Introduction
Colorectal cancer is the most common malignant tumor
of the digestive tract, more common in people over 45 years old and
more in male than female, as one of the main causes of human death
(1,2).
With changes in people's eating habits and lack of physical
exercise, the incidence of colorectal cancer has been increasing
year by year, with nearly 1,000,000 new patients each year
worldwide (3,4). Approximately 81% of its sites are near
the anal sphincter. Surgical resection is a very effective
treatment method for malignant tumors. However, for most colorectal
cancer patients, the preservation of anus and its function is a
difficult problem to be treated by surgical treatment, and the most
controversial disease in surgical treatment (5,6).
Chemotherapy has always been one of the basic
treatment methods for colorectal cancer patients, but as it
continues, it becomes unbearable for patients due to its toxic side
effects, and decreased efficacy over time. For decades, there has
been no new breakthrough in the treatment for colorectal cancer
(7,8).
FOLFIRI chemotherapy regimen is currently the main chemotherapy
drug regimen in middle and advanced colon cancer (9), and some scholars have been trying to
improve it. High frequency hyperthermia is a physical therapy, and
the energy transmitted is absorbed by cells through the high
frequency electromagnetic field. Due to its characteristics of
rapid growth, the tumor tissue has disordered structure and
abnormal blood vessel growth, which is often squeezed together,
with slow heat dissipation. The sustained energy absorption of
tumor cells causes a rapid rise in temperature, ultimately
resulting in their irreversible damage and death, thereby treating
tumors (10,11). However, worldwide there have been few
studies related to high frequency hyperthermia combined with
chemotherapy.
Therefore, in this study, the clinical effects of
high frequency hyperthermia-assisted irinotecan chemotherapy on
patients with middle and advanced colorectal cancer were
investigated, to analyze the application of high frequency
hyperthermia in patients with middle and advanced colorectal
cancer.
Materials and methods
Clinical information
A retrospective analysis was performed on the
medical records of 103 patients with middle and advanced colorectal
cancer treated in Cancer Center of Guangzhou Medical University
(Guangzhou, China) from May 2011 to June 2015, including 48
patients receiving irinotecan plus conventional treatment
(irinotecan group) and 55 patients receiving high frequency
hyperthermia-assisted irinotecan plus conventional treatment
(combination group). All patients were diagnosed with middle and
advanced colorectal cancer by pathology combined with imaging in
Cancer Center of Guangzhou Medical University. Patients without
abnormal leucocyte and lymphocyte counts were eligible for
chemotherapy; without drug allergy history and contraindications;
without organ dysfunction such as the heart and kidney before
operation; without abnormal bleeding or coagulation dysfunction
before operation; without portal hypertension, gastrointestinal
diseases and past history of tumors; receiving a series of
examinations and treatments in the Cancer Center of Guangzhou
Medical University after diagnosis; willing to cooperate with
medical staff in the Cancer Center of Guangzhou Medical University;
and with complete medical records. Patients with a preoperative
simple intelligence scale MMSE score of <24 points were
excluded; with incomplete medical records; with a history of
hepatitis; with mental or learning dysfunction; with excessive
diameter of the tumor; with other cardiovascular and
cerebrovascular diseases; with other respiratory tract diseases;
with other digestive tract diseases; transferred to other hospitals
halfway; and taking antibiotics prescribed by other hospitals or
with rehabilitation treatment arranged by other hospitals during
treatment. The study was approved by the Ethics Committee of Cancer
Center of Guangzhou Medical University, Guangzhou, China. Patients
or their families signed an informed consent form.
