Introduction
The incidence of children's renal cell carcinoma
(RCC) is low. This disease affects 2.2 in 100,000 individuals, and
the age of onset is usually older than 5 years, but the prognosis
is poor (1). Malignant kidney tumor
in children is mainly nephroblastoma (2). Some studies (3) have pointed out that chromosome Xp11.2
translocation induced-fracture of transcription plays an important
role in the development of RCC in children, suggesting that the
occurrence of RCC in children may be related to genetic variations.
Recent studies shown that tumor suppressor gene phosphatase and
tensin homolog deletions on chromosome ten (PTEN) is closely
related to the occurrence and development of multiple malignant
tumors such as glioma (4), breast
cancer (5), liver cancer (6), colon cancer (7) and prostate cancer (8), and plays an important role in the
inhibition of cell proliferation, cell migration and cell adhesion
(9), and induction of apoptosis
(10), embryonic development
(11) and angiogenesis (12). PI3K/AKT is an important downstream
target of PTEN, and PTEN/PI3K/AKT cell signaling pathway may play
an important role in the occurrence and development of multiple
tumors. This study investigated the expression pattern of PTEN and
PTEN/PI3K/AKT cell signaling pathway in RCC in children.
Patients and methods
Patient information
A total of 5 cases of RCC (observation group) in
children and 10 cases of benign renal tumors (control group)
diagnosed by pathological examinations were selected in Zibo
Maternal and Child Health Care Hospital (Zibo, China) from June
2013 to June 2017. There were 3 boys and 2 girls in the observation
group, with an age range from 5 to 10 years, with an average age of
7.5±2.3 years. For clinical tumor TNM staging, there were 2 cases
of Stage I–II and 3 cases of Stage III–IV. The maximum tumor
diameter ranged from 1.3 to 4.3 cm, with an average maximum tumor
diameter of 2.7±1.5 cm. There was no significant difference in sex,
age and maximum tumor diameter between the two groups
(P>0.05).
The study was approved by the Ethics Committee of
Zibo Maternal and Child Health Care Hospital and the
parents/guardians of the children signed the informed consent.
Research methods
Tumor specimens were obtained by surgery, and
preserved in −70°C liquid nitrogen. The expression of PTEN mRNA in
tumor tissue was detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). The protein expression of
PTEN, PI3K and AKT in tumor tissues was detected by western
blotting. The relationship between the expression level of PTEN
mRNA and the clinical features of RCC was analyzed. The procedure
was repeated 3 times.
RT-qPCR method
Total RNA was extracted using TRIzol reagent
(Beijing Zhongshan Goldenbridge Co., Ltd., Beijing, China)
according to the manufacturer's protocol. RNA quality was checked
by 1.5% agarose gel electrophoresis. SYBR-Green I real-time
fluorescence PCR kit were used (Thermo Fisher Scientific, Inc.
Waltham, MA, USA). Total RNA (2 µg) was used in reverse
transcription as template to synthesize cDNA according to the
manufacturer's protocol of the reverse transcription kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Then, 2 µl cDNA
was used in PCR reaction. Primers were synthesized by Sangon
Biotech (Shanghai) Co., Ltd., Shanghai, China. Primers used in PCR
reactions were: 5′-TTGATTGCATCTCCATCTCCT-3′ (forward) and
5′-AAGAGATGGCCACGGCTGCT-3′ (reverse) for β-actin, and length of PCR
product was 421 bp; primer: 5′-TTGATTGCATCTCCATCTCCT-3′ (forward)
and 5′-TTCGCTTTCTCTGAGCATTCT-3′ (reverse) for PTEN, and length of
PCR product was 249 bp. Reaction conditions were: 94°C for 2 min,
followed by 30 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C
for 1 min. PCR product (6 µl) was subjected to 1.5% agarose gel
electrophoresis, and gray value quantification was carried out with
ultraviolet imaging system (Olympus, Tokyo, Japan). The data was
quantified using the 2−ΔΔCq method (13).
