Introduction
Nasopharyngeal carcinoma (NPC), also known as
‘Guangdong tumor’, is a malignant tumor that commonly occurs in
southern China, particularly in Guangdong Province (1). Intensity-modulated radiation
therapy-based comprehensive treatment is effective in the treatment
of early NPC (2). However, as
diagnosis of NPC is difficult at early stages with a marked
potential for metastasis, ~75% of patients with NPC are diagnosed
with late-stage NPC on their initial presentation to the doctor,
with local lymph node and/or distant metastasis (3). The early diagnosis of this disease is a
major clinical problem; there is a poor prognosis due to recurrence
or metastasis following treatment, accounting for the majority of
cases of failed NPC treatment and the low survival rate (4). Therefore, screening NPC tumor markers
for early detection, reasonable treatment, prognosis prediction and
recurrence monitoring may be of marked importance to the clinical
diagnosis and treatment of NPC (4).
As microRNA (miRNA) serves an important function in
the incidence and development of tumors, it has become a hotspot in
cancer research (5). Previous studies
have identified that circulating miRNA expression level
dysregulation is common in hematological tumors, and its expression
in lung cancer, liver cancer, and head and neck cancer, as well as
in other solid tumors, also differs markedly, and is associated
with the clinical features and prognosis of tumors (6,7). Research
into the association between circulating miRNA and NPC is at a
preliminary stage (5). It has been
identified that determining serum miRNA levels offered marked
potential in the early diagnosis and prediction of NPC (7). Although the results of the previous
study differed, they all confirmed that certain circulating miRNAs
are expressed specifically in NPC (7).
Various cytokines, including hypoxia-inducible
factor, insulin-like growth factor, epidermal growth factor,
hepatocyte growth factor, fibroblast growth factor, vascular
endothelial growth factor and transforming growth factor (TGF),
induce epithelial-mesenchymal transition (EMT) and promote the
metastasis of tumor cells (8).
TGF-β1, one of the most important TGF family members, is
a ‘double-edged sword’, as it is able to inhibit cell proliferation
and induce cell apoptosis in the early stages of primary tumors,
but also promote the invasion and metastasis of cancer cells at
later stages (9). In addition, a
number of studies have indicated that TGF-β2 is the
primary factor inducing EMT, regulating the incidence and
development of EMT through Smad and non-Smad signaling pathways
(10). In addition, it is involved in
normal embryonic development, and also associated with organ
fibrosis and a variety of malignant tumors, including lung, breast,
extrahepatic bile duct and skin cancer; however, it has not yet
been identified in NPC.
Chen et al (11) identified that the expression of
miR-153 was decreased in patients with non-small cell lung cancer
relative to the adjacent tissues. The aim of the present study was
to investigate the molecular mechanism underlying the effect of
microRNA-153 (miR-153) on the growth of NPC and experimental
validation.
Materials and methods
Culture of NPC
The human NPC 13-9B cell line was purchased from the
Chinese Academy of Sciences (Shanghai, China) and was cultured in
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare, Logan,
UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
Tissue samples
The present study was approved by the Institute
Research Ethics Committee of Beijing Army General Hospital
(Beijing, China). Written informed consent was provided by all
enrolled patients (n=48, 56-78 years age) at June 2014 to December
2014. The number of patients were 48, number of male patients were
35, number of female patients were 13; mean age of patients were
56–78 years age. All cancer tissue samples and para-carcinoma
tissue were collected by surgical resection and were stored at
−80°C until subsequent experimentation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of miR-153
expression
Total RNA from NPC tissue samples was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. cDNA
synthesis was performed using an Oligo-dT kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. miR-153 expression level analysis was performed using a
Power SYBR Green PCR Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
miR-153: Forward, 5′-TTGCATAGTCACAAAAGTGAT-3′, Reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; U6: Forward,
5′-CTCGCTTCGGCAGCACATATACT-3′, Reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. Fold changes in mRNA expression were
quantified using the 2−ΔΔCq relative quantification
method. PCR conditions included an initial holding period at 95°C
for 15 sec and 60°C for 30 sec for 40 cycles {Livak, 2001
#5460}.
miR-153 overexpression and
TGF-β2 inhibitor
Human miR-153 mimic (5′-UUGCAUAGUCACAAAAGUGAUC-3′
and 5′-UUCUCCGAACGUGUCACGUTT-3′) and negative control mimic
(5′-CCCCCCCCCCCCCCCCCCC-3′ and 5′-AAAAAAAAAAAAAAAA-3′) were
obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China).
13-9B cells were cultured in a 6- or 96-well plate and transiently
transfected with the mimics using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. In addition, 1 µM pirfenidone
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), a
TGF-β2 inhibitor, was added to transfection of 13-9B
cells with miR-153 for 48 h.