Treatment methods
Irinotecan group was treated with irinotecan plus
conventional treatment, and combination group with high frequency
hyperthermia-assisted irinotecan plus conventional treatment. Both
groups of patients were treated with FOLFIRI chemotherapy regimen,
with irinotecan (guoyaozhunzi: H20020687, manufacturer: Jiangsu
Hengrui Medicine Co., Ltd., Jiangsu, China) 180 mg/m2
and calcium folinate (guoyaozhunzi: H33020913, Zhejiang Wansheng
Pharmaceutical Co., Ltd., Zhejiang, China) 400 mg/m2
intravenously infused for 2 h at the same time. 5-fluorouracil
(guoyaozhunzi: 05501H201, Xi'an Haixin Pharmaceutical Co., Ltd.,
Xi'an, China) 400 mg/m2 was intravenously injected, and
then 5-fluorouracil 2,400-3,000 mg/m2 was pumped within
46 h. Based on this, combination group was treated with high
frequency hyperthermia (NRL-004 endogenous field tumor hyperthermia
system, Jilin Maida Medical Equipment Co., Ltd., Jilin, China). The
height of the electrode plate was adjusted, to lower the upper
electrode plate to 5–7 cm on the skin surface corresponding to the
lesion, with a working frequency of 13.56 MHz and a treatment power
of 1,300-1,400 W. The computer fitting temperature was adjusted to
41–43°C according to the patient's condition and tolerance, each
treatment time for 40–60 min, 2–3 times a week. All treatment
methods were every 2 weeks for one course, for a total of 4
courses.
Observation indicators
The Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1 (12) was used
to evaluate treatment effects on the two groups of patients after 4
courses of treatment. Complete remission: Τhe target lesion
disappears, and the short diameter of the lymph node does not
exceed 10 mm. Partial remission: Τhe sum of the measurement results
of the target lesion diameter is reduced by no less than 30%
compared to baseline measurements. Stable disease: Τhe measurement
results of the target lesion after treatment are between those of
partial remission and disease progression. Disease progression: Τhe
sum of the measurement results of target lesion diameter is
increased by no less than 20% compared to baseline measurements,
and the absolute increase is no less than 5 mm. The objective
remission rate is the ratio of the sum of patients with complete
remission and partial remission in the total number of patients.
The CTCAE version 4.0 (13) was used
to evaluate the severity of adverse reactions in the two groups of
patients, divided into 1–5 grades. The higher the grade is, the
more severe the adverse reactions are. The incidence of adverse
reactions in the two groups of patients was recorded (neutrophil
deficiency and leukopenia), and the treatment safety was evaluated.
The QLQ-C30 scale was used to evaluate the quality of life
(emotional, role, cognitive, physiological and social function) in
the two groups of patients before and after treatment. The higher
the score is, the higher the quality of life is.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used.
Count data were expressed as n (%). χ2 test was used for
the comparison of ratio. Measurement data were expressed as mean ±
SD. Α t-test was used for comparison between the two groups, paired
t-test for comparison before and after treatment, K-M survival
curve for analyzing the 3-year survival rate of the two groups of
patients. P<0.05, indicates a statistically significant
difference.
Results
General information
Altogether 48 patients were in irinotecan group,
including 26 male and 22 female patients, aged 53.76±9.48 years,
with a BMI of 23.42±3.47 kg/m2. Fifty-five patients were
in combination group, including 30 male and 25 female patients,
aged 54.13±11.29 years, with a BMI of 24.25±3.14 kg/m2.
There was no statistically significant difference in sex, age and
BMI between the two groups of patients (all P>0.05), and in
tumor pathological type, metastasis, site, α-fetoprotein level,
hemoglobin level, smoking history, drinking history, education
status and place of residence (all P>0.05) (Table I).
 | Table I.General information. |
Table I.
General information.
| Variables | Irinotecan group
(n=48) | Combination group
(n=55) | χ2/t
value | P-value |
|---|
| Sex [n (%)] |
|
| 0.001 | 0.969 |
| Male | 26 (54.17) | 30 (54.55) |
|
|
|
Female | 22 (45.83) | 25 (45.45) |
|
|
| Age (years) | 53.76±9.48 |
54.13±11.29 | 0.179 | 0.859 |
| BMI
(kg/m2) | 23.42±3.47 | 24.25±3.14 | 1.274 | 0.206 |
| Pathological type [n
(%)] |
|
| 0.406 | 0.939 |
|
Adenocarcinoma | 32 (66.67) | 34 (61.82) |
|
|
| Squamous
carcinoma | 1 (2.08) | 2 (3.64) |
|
|
| Mucous
carcinoma | 6
(12.50) | 8
(14.55) |
|
|
|
Undifferentiated
carcinoma | 9
(18.75) | 11 (20.00) |
|
|
| α-fetoprotein
(ng/ml) | 19.42±3.25 | 19.58±3.64 | 0.234 | 0.816 |
| Hemoglobin (g/l) | 12.33±3.86 | 13.49±3.73 | 1.549 | 0.125 |
| Metastasis [n
(%)] |
|
| 1.334 | 0.248 |
| Yes | 40 (83.33) | 50 (90.91) |
|
|
| No | 8
(16.67) | 5 (9.09) |
|
|
| Site [n (%)] |
|
| 0.733 | 0.693 |
|
Colon | 32 (66.67) | 40 (72.73) |
|
|
|
Rectal | 9
(18.75) | 7
(12.73) |
|
|
| Colon
plus rectal | 7
(14.58) | 8
(14.55) |
|
|
| Smoking history [n
(%)] |
|
| 0.149 | 0.699 |
| Yes | 28 (58.33) | 30 (54.55) |
|
|
| No | 20 (41.67) | 25 (45.45) |
|
|
| Drinking history [n
(%)] |
|
| 1.258 | 0.262 |
| Yes | 19 (39.58) | 16 (29.09) |
|
|
| No | 29 (60.42) | 39 (70.91) |
|
|
| Education status [n
(%)] |
|
| 1.324 | 0.250 |
| High
school and below | 21 (43.75) | 18 (32.73) |
|
|
| High
school above | 27 (56.25) | 37 (67.27) |
|
|
| Place of residence [n
(%)] |
|
| 0.014 | 0.905 |
|
Rural | 23 (47.92) | 27 (49.09) |
|
|
|
Urban | 25 (52.08) | 28 (50.91) |
|
|
Analysis of treatment effects on two
groups of patients
After 4 courses of treatment, there was no
statistically significant difference in the proportions of patients
with complete remission, stable disease and disease progression
between the two groups of patients (all P>0.05), but there were
statistically significant differences in the proportions of
patients with partial remission and objective remission, with those
in combination group higher than those in irinotecan group (both
P<0.05) (Table II).
 | Table II.Analysis of treatment effects on two
groups of patients. |
Table II.
Analysis of treatment effects on two
groups of patients.
| Variables | Irinotecan group
(n=48) | Combination group
(n=55) | χ2
value | P-value |
|---|
| Complete
remission | 2 (4.17) | 5 (9.09) | 0.981 | 0.322 |
| Partial
remission | 15 (31.25) | 29 (52.73) | 4.832 | 0.028 |
| Stable disease | 23 (47.92) | 17 (30.91) | 3.121 | 0.077 |
| Disease
progression | 8
(16.67) | 4 (7.27) | 2.197 | 0.138 |
| Objective
remission | 17 (35.42) | 34 (61.82) | 7.147 | 0.008 |
Safety assessment of two treatment
methods
After 4 courses of treatment, no patient died. There
was no statistically significant difference in the severity of
adverse reactions between the two groups of patients (P>0.05).
The results of the statistical analysis of the incidence of
complications in the two groups of patients showed that there was
no statistically significant difference in the proportions of
patients with neutrophil deficiency, leukopenia, nausea and
vomiting, diarrhea, scald, anemia and fatigue between the two
groups of patients (all P>0.05) (Tables III and IV).
 | Table III.Assessment of severity of adverse
reactions after 4 courses of treatment in two groups of
patients. |
Table III.
Assessment of severity of adverse
reactions after 4 courses of treatment in two groups of
patients.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Irinotecan
(n=48) | Combination (n=
55) | χ2
value | P-value |
|---|
| 1 | 12 (25.00) | 14 (25.45) | 0.016 | 0.901 |
| 2 | 20 (41.67) | 22 (40.00) | 0.029 | 0.864 |
| 3 | 12 (25.00) | 13 (23.64) | 0.026 | 0.872 |
| 4 | 4 (8.33) | 6
(10.91) | 0.194 | 0.660 |
| 5 | 0 (0.00) | 0 (0.00) |
|
|
 | Table IV.Statistics on incidence of adverse
reactions after 4 courses of treatment in two groups of
patients. |
Table IV.
Statistics on incidence of adverse
reactions after 4 courses of treatment in two groups of
patients.
| Variables | Irinotecan group
(n=48) | Combination group
(n=55) | χ2
value | P-value |
|---|
| Neutrophil
deficiency | 19 (39.58) | 25 (45.45) | 0.361 | 0.548 |
| Leukopenia | 9
(18.75) | 10 (18.18) | 0.006 | 0.941 |
| Nausea and
vomiting | 9
(18.75) | 8
(14.55) | 0.329 | 0.566 |
| Diarrhea | 1 (2.08) | 2 (3.64) | 0.219 | 0.640 |
| Scald | 0 (0.00) | 1 (1.82) |
|
|
| Anemia | 2 (4.17) | 2 (3.64) | 0.019 | 0.889 |
| Fatigue | 8
(16.67) | 7
(12.73) | 0.320 | 0.572 |
Analysis of quality of life in two
groups of patients
There was no statistically significant difference in
emotional, role, cognitive, physiological and social function
scores between the two groups of patients before treatment (all
P>0.05). After 4 courses of treatment, those that increased in
different degrees (all P<0.05), compared to those in combination
group were significantly higher than those in irinotecan group (all
P<0.05) (Table V).
 | Table V.Analysis of quality of life in two
groups of patients before and after treatment. |
Table V.
Analysis of quality of life in two
groups of patients before and after treatment.
| Variables | Irinotecan group
(n=48) | Combination group
(n=55) | t value | P-value |
|---|
| Emotional
function |
| Before
treatment | 50.69±11.47 | 51.33±11.24 | 0.286 | 0.776 |
| After
treatment |
59.72±11.05a |
64.38±11.67a | 2.072 | 0.041 |
| Role function |
| Before
treatment | 46.54±11.25 | 48.31±11.87 | 0.774 | 0.441 |
| After
treatment |
61.28±11.69a |
69.84±11.72a | 3.705 | <0.001 |
| Cognitive
function |
| Before
treatment | 50.42±12.03 | 51.75±11.54 | 0.572 | 0.569 |
| After
treatment |
62.74±9.87a |
74.59±10.66a | 5.825 | <0.001 |
| Physiological
function |
| Before
treatment | 45.33±8.72 | 44.96±9.28 | 0.208 | 0.836 |
| After
treatment |
59.86±9.35a |
67.33±10.24a | 3.845 | <0.001 |
| Social
function |
| Before
treatment | 49.64±10.65 | 48.27±10.43 | 0.659 | 0.512 |
| After
treatment |
62.78±10.68a |
72.24±11.45a | 4.315 | <0.001 |
Analysis of 3-year survival rate in
two groups of patients
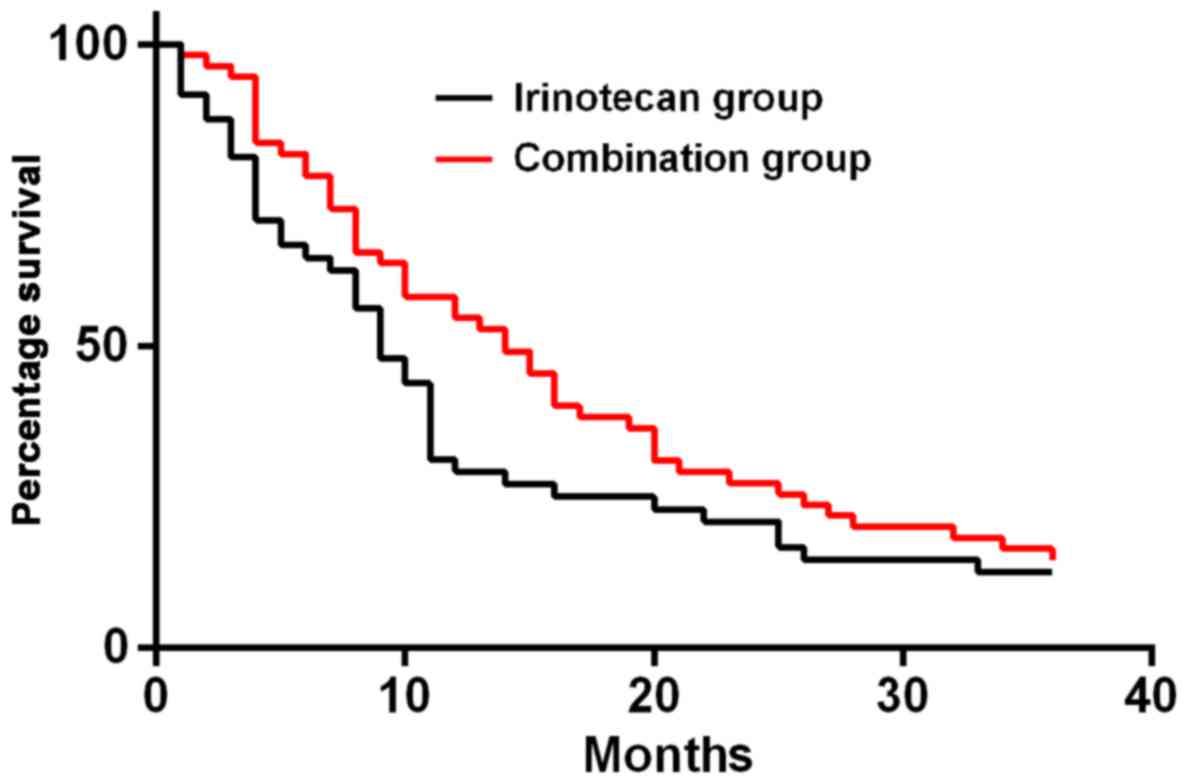
The 1-, 2- and 3-year survival rates of patients in
irinotecan group were 31.25% (15 cases), 22.92% (11 cases) and
12.50% (6 cases), respectively. Those in combination group were
58.18% (32 cases), 29.09% (16 cases) and 16.36% (9 cases),
respectively. The results of K-M survival curve analysis showed
that there was no statistically significant difference in survival
rate between the two groups of patients (P=0.05). (Fig. 1).
Discussion
Colorectal cancer is a type of malignant tumor with
high incidence. In some studies, the proportion of colorectal
cancer patients that can be found in the early stage is only
approximately 2% of all colorectal cancer patients (14). The current chemotherapy regimens for
patients with middle and advanced colorectal cancer are mainly
FOLFIRI regimen containing irinotecan and FOLFOX regimen containing
oxaliplatin. They effectively improve the survival and prognosis of
patients, but adverse reactions caused by them in nervous, blood
and digestive systems also increase with the increase of
chemotherapy course (15,16). In recent years, hyperthermia has been
confirmed not to cause adverse reactions such as bone marrow
suppression in patients, which has better killing effects on tumor
cells (17). However, there are few
studies on hyperthermia combined with chemotherapy for patients
with middle and advanced colorectal cancer. In this study, the
application effects of high frequency hyperthermia combined with
FOLFIRI regimen containing irinotecan on patients with middle and
advanced colorectal cancer were analyzed, to provide references for
the clinical treatment of colorectal cancer.
In this study, the medical records of 103 patients
with middle and advanced colorectal cancer were included in strict
accordance with inclusion and exclusion criteria. There was no
statistically significant difference in the basic data between the
two groups of patients after grouping, suggesting that they are
comparable in this study, and the results of the study are
credible. The results of their efficacy analysis after 4 courses of
treatment showed that the proportion of patients with partial
remission was significantly higher in combination group than that
in irinotecan group, and the objective remission rate was also
higher in combination group than that in irinotecan group. It is
indicated that high frequency hyperthermia can improve the
treatment effects of irinotecan chemotherapy on patients with
middle and advanced colorectal cancer.
In recent years, there have been some studies on
hyperthermia combined with chemotherapy improving the treatment
effects on tumors, which confirm the positive effects of
hyperthermia as an adjunctive therapy for treating tumors (18,19).
Hyperthermia is one of the oldest cancer treatment methods. The
tumor tissue is similar to heat storage due to its structure.
Hyperthermia regulates the temperature to 39–45°C to selectively
heat the tumor tissue, thereby killing tumor cells under high
temperature. Recent advances based on its biological principles
suggest that hyperthermia is an effective radiation sensitizer that
is considered as a safe and effective supplementary method for
treating tumors (20,21). Adverse reactions caused by
chemotherapy have always been one of the most important problems
that clinicians want to overcome, seriously influencing the
treatment effects on tumors and the use of drugs, which is one of
the important reasons that hinder the improvement of chemotherapy
regimens in recent decades (22). The
results of this study showed that there was no statistically
significant difference in the severity and incidence of adverse
reactions between the two groups of patients, suggesting that high
frequency hyperthermia as an adjunctive therapy does not change the
safety of current chemotherapy regimens. However, there was one
scalded patient in combination group, which should also be taken
seriously.
It is necessary to pay close attention to changes in
patients and make more detailed planning for temperature regulation
in hyperthermia. Nevertheless, the effects of hyperthermia on the
treatment safety have not been found in some hyperthermia-related
reports (23,24). Patients' quality of life in the two
groups was also analyzed. For patients with middle and advanced
colorectal cancer, improving their quality of life is one of the
most important goals of the treatment. The results of this study
also showed that the quality of life scores of patients after
treatment were higher in combination group than those in irinotecan
group. This should be related to the treatment effects of the two
methods. The better recovery of patients in combination group can
effectively ςimprove their quality of life. Some studies (25) have reported that promoting blood
circulation and enhancing immune function, hyperthermia can reduce
muscle tone, thereby alleviating cancer pain, which should also be
an important reason for patients' higher quality of life in
combination group. In this study, the results of the 3-year
survival rate analysis showed that there was no statistically
significant difference in it between the two groups of patients. In
related reports, hyperthermia is also found to be able to reduce
the risk of poor prognosis of patients, and no difference in the
survival rate was found (26,27). This is similar to our findings.
In summary, high frequency hyperthermia-assisted
chemotherapy for patients with middle and advanced colorectal
cancer can effectively improve its treatment effects and patients'
quality of life, with better treatment safety, worthy of clinical
promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived and designed the study, collected and
interpreted the data, and treated patients. The author read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Cancer Center of Guangzhou Medical University (Guangzhou, China).
Patients who participated in this research, signed the informed
consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al; RECOURSE Study Group, . Randomized trial of
TAS-102 for refractory metastatic colorectal cancer. N Engl J Med.
372:1909–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imperiale TF, Ransohoff DF, Itzkowitz SH,
Levin TR, Lavin P, Lidgard GP, Ahlquist DA and Berger BM:
Multitarget stool DNA testing for colorectal-cancer screening. N
Engl J Med. 370:1287–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bibbins-Domingo K, Grossman DC, Curry SJ,
Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM,
Kemper AR, Krist AH, et al; US Preventive Services Task Force, .
Screening for colorectal cancer: US Preventive Services Task Force
recommendation statement. JAMA. 315:2564–2575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koehler BC, Jassowicz A, Scherr AL, Lorenz
S, Radhakrishnan P, Kautz N, Elssner C, Weiss J, Jaeger D,
Schneider M, et al: Pan-Bcl-2 inhibitor Obatoclax is a potent late
stage autophagy inhibitor in colorectal cancer cells independent of
canonical autophagy signaling. BMC Cancer. 15:9192015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown DG, Rao S, Weir TL, O'Malia J, Bazan
M, Brown RJ and Ryan EP: Metabolomics and metabolic pathway
networks from human colorectal cancers, adjacent mucosa, and stool.
Cancer Metab. 4:112016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuda C, Munemoto Y, Mishima H, Nagata
N, Oshiro M, Kataoka M, Sakamoto J, Aoyama T, Morita S and Kono T:
Double-blind, placebo-controlled, randomized phase II study of
TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based
colorectal cancer chemotherapy-induced oral mucositis. Cancer
Chemother Pharmacol. 76:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji J, Weng Q, Zhang F, Xiong F, Jin Y, Hui
J, Song J, Gao J, Chen M, Li Q, et al: Non-small-cell lung cancer:
Feasibility of intratumoral radiofrequency hyperthermia-enhanced
herpes simplex virus thymidine kinase gene therapy. Radiology.
288:612–620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas RG, Moon MJ, Lee H, Sasikala AR,
Kim CS, Park IK and Jeong YY: Hyaluronic acid conjugated
superparamagnetic iron oxide nanoparticle for cancer diagnosis and
hyperthermia therapy. Carbohydr Polym. 131:439–446. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beriwal S, Shukla G, Shinde A, Heron DE,
Kelley JL, Edwards RP, Sukumvanich P, Richards S, