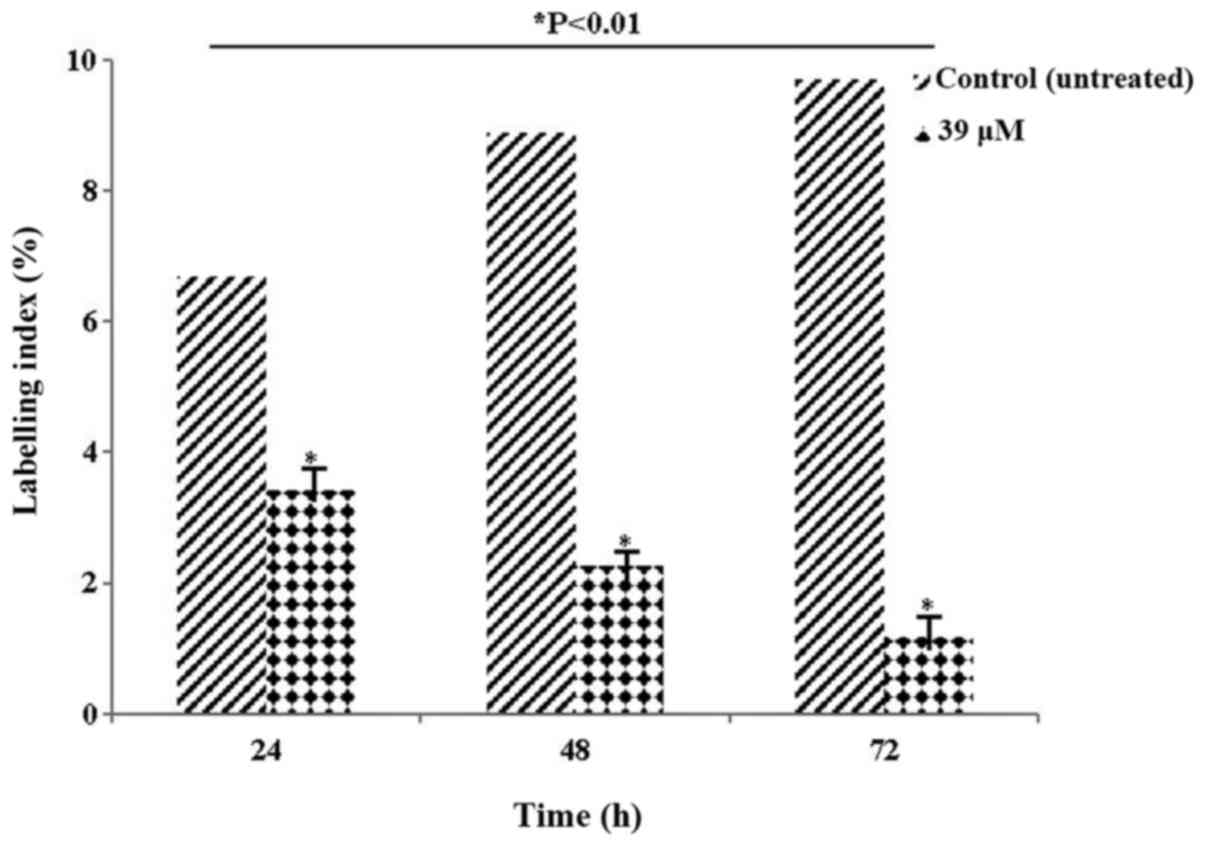
Table II and Fig. 4 present the LI values. The LI
parameter, which determines the ratio of cells in the synthesis
phase, was evaluated. The decrease in the rate of labelling,
particularly at 72 h, indicated that the cells were blocked at the
synthesis phase. The decrease in the number of HeLa cells following
exposure to 39 µM LY2109761 concentration was statistically
significant compared with control (P<0.01).
 | Table II.Labelling index (%) values of HeLa
cells treated with 39 µM of LY2109761 for 24, 48 and 72 h. |
Table II.
Labelling index (%) values of HeLa
cells treated with 39 µM of LY2109761 for 24, 48 and 72 h.