MTT assay of cell viability
13-9B cells were transfected with miR-153 with or
without TGF-β2 inhibitor treatment, and were cultured in
a 96-well plate. The cell viability was determined using an MTT
(Beyotime Institute of Biotechnology, Haimen, China) assay at 0, 24
and 48 h. MTT (0.5 mg/ml) was added for 4 h and 150 µl/well DMSO
was added to dissolve the formazan crystals that formed. The
optical density at 492 nm was determined using a colorimetric
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to the manufacturer's protocol.
Flow cytometric analysis of
apoptosis
13-9B cells were transfected with miR-153 with or
without TGF-β2 inhibitor treatment, and were cultured
following transfection for 48 h in a 6-well plate. Cells were
stained with annexin V (1 µM) and propidium iodide (5 µM) (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) for 30 min in darkness.
The rate of apoptosis was determined using flow cytometry (BD
FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
ELISA
13-9B cells were transfected with miR-153 with or
without TGF-β2 inhibitor treatment, and were cultured in
a 6-well plate. Caspase-3/9 activity of the cells was determined
using ELISA kits (catalog nos. C1115 and C1158; Beyotime Institute
of Biotechnology). Cells were incubated with
N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (caspase-3 substrate) and
N-acetyl-Leu-Glu-His-Asp-p-nitroanilide (caspase-9 substrate) at
37°C for 2 h. The optical density at 405 nm was determined using a
colorimetric microplate reader.
Western blotting
13-9B cells were transfected with miR-153 with or
without TGF-β2 inhibitor treatment, and were cultured
following transfection for 48 h in a 6-well plate. Subsequently,
cells were lysed with lysis buffer (Beyotime Institute of
Biotechnology) at 4°C for 30 min. Proteins were quantified using
the bicinchoninic acid method (Beyotime Institute of Biotechnology)
and 50 µg protein was separated by SDS/PAGE (8–12% gel) and
transferred onto polyvinylidene fluoride membranes (GE Healthcare,
Chicago, IL, USA). Membranes were blocked with 5% skimmed milk
powder in Tris-buffered saline containing 0.1% Tween-20 followed by
incubation with the following primary antibodies: Anti-B-cell
lymphoma 2 (Bcl-2, sc-23960, 1:1,000, Santa Cruz Biotechnology,
Inc.), anti-Bcl-2-associated X protein (Bax, sc-6236, 1:1,000,
Santa Cruz Biotechnology, Inc.), anti-TGF-β2 (sc-374658,
1:1,000, Santa Cruz Biotechnology, Inc.), anti-phospho-Smad2
(ab53100, 1:1,000, Abcam) and anti-GAPDH (sc-51631, 1:50,000, Santa
Cruz Biotechnology, Inc.) at 4°C overnight. Membranes were
incubated with horseradish peroxidase conjugated anti-rabbit
immunoglobulin G secondary antibody (sc-2004, 1:5,000, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 37°C for 1 h and
SuperSignal West Pico Enhanced Chemiluminescent Substrate (Beyotime
institute of Biotechnology). The intensity of each band was
quantified using ImageJ software (version 3.0; National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
Results are presented as the mean ± standard
deviation. Differences between groups were analyzed using Student's
t-test or one-way analysis of variance with Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
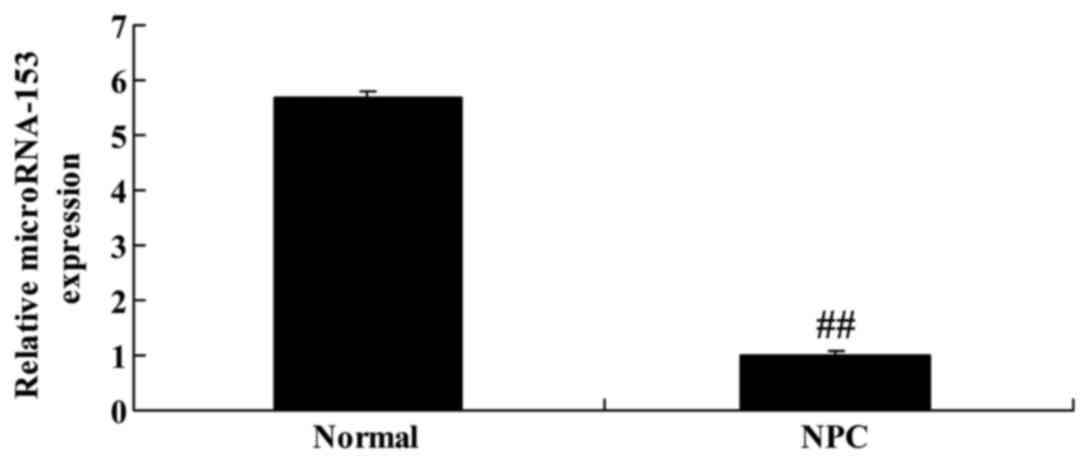
Expression of miR-153 in patients with
NPC
To identify the expression of miR-153 in patients
with NPC, RT-qPCR was performed. It was identified that miR-153
expression in patients with NPC was significantly decreased
compared with that of paracarcinoma tissue (Fig. 1).
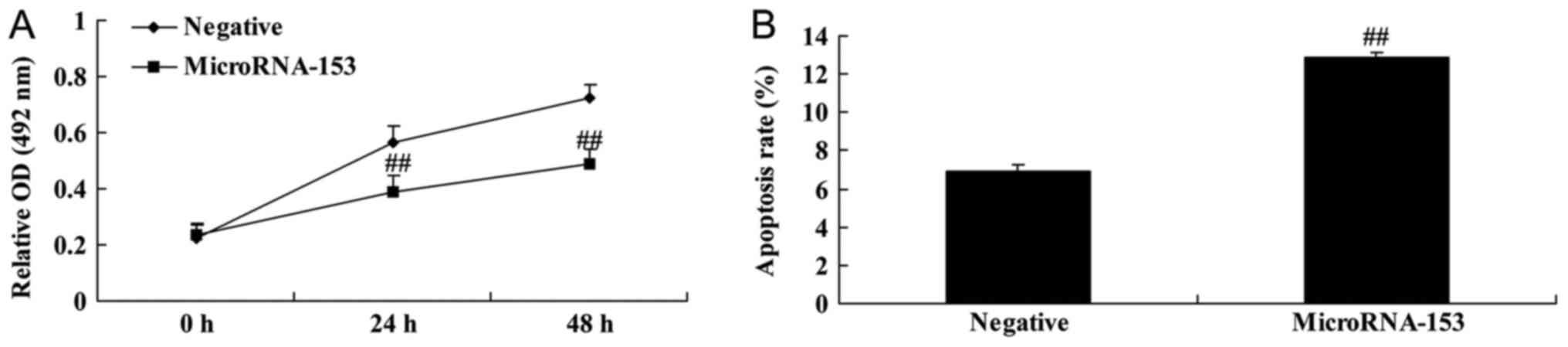
Upregulation of miR-153 decreases cell
viability and induces apoptosis of 13-9B cells
miR-153 mimic and negative control mimic were
transfected into 13-9B cells in order to determine the effect of
miR-153. Upregulation of miR-153 significantly decreased cell
viability and significantly induced apoptosis of 13-9B cells,
compared with control negative mimic (Fig. 2).
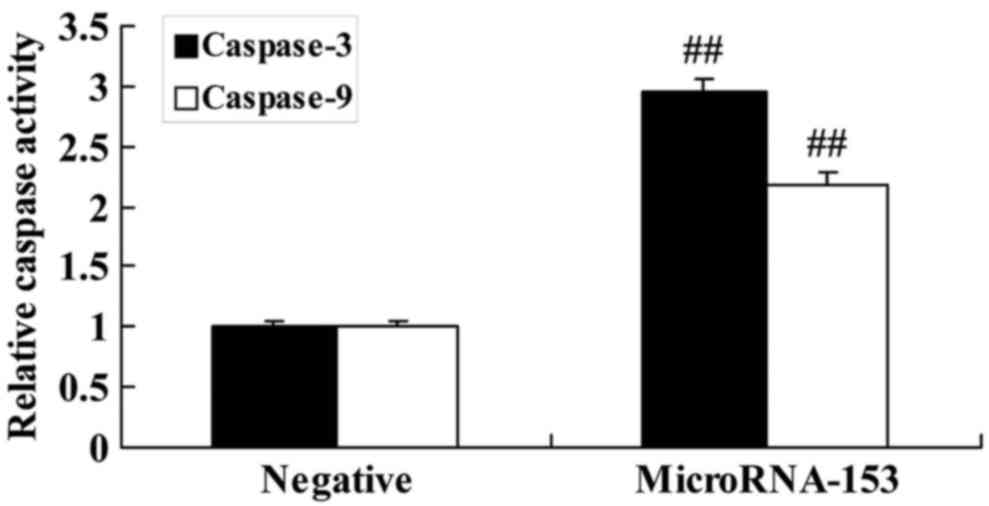
Upregulation of miR-153 induces caspase-3 and −9
activity of 13-9B cells. To determine whether the upregulation of
miR-153 induced caspase-3 and −9 activity in 13-9B cells, caspase
activity of 13-9B cells was determined using ELISA. 13-9B cells
transfected with miR-153 mimic exhibited significantly increased
caspase-3 and −9 activity, compared with 13-9B cells transfected
with negative control mimic (Fig.
3).
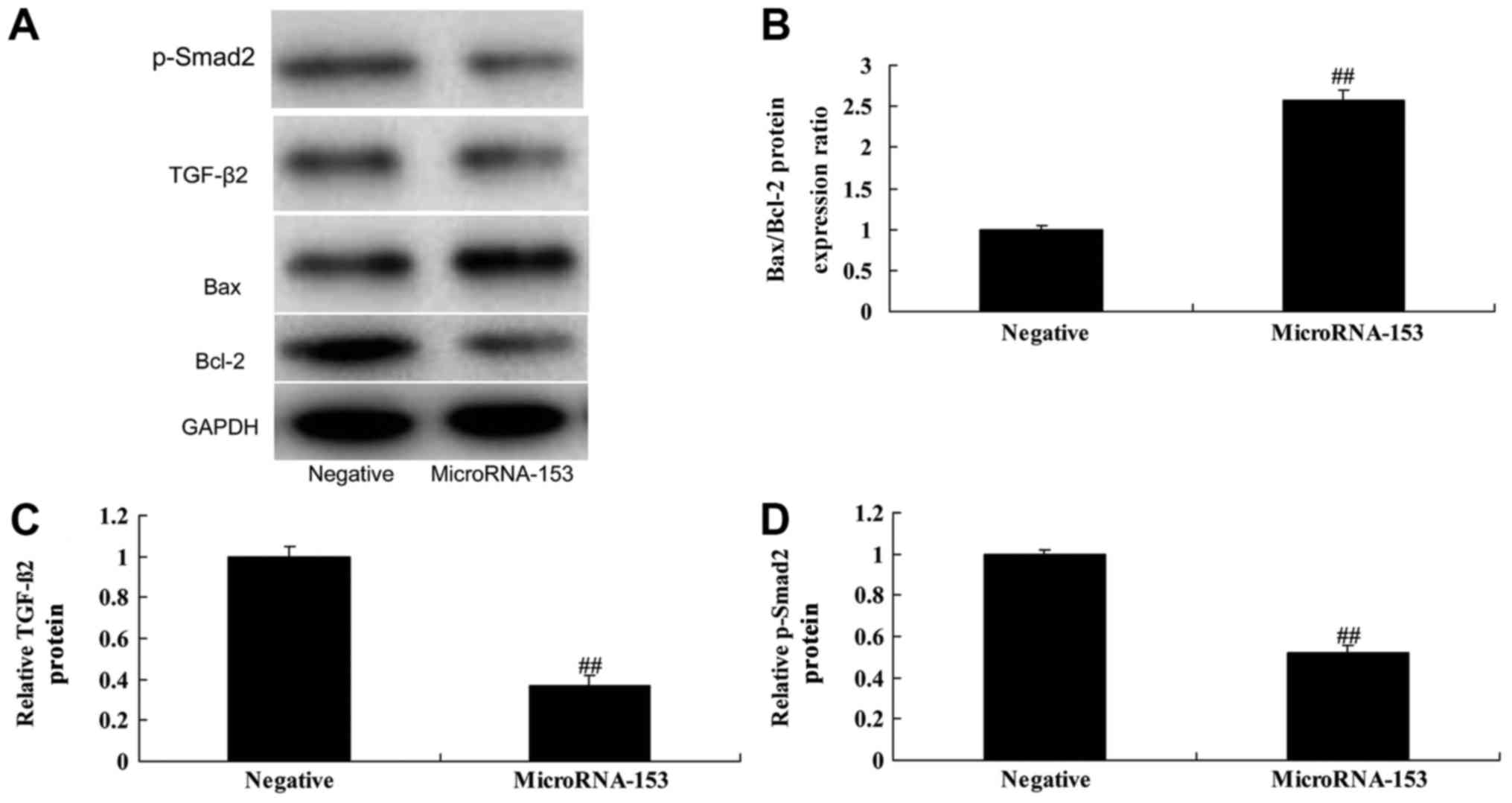
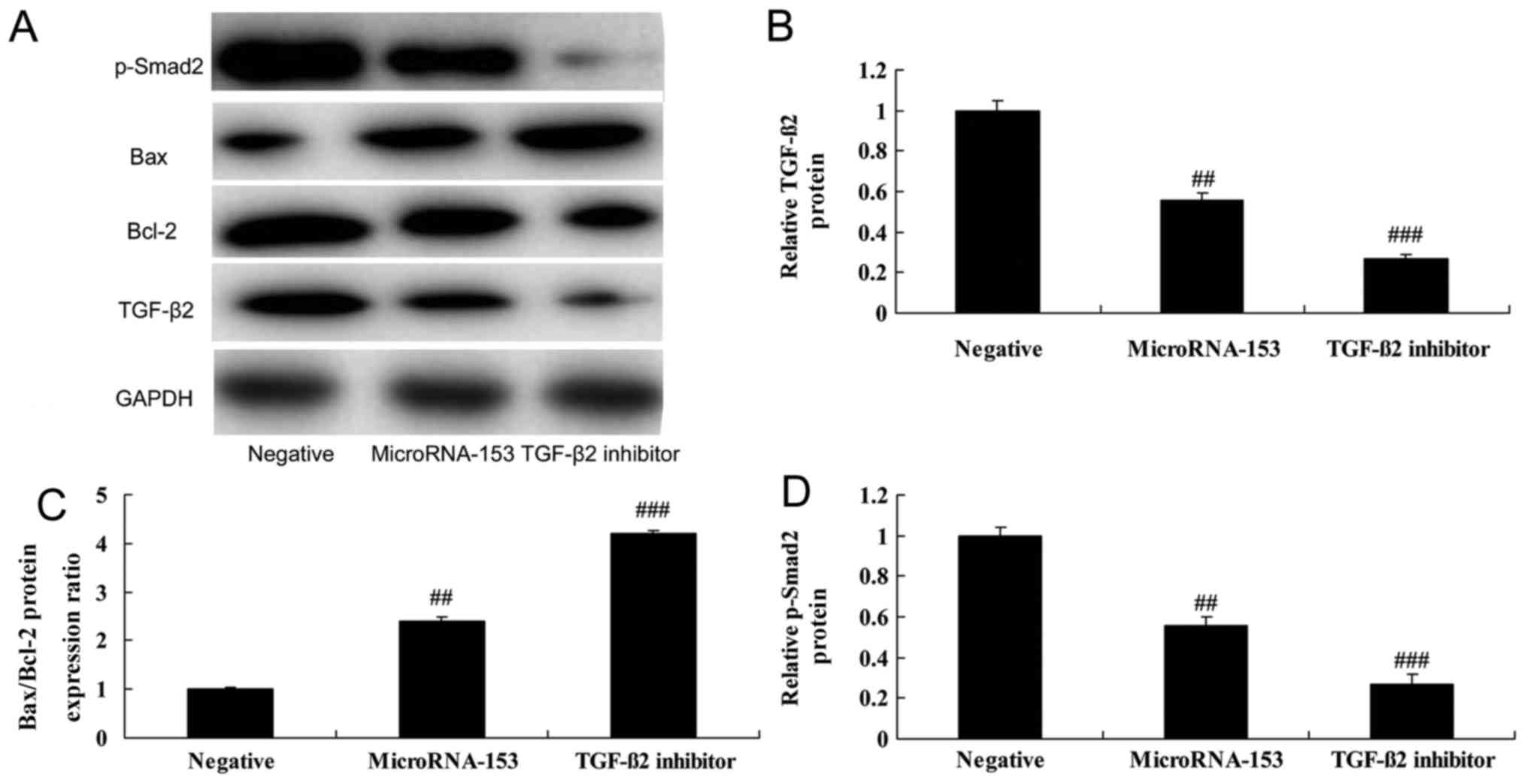
Upregulation of miR-153 increases the
Bax/Bcl-2 protein expression ratio, and suppresses
TGF-β2 and p-Smad2 protein expression in 13-9B
cells
To further determine the effect of upregulating
miR-153 on the Bax/Bcl-2 protein expression ratio and
TGF-β2 and Smad2 expression in 13-9B cells, western
blotting was used. The Bax/Bcl-2 protein expression ratio was
significantly increased, and TGF-β2 and p-Smad2 protein
expression was suppressed in 13-9B cells following miR-153
upregulation, compared with cells transfected with negative control
mimic (Fig. 4).
TGF-β2 inhibitor enhances
the effect of miR-153 upregulation on the Bax/Bcl-2 protein
expression ratio, and TGF-β2 and p-Smad2 protein
expression of 13-9B cells
To investigate whether TGF-β2 is involved
in the effect of miR-153 in 13-9B cells, 1 µM pirfenidone, a
TGF-β2 inhibitor, was added to cells transfected with
miR-153 mimic. In miR-153-transfected 13-9B cells, pirfenidone was
able to further suppress TGF-β2 and p-Smad2 protein expression, and
induced Bax/Bcl-2 ratio caused by miR-153 upregulation (Fig. 5).
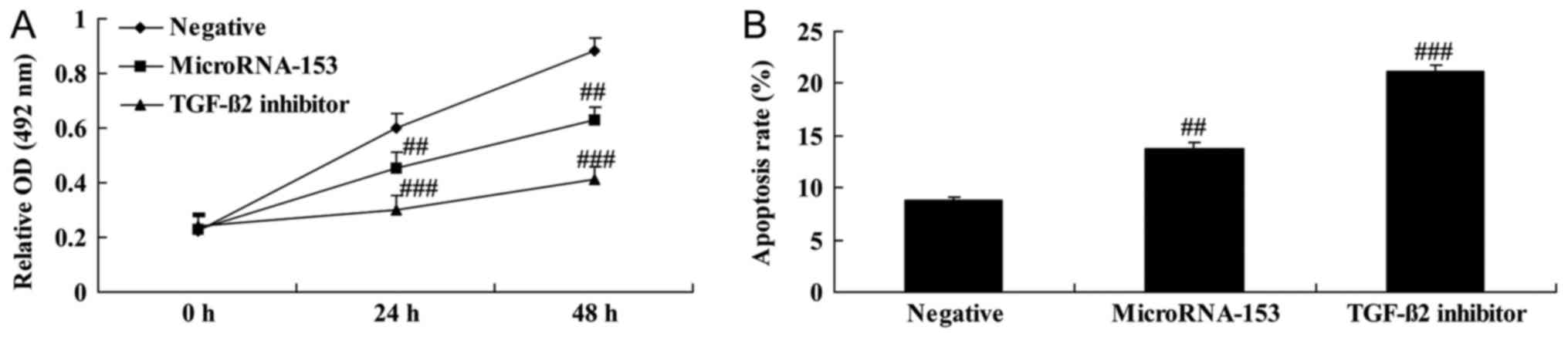
Effect of TGF-β2 inhibitor
on cell viability and apoptosis of 13-9B cells following miR-153
upregulation
The effect of TGF-β2 inhibitor on the
viability of miR-153-upregulated 13-9B cells. Following
transfection with miR-153 mimic, 13-9B cells exhibited
significantly decreased cell viability and significantly increased
apoptosis which was enhanced by the inhibition of TGF-β2
(Fig. 6).
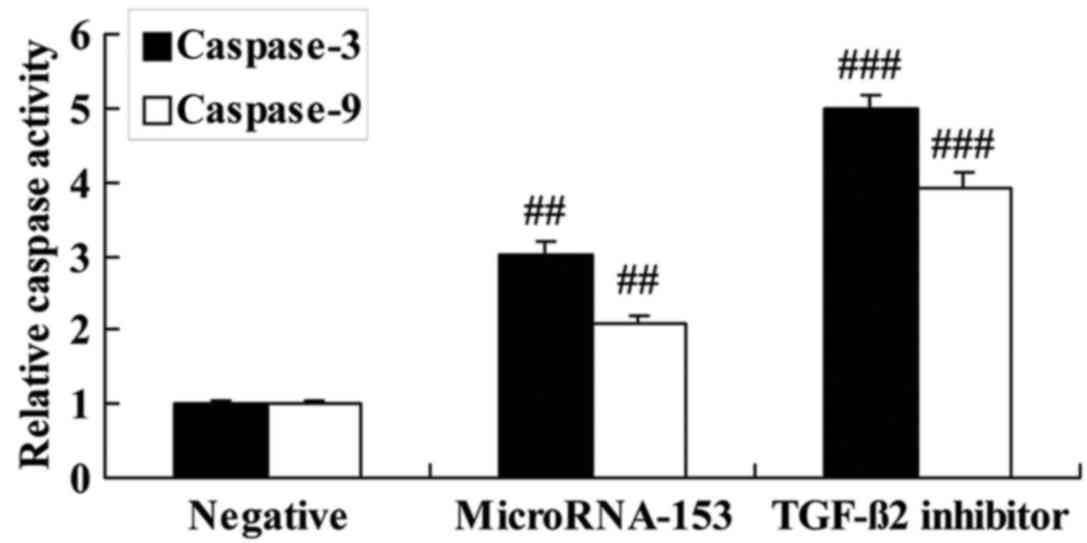
Effect of TGF-β2 inhibitor
on caspase-3 and −9 activity of 13-9B cells following miR-153
upregulation
To investigate the effect of TGF-β2
inhibitor on apoptosis of miR-153-upregulated 13-9B cells,
caspase-3 and −9 activity levels were determined using ELISA. The
inhibition of TGF-β2 significantly enhanced the increase
in caspase-3 and −9 activity of 13-9B cells caused by miR-153
upregulation (Fig. 7).
Discussion
NPC, also known as ‘Guangdong tumor’, is a malignant
tumor commonly occurring in southern China and southeast Asia,
particularly in Guangdong Province. It has been identified that NPC
is associated with genetic factors, Epstein-Barr virus infection
and environmental factors (12).
Early diagnosis and early treatment is the most effective means to
prolong the lives of patients and improve their quality of life
(13). Unfortunately, diagnosis of
NPC is difficult in its early stage, with a high likelihood of
metastasis (14). In the present
study, the expression of miR-153 was identified to be suppressed in
NPC tissues. Notably, it was identified that the upregulation of
miR-153 significantly decreased cell viability and induced
apoptosis of 13-9B cells. Chen et al (11) identified that the expression of
miR-153 was decreased in patients with non-small cell lung cancer
relative to the adjacent tissues (14). Therefore, miR-153 may be involved in
the proliferation of NPC cells and patient mortality.
In addition to tumor-associated proteins and their
coding genes, non-coding genes are also associated with the
incidence and development of tumors. In particular, markedly
conserved miRNAs are able to pair with 3′-untranslated regions
incompletely, to inhibit gene expression post-transcriptionally
(7). It has been identified that
>50% miRNA are located in tumor-associated genomes, and
chromosomal abnormalities directly led to an alteration in miRNA
gene copy number, resulting in the disordered expression of miRNAs
in a variety of tumor types, promoting or inhibiting
cancer-associated genes (15,16).
In the process of tumor cell apoptosis, the
apoptotic signal is transmitted through the intrinsic pathway, and
mitochondria serve an important function (17). Mitochondria generally transmit the
apoptotic signal through the caspase cascade signaling pathway,
which may be inhibited by the overexpression of Bcl-2/B-cell
lymphoma extra-large (18). Apoptosis
is promoted through the mitochondrial pathway to activate caspases
and form DNA fragments, thus interfering with the functions of
mitochondria (19). In the present
study, it was identified that miR-153 upregulation significantly
increased caspase-3 and −9 activity, and promoted the Bax/Bcl-2
protein expression ratio of 13-9B cell. Anaya-Ruiz et al
(20) identified that miR-153 induces
apoptosis in the MDA-MB-231 breast cancer cell line through
activating caspase 3/7.
TGF-β inhibits the malignant proliferation of
epithelial cells at an early stage, while promoting tumor growth
and metastasis at the late stage (8).
It has been identified previously that patients with increased
TGF-β had a relatively poor prognosis. TGF-β exerts its biological
functions mainly through the Smad protein family: TGF-β binds to
its receptor to phosphorylate Smad2/3, and then the latter binds to
Smad4 and enters the nucleus where the Smad transcription complex
regulates the expression of targeted genes (21). When TGF-β induces EMT through Smad
proteins, Smad3 and Smad4 interact with each other, and form a
transcription complex with Snail (22). Snail-Smad3/4 binds to the promoter
regions of epithelial cadherin, coxsackie adenovirus receptor and
occludin genes, to inhibit their transcriptional activity and
thereby induce EMT (22).
In the present study, it was identified that miR-153
upregulation significantly suppressed TGF-β2 and Smad2
protein expression of 13-9B cells. Niu et al (23) suggested that miR-153 inhibits
osteosarcoma cell viability and invasion through targeting
TGF-β2. Liang et al (24) also identified that miR-153 disturbs
TGF-β1/p-SMAD2/3 signal transduction, acting as an
anti-fibrotic element in the development of pulmonary fibrosis.
In conclusion, the results of the present study
indicate that miR-153 affects NPC cell viability by targeting
TGF-β2/Smad2. Therefore, miR-153 may be a target for the
treatment of NPC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XB designed the experiment; GG, YZ and LH performed
the experiment; XB and GG analyzed the data; XB wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institute
Research Ethics Committee of Beijing Army General Hospital
(Beijing, China). Written informed consent was provided by all
enrolled patients.
Patient consent for publication
All patients provided consented for the publication
of this data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Pan K, Liu LZ, Li YQ, Gu MF, Zhang
H, Shen WX, Xia JC and Li JJ: Sequential cytokine-induced killer
cell immunotherapy enhances the efficacy of the gemcitabine plus
cisplatin chemotherapy regimen for metastatic nasopharyngeal
carcinoma. PLoS One. 10:e01306202015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee FK, Yip CW, Cheung FC, Leung AK, Chau
RM and Ngan RK: Dosimetric difference amongst 3 techniques:
TomoTherapy, sliding-window intensity-modulated radiotherapy
(IMRT), and RapidArc radiotherapy in the treatment of late-stage
nasopharyngeal carcinoma (NPC). Med Dosim. 39:44–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang H, Lu H, Yuan H, Huang H, Wei Y,
Zhang Y and Liu X: Dosimetric benefits of placing dose constraints
on the brachial plexus in patients with nasopharyngeal carcinoma
receiving intensity-modulated radiation therapy: A comparative
study. J Radiat Res. 56:114–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng G, Wang T, Yang KY, Zhang S, Zhang T,
Li Q, Han J and Wu G: A prospective, randomized study comparing
outcomes and toxicities of intensity-modulated radiotherapy vs.
conventional two-dimensional radiotherapy for the treatment of
nasopharyngeal carcinoma. Radiother Oncol. 104:286–293. 2012.
|
|
5
|
Liu N, Cui RX, Sun Y, Guo R, Mao YP, Tang
LL, Jiang W, Liu X, Cheng YK, He QM, et al: A four-miRNA signature
identified from genome-wide serum miRNA profiling predicts survival
in patients with nasopharyngeal carcinoma. Int J Cancer.
134:1359–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lung RW, Wang X, Tong JH, Chau SL, Lau KM,
Cheng SH, Woo JK, Woo J, Leung PC, Ng MH, et al: A single
nucleotide polymorphism in microRNA-146a is associated with the
risk for nasopharyngeal carcinoma. Mol Carcinog. 52 (Suppl
1):E28–E38. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spence T, Bruce J, Yip KW and Liu FF:
MicroRNAs in nasopharyngeal carcinoma. Chin Clin Oncol. 5:172016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J
and Liao W: Flotillin-2 promotes nasopharyngeal carcinoma
metastasis and is necessary for the epithelial-mesenchymal
transition induced by transforming growth factor-β. Oncotarget.
6:9781–9793. 2015.PubMed/NCBI
|
|
9
|
Kan R, Shuen WH, Lung HL, Cheung AK, Dai
W, Kwong DL, Ng WT, Lee AW, Yau CC, Ngan RK, et al: NF-κB p65
subunit is modulated by latent transforming growth factor-β binding
protein 2 (LTBP2) in nasopharyngeal carcinoma HONE1 and HK1 cells.
PLoS One. 10:e01272392015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Zeng Z, Fan S, Wang J, Yang J,
Zhou Y, Li X and Huang D: Evaluation of the prognostic value of
TGF-β superfamily type I receptor and TGF-β type II receptor
expression in nasopharyngeal carcinoma using high-throughput tissue
microarrays. J Mol Histol. 43:297–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen WJ, Zhang EN, Zhong ZK, Jiang MZ,
Yang XF, Zhou DM and Wang XW: MicroRNA-153 expression and prognosis
in non-small cell lung cancer. Int J Clin Exp Pathol. 8:8671–8675.
2015.PubMed/NCBI
|
|
12
|
Wang W, Feng M, Fan Z, Li J and Lang J:
Clinical outcomes and prognostic factors of 695 nasopharyngeal
carcinoma patients treated with intensity-modulated radiotherapy.
Biomed Res Int. 2014:8149482014.PubMed/NCBI
|
|
13
|
Wang J, Zheng J, Tang T, Zhu F, Yao Y, Xu
J, Wang AZ and Zhang L: A randomized pilot trial comparing position
emission tomography (PET)-guided dose escalation radiotherapy to
conventional radiotherapy in chemoradiotherapy treatment of locally
advanced nasopharyngeal carcinoma. PLoS One. 10:e01240182015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long GX, Lin JW, Liu DB, Zhou XY, Yuan XL,
Hu GY, Mei Q and Hu GQ: Single-arm, multi-centre phase II study of
lobaplatin combined with docetaxel for recurrent and metastatic
nasopharyngeal carcinoma patients. Oral Oncol. 50:717–720. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruce JP, Yip K, Bratman SV, Ito E and Liu
FF: Nasopharyngeal cancer: Molecular landscape. J Clin Oncol.
33:3346–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu LH, Miao XT and Wang NY: Integrated
miRNA-mRNA analysis of Epstein-Barr virus-positive nasopharyngeal
carcinoma. Genet Mol Res. 14:6028–6036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Low SY, Tan BS, Choo HL, Tiong KH, Khoo AS
and Leong CO: Suppression of BCL-2 synergizes cisplatin sensitivity
in nasopharyngeal carcinoma cells. Cancer Lett. 314:166–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li SS, Tang QL, Wang SH, Chen YH, Liu JJ
and Yang XM: Simultaneously targeting Bcl-2 and Akt pathways
reverses resistance of nasopharyngeal carcinoma to TRAIL
synergistically. Tumori. 97:762–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anaya-Ruiz M, Cebada J, Delgado-Lopez G,
Sánchez-Vázquez ML and Pérez-Santos JL: miR-153 silencing induces
apoptosis in the MDA-MB-231 breast cancer cell line. Asian Pac J
Cancer Prev. 14:2983–2986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyu X, Fang W, Cai L, Zheng H, Ye Y, Zhang
L, Li J, Peng H, Cho WCS, Wang E, et al: TGFβR2 is a major target
of miR-93 in nasopharyngeal carcinoma aggressiveness. Mol Cancer.
13:512014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poh YW, Gan SY and Tan EL: Effects of
IL-6, IL-10 and TGF-β on the expression of survivin and apoptosis
in nasopharyngeal carcinoma TW01 cells. Exp Oncol. 34:85–89.
2012.PubMed/NCBI
|
|
23
|
Niu G, Li B, Sun L and An C: MicroRNA-153
inhibits osteosarcoma cells proliferation and invasion by targeting
TGF-β2. PLoS One. 10:e01192252015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang C, Li X, Zhang L, Cui D, Quan X and
Yang W: The anti-fibrotic effects of microRNA-153 by targeting
TGFBR-2 in pulmonary fibrosis. Exp Mol Pathol. 99:279–285. 2015.
View Article : Google Scholar : PubMed/NCBI
|