Western blotting
Total protein was extracted from tumor tissue, and
then protein samples were subjected to 10% SDS-PAGE gel
electrophoresis. The PVDF membranes were blocked and incubated with
mouse anti-human monoclonal PTEN, PI3K and AKT protein primary
antibodies (1:2,000; cat. nos. P3487, SAB5300225 and SAB4100001;
Sigma-Aldrich; Merck KGaA), overnight at 4°C. After washing with
phosphate-buffered saline (PBS), membranes were incubated with
rabbit anti-mouse polyclonal anti-immunoglobulin G (anti-IgG)
secondary antibody (1:500; cat. no. SAB3701023; Sigma-Aldrich;
Merck KGaA) at 37°C for 4 h. Signal detection was performed by ECL
(Jiangsu Beyotime Biotechnology Co., Ltd., Jiangsu, China) method,
and signals were analyzed using image analysis software
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to
calculate the gray values of each group, and expression of each
protein was normalized to endogenous control β-actin.
Statistical analysis
Statistical Product and Service Solutions (SPSS
Inc., Chicago, IL, USA) 20.0 software was used for statistical
analysis. Measurement data were expressed as mean ± standard
deviation, and independent sample t-test (Student's t-test) was
used for comparisons among groups. Enumeration data were expressed
as cases or (%), and χ2 test was used for comparisons
among groups. P<0.05 indicated that the difference was
statistically significant.
Results
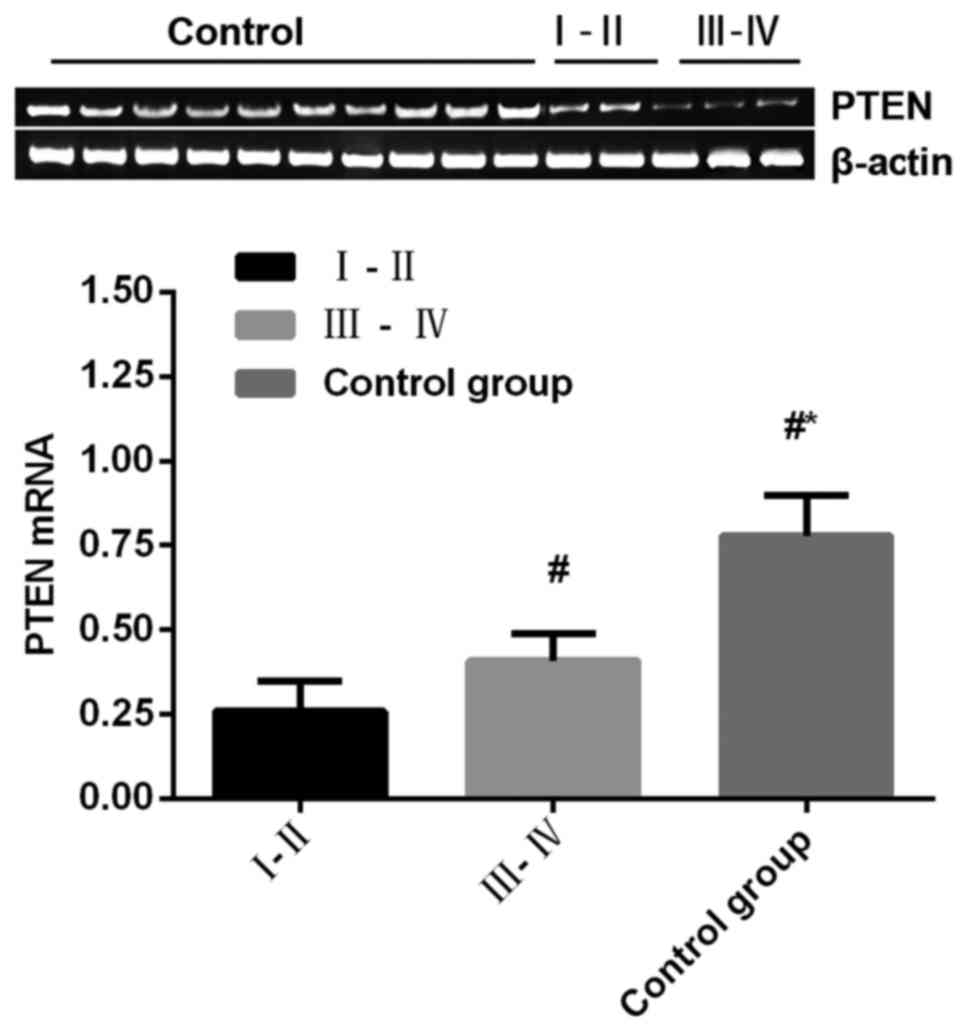
Comparison of PTEN mRNA expression
level among groups
The expression level of PTEN mRNA in the observation
group was significantly lower than that in control group
(P<0.05) (Fig. 1).
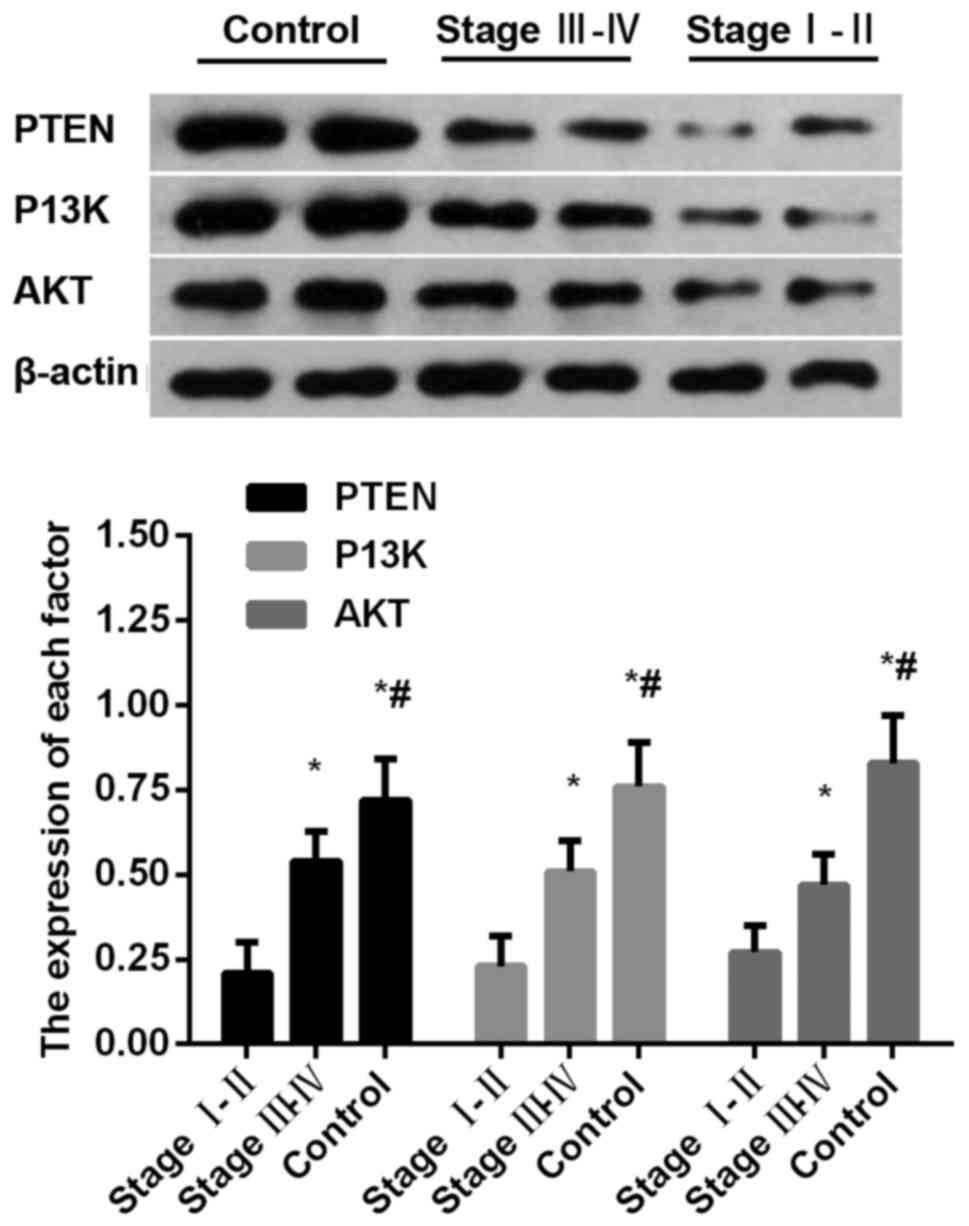
Comparison of protein expression
levels of PTEN, PI3K and AKT among groups
Protein expression levels of PTEN, PI3K and AKT in
observation group were significantly lower than those in control
group (P<0.05) (Fig. 2).
Relationship between expression levels
of PTEN mRNA and clinical features of RCC in the tissue
Expression levels of PTEN mRNA decreased with the
increased clinical stages of RCC (P=0.003), and were not related to
sex (P=0.865), age (P=0.765) and maximum tumor diameter (P=0.649)
(Table I).
 | Table I.The relationship between expression
level of PTEN mRNA and clinical features of RCC in the tissues. |
Table I.
The relationship between expression
level of PTEN mRNA and clinical features of RCC in the tissues.
| Clinical
features | Cases | PTEN mRNA | t value | P-value |
|---|
| Clinical stages |
|
| 10.326 | <0.001 |
| I–II | 2 | 0.4326±0.1322 |
|
|
|
III–IV | 3 | 0.2053±0.0968 |
|
|
| Age (years) |
|
|
0.152 | 0.865 |
|
<7.5 | 2 | 0.2465±0.0865 |
|
|
| ≥7.5 | 3 | 0.2596±0.0759 |
|
|
| Sex |
|
|
0.263 | 0.765 |
| Boys | 3 | 0.2365±0.0965 |
|
|
|
Girls | 2 | 0.2642±0.0825 |
|
|
| Max tumor diameters
(cm) |
|
|
0.325 | 0.649 |
|
<2.9 | 3 | 0.2323±0.0854 |
|
|
| ≥2.9 | 2 | 0.2706±0.0926 |
|
|
Discussion
Although clinical incidence of RCC in children is
relatively low, prognosis is very poor. Pathogenesis of RCC in
children is still unclear. Some studies have shown that occurrence
of RCC is closely correlated with genetic variation (14). In this study, expression levels of
PTEN mRNA in observation group were significantly lower than those
in control group, protein expression levels of PTEN, PI3K and AKT
were significantly lower in observation group than those in control
group expression level of PTEN mRNA decreased with the increased
clinical stages of RCC, and was not related to the sex, age and
maximum tumor diameter. Therefore, it is speculated that reduced
expression level of tumor suppressor gene PTEN and the inhibition
of PTEN/PI3K/AKT cell signaling pathway was very likely to be
involved in the occurrence and development of RCC in children.
PTEN is located on chromosome 10q23, and its reduced
expression level is related to tumorigenesis (15). In vitro experiments showed that
PTEN could rapidly block the cell cycle arrest in G1 phase and
induce cell apoptosis (16). PTEN can
also inhibit the expression of PIP3 and block PI3K/AKT signaling
pathway (17), alter cell morphology,
downregulate actin filament protein expression, and participate in
tumor cell migration and local adhesion (18). The multiple biological activities of
PTEN were achieved through focal adhesion kinase (FAK), PIP3,
mitogen activated protein kinase (MAPK), cyclin and other signaling
pathways. Zhang et al (19)
have shown that PTEN can dephosphorylate FAK, downregulate the
expression of downstream target gene p130CAS of FAK, thereby
inhibiting tumor growth, infiltration and metastasis. PTEN can also
affect the activity of FAK then inhibiting the activation of
PI3K/AKT pathway. PIP3 is transformed into PIP2 by
dephosphorylation, thereby blocking PI3K/PKB/AKT pathway, and
regulating cell proliferation and apoptosis (20). PIP3 is an important reaction substrate
of PTEN, so expression of PIP3 protein is closely related to active
state of lipid phosphatase (21).
Some studies have pointed out that (22) PTEN can inhibit the biological activity
of extracellular regulated protein kinases (ERK) in MAPK pathway.
PTEN can regulate the expression of cyclin-dependent protein kinase
(CDK). Overexpression of PTEN can dephosphorylate substrate
retinoblastoma protein (pRb), and moreover combine with
transcription factor E2F to significantly reduce cell proliferative
potential (23). Some studies have
shown that (17,24) PTEN/PI3K/AKT cell signaling pathway can
activate kinase system, and then regulates expression of multiple
cytokines such as VEGF and hypoxia inducible factor 1α (HIF-1α), so
as to participate in tumorigenesis. Activation of AKT depends on
upstream PI3K. PI3K can regulate cell metabolism, cell
proliferation and cell apoptosis. AKT is the central downstream
effector of PI3K. The activated AKT can phosphorylate multiple
proteins to participate in cell growth, cell development and
vascular regulation. AKT activity is closely related to neuronal
cell death. AKT promotes cell apoptosis through the opposite effect
of PTEN. AKT activity may decrease with time. The long-term
specimen preservation may affect experimental results.
Regarding AKT phosphorylation, the occurrence and
development of renal cell carcinoma is related to various growth
factors such as platelet-derived growth factor (PDGF) and epidermal
growth factor (EGF), while AKT phosphorylation is mainly regulated
by P13K-AKT. AKT is translocated into the plasma membrane and then
binds to PtdIns(3,4,5)P3 or PtdIns(3,4)P2 generated by P13K
activation to be phosphorylated, whereas PtdIns(3,4,5)P3 and
PtdIns(3,4) P2 are active factors and can easily react with various
cellular factors. In the course of the preservation of the
specimens in this experiment, it was not ruled out that the
phosphorylation ability of AKT varied due to the activation of the
two factors. Thus, we did not focus on the phosphorylated AKT, we
will work on this problem in the following study.
RT-qPCR was performed due to the limited resources,
which can cause errors in our data. Incidence of renal cell
carcinoma in children is not high, and only 8 patients were
admitted by our hospital in past 3 years. Among those 8 cases, 3
patients were transferred to other hospitals during treatment, so
we cannot include them in this study; therefore, only 5 tumor
samples were utilized in this study, which is a limitation.
Pediatric renal cell carcinoma is an incurable disease that is
extremely rare in clinical practice. Studies on the pathogenesis,
diagnosis, and treatment methods of this disease are also rare.
PTEN plays pivotal roles in this disease and may serve as a
potential target for the treatment. The small sample size may
affect the reliability of our data. We will try to include more
participants in our future studies to further confirm our
conclusions.
Results of this study showed that PTEN is very
likely to be involved in the occurrence and development of RCC in
children. Our study provided references for the diagnosis of RCC
and development of targeted therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. The datasets used and/or
analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
HL and YT performed RT-qPCR and western blotting. LC
extracted total RNA. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zibo Maternal and Child Health Care Hospital (Zibo, China) and
informed consents were signed by the parents of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Graves A, Hessamodini H, Wong G and Lim
WH: Metastatic renal cell carcinoma: Update on epidemiology,
genetics, and therapeutic modalities. Immunotargets Ther. 2:73–90.
2013.PubMed/NCBI
|
|
2
|
Szychot E, Apps J and Pritchard-Jones K:
Wilms' tumor: Biology, diagnosis and treatment. Transl Pediatr.
3:12–24. 2014.PubMed/NCBI
|
|
3
|
Silberstein J, Grabowski J, Saltzstein SL
and Kane CJ: Renal cell carcinoma in the pediatric population:
Results from the California Cancer Registry. Pediatr Blood Cancer.
52:237–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González-Sánchez A, Jaraíz-Rodríguez M,
Domínguez-Prieto M, Herrero-González S, Medina JM and Tabernero A:
Connexin43 recruits PTEN and Csk to inhibit c-Src activity in
glioma cells and astrocytes. Oncotarget. 7:49819–49833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo X, Liao R, Hanley KL, Zhu HH, Malo KN,
Hernandez C, Wei X, Varki NM, Alderson N, Chu C, et al: Dual shp2
and pten deficiencies promote non-alcoholic steatohepatitis and
genesis of liver tumor-initiating cells. Cell Rep. 17:2979–2993.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao F, Huang W, Zhang Y, Tang S, Zheng L,
Ma F, Wang Y, Tang H and Li X: Hes1 promotes cell proliferation and
migration by activating Bmi-1 and PTEN/Akt/GSK3β pathway in human
colon cancer. Oncotarget. 6:38667–38680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwak MK, Johnson DT, Zhu C, Lee SH, Ye DW,
Luong R and Sun Z: Conditional deletion of the Pten gene in the
mouse prostate induces prostatic intraepithelial neoplasms at early
ages but a slow progression to prostate tumors. PLoS One.
8:e534762013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Qiu Y, Shi NW, Zhao JN, Wang YC,
Jiang H and Qian HB: microRNA-21 mediates the TGF-β1-induced
migration of keratinocytes via targeting PTEN. Eur Rev Med
Pharmacol Sci. 20:3748–3759. 2016.PubMed/NCBI
|
|
10
|
Qi Y, Liu J, Saadat S, Tian X, Han Y, Fong
GH, Pandolfi PP, Lee LY and Li S: PTEN induces apoptosis and
cavitation via HIF-2-dependent Bnip3 upregulation during epithelial
lumen formation. Cell Death Differ. 22:875–884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stumpf M and den Hertog J: Differential
requirement for pten lipid and protein phosphatase activity during
zebrafish embryonic development. PLoS One. 11:e01485082016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stumpf M, Blokzijl-Franke S and den Hertog
J: Fine-tuning of pten localization and phosphatase activity is
essential for zebrafish angiogenesis. PLoS One. 11:e01547712016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 225:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Ragos V, Fotiades PP, Tsiambas E and
Peschos D: PTEN in laryngeal carcinomas. J BUON. 21:1024–1025.
2016.PubMed/NCBI
|
|
15
|
Heymont J, Berenfeld L, Collins J,
Kaganovich A, Maynes B, Moulin A, Ratskovskaya I, Poon PP, Johnston
GC, Kamenetsky M, et al: TEP1, the yeast homolog of the human tumor
suppressor gene PTEN/MMAC1/TEP1, is linked to the
phosphatidylinositol pathway and plays a role in the developmental
process of sporulation. Proc Natl Acad Sci USA. 97:12672–12677.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis,
and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma a549 cells. BioMed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu KL, Wu CA, Wu CW, Chan SH, Chang AY and
Chan JY: Redox-sensitive oxidation and phosphorylation of PTEN
contribute to enhanced activation of PI3K/Akt signaling in rostral
ventrolateral medulla and neurogenic hypertension in spontaneously
hypertensive rats. Antioxid Redox Signal. 18:36–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shojaee S, Chan LN, Buchner M, Cazzaniga
V, Cosgun KN, Geng H, Qiu YH, von Minden MD, Ernst T, Hochhaus A,
et al: PTEN opposes negative selection and enables oncogenic
trans-formation of pre-B cells. Nat Med. 22:379–387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Yu Q, He J and Zha X: Study of
the PTEN gene expression and FAK phosphorylation in human
hepatocarcinoma tissues and cell lines. Mol Cell Biochem.
262:25–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta A and Dey CS: PTEN, a widely known
negative regulator of insulin/PI3K signaling, positively regulates
neuronal insulin resistance. Mol Biol Cell. 23:3882–3898. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gerisch G, Schroth-Diez B,
Müller-Taubenberger A and Ecke M: PIP3 waves and PTEN dynamics in
the emergence of cell polarity. Biophys J. 103:1170–1178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebbesen SH, Scaltriti M, Bialucha CU,
Morse N, Kastenhuber ER, Wen HY, Dow LE, Baselga J and Lowe SW:
Pten loss promotes MAPK pathway dependency in HER2/neu breast
carcinomas. Proc Natl Acad Sci USA. 113:3030–3035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Yin LL, Su KL, Zhang GF and Wang J:
Concomitant depletion of PTEN and p27 and overexpression of cyclin
D1 may predict a worse prognosis for patients with post-operative
stage II and III colorectal cancer. Oncol Lett. 8:1543–1550. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu W, Fu JD, Yang F, Yang GL, Zhang YL,
Wang XY, Gu HX, Zhang HY and Wang L: Clinical implications of PTEN
andVEGF expression status, as well as microvessel density
inesophageal squamous cell carcinoma. Oncol Lett. 10:1409–1415.
2015. View Article : Google Scholar : PubMed/NCBI
|