|
| Labelling index
(%) |
|---|
|
|
|
|---|
| Time (h) | Control | 39 µM |
|---|
| 24 | 6.7±0.03 |
3.42±0.06a |
| 48 | 8.9±0.05 |
2.26±0.04a |
| 72 | 9.7±0.04 |
1.18±0.01a |
LY2109761 increases the rate of
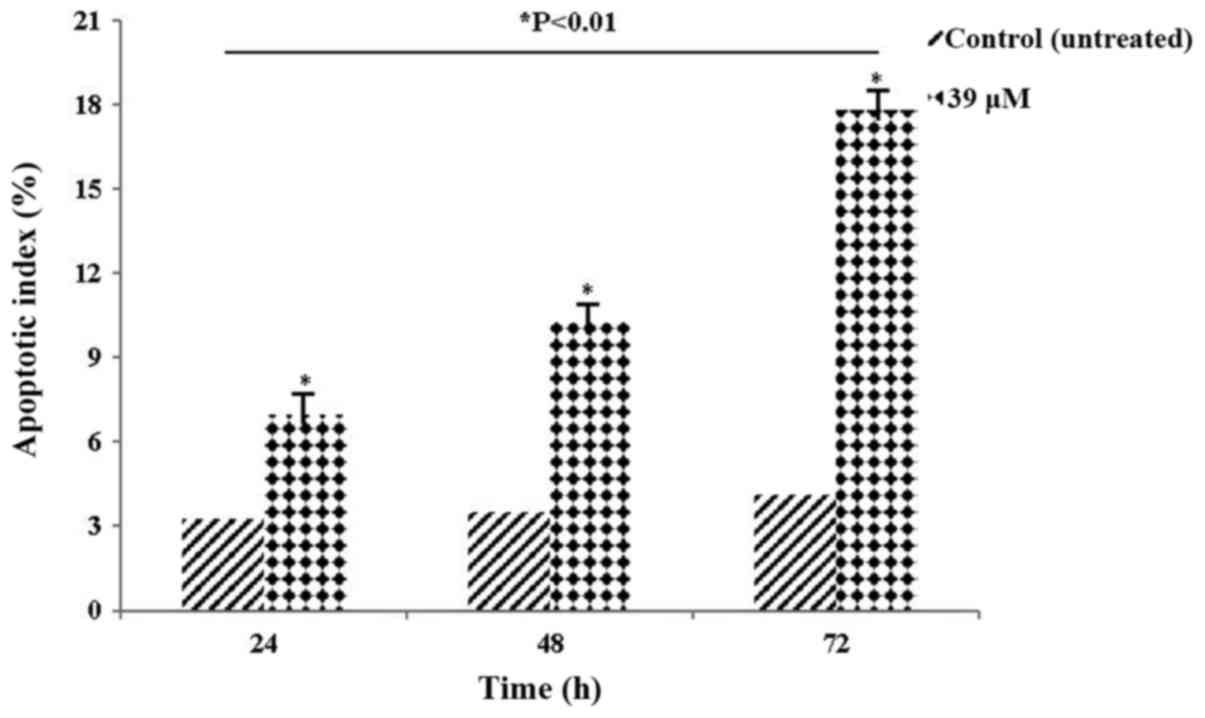
apoptotic cells in HeLa cell culture
The AI values of HeLa cells treated with 39 µM
LY2109761 are presented in Table
III and Fig. 5. The significant
increase in AI values indicated that HeLa cells activated the
apoptotic death pathway (P<0.01), suggesting that they may serve
an important role in cancer treatment and prognosis.
 | Table III.Apoptotic index (%) values of HeLa
cells treated with 39 µM of LY2109761 for 24, 48 and 72 h. |
Table III.
Apoptotic index (%) values of HeLa
cells treated with 39 µM of LY2109761 for 24, 48 and 72 h.
|
| Apoptotic index
(%) |
|---|
|
|
|
|---|
| Time (h) | Control | 39 µM |
|---|
| 24 | 3.26±0.03 |
6.98±0.07a |
| 48 | 3.48±0.02 |
10.26±0.08a |
| 72 | 4.12±0.06 |
17.82±0.12a |
Discussion
In the present study, the cytotoxic effects of
LY2109761 on HeLa cells were evaluated. The cell kinetic parameters
used in the present study demonstrated significant alterations to
the CI, MI, LI and MI following treatment with LY2109761. The data
of the present study indicate changes in proliferation at a
cellular level, thus providing information on the effects of
LY2109761 on cell activity. The function of TGF-β in tumour
metastasis has previously been demonstrated by researchers in a
series of cancer models (11). TGF-β
has been reported to be a significant contributor to bone
metastasis (12), and blockade of the
TGF-β signalling pathway with TGF-β inhibitor LY2109761 has been
demonstrated to inhibit connective tissue growth factor production
and tumour growth (13).
In a study by Gao et al (14), LY2109761 was indicated to increase the
apoptotic rate of cisplatin-resistant cells. The combination
treatment of LY2109761 and cisplatin demonstrated an
antiproliferative effect, in addition to inducing an increase in
the apoptotic rate compared with the apoptotic rates following
treatment with each drug separately. Furthermore, this combined
treatment has been demonstrated to promote tumour regression in
established parental and cisplatin-resistant ovarian cancer
xenograft models. TGF-β inhibition by various agents, including
LY2109761, SD-208 and trabedersen, has been demonstrated to reduce
pancreatic ductal adenocarcinoma cell invasion in vitro and
metastasis in vivo (15–17).
LY2109761 has also been reported to exhibit
anti-tumour activity against solid and haematological malignancies,
and to suppress pancreatic cancer metastasis to the stomach
(16). LY2109761 has been indicated
to reduce tumour mass and increase the survival rate of orthotopic
murine model of metastatic pancreatic cancer (16). In another study by Xu et al
(18), LY2109761 was demonstrated to
inhibit the survival of leukaemia cells and the amount of TGF-β1
produced by bone marrow stromal cells that maintains chemotherapy
resistance.
A limitation of the present study is that only
tissue culture experiments were conducted. Animal experiments
should be performed in future studies and the data should be
evaluated to determine whether it is compatible with the present
study.
The results of the present study indicated that
LY2109761 affected the cytoskeleton of HeLa cells. In addition,
while LY2109761 significantly reduced MI and LI values in the HeLa
cell line, it increased AI values. Therefore, the results of the
present study appear to be concordant with the aforementioned
studies. As a result, when the alterations in AI, CI, LI and MI
values were examined, 39 µM LY2109761 caused regression in
proliferation. Changes at a cellular level suggest that this
inhibitor may also regress cancer on a clinical level in human
cervical carcinoma. For this reason, LY2109761 offers a promising
treatment option for cervical carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific
Research Projects Coordination Unit of Istanbul University (grant
no, 49748 and 41832; Istanbul, Turkey).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
İÇ and MT designed the study. İÇ and MT performed
the experiments. İÇ analyzed the data. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neuzillet C, Tijeras-Raballand A, Cohenb
R, Cros J, Faivre S, Raymond E and de Gramont A: Targeting the TGFβ
pathway for cancer therapy. Pharmacol Ther. 147:22–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inman GJ: Switching TGFβ from a tumour
suppressor to a tumour promoter. Curr Opin Genet Dev. 21:93–99.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Principe DR, Doll JA, Bauer J, Jung B,
Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β:
Duality of function between tumour prevention and carcinogenesis. J
Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jakowlew SB: Transforming growth
factor-beta in cancer and metastasis. Cancer Metastasis Rev.
25:435–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian M, Neil JR and Schiemann WP:
Transforming growth factor-β and the hallmarks of cancer. Cell
Signal. 23:951–962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drabsch Y and ten Dijke P: TGF-β
signalling and its role in cancer progression and metastasis.
Cancer Metastasis Rev. 31:553–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wendt MK, Tian M and Schiemann WP:
Deconstructing the mechanisms and consequences of TGF-β-induced EMT
during cancer progression. Cell Tissue Res. 347:85–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhurst RJ and Hata A: Targeting the TGFβ
signalling pathway in disease. Nat Rev Drug Discov. 11:790–811.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-beta signalling inhibitors for cancer therapy.
Nat Rev Drug Discov. 3:1011–1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiercinska E, Naber HP, Pardali E, van der
Pluijm G, van Dam H and ten Dijke P: The TGF-β/Smad pathway induces
breast cancer cell invasion through the up-regulation of matrix
metalloproteinase 2 and 9 in a spheroid invasion model system.
Breast Cancer Res Treat. 128:657–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kingsley LA, Fournier PG, Chirgwin JM and
Guise TA: Molecular biology of bone metastasis. Mol Cancer Ther.
6:2609–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bennewith KL, Huang X, Ham CM, Graves EE,
Erler JT, Kambham N, Feazell J, Yang GP, Koong A and Giaccia AJ:
The role of tumor cell derived connective tissue growth factor
(CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 69:775–784.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Shan N, Zhao C, Wang Y, Xu F, Li J,
Yu X, Gao L and Yi Z: LY2109761 enhances cisplatin antitumor
activity in ovarian cancer cells. Int J Clin Exp Pathol.
8:4923–4932. 2015.PubMed/NCBI
|
|
15
|
Gaspar NJ, Li L, Kapoun AM, Medicherla S,
Reddy M, Li G, O'Young G, Quon D, Henson M, Damm DL, et al:
Inhibition of transforming growth factor beta signaling reduces
pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol.
72:152–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Melisi D, Ishiyama S, Sclabas GM, Fleming
JB, Xia Q, Tortora G, Abbruzzese JL and Chiao PJ: LY2109761, a
novel transforming growth factor beta receptor type I and type II
dual inhibitor, as a therapeutic approach to suppressing pancreatic
cancer metastasis. Mol Cancer Ther. 7:829–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlingensiepen KH, Jaschinski F, Lang SA,
Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M and Schneider A:
Transforming growth factor-beta 2 gene silencing with trabedersen
(AP 12009) in pancreatic cancer. Cancer Sci. 102:1193–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Tabe Y, Jin L, Watt J, McQueen T,
Ohsaka A, Andreeff M and Konopleva M: TGF-beta receptor kinase
inhibitor LY2109761 reverses the anti-apoptotic effects of
TGF-beta1 in myelo-monocytic leukaemic cells co-cultured with
stromal cells. Br J Haematol. 142:192–201. 2008. View Article : Google Scholar : PubMed/NCBI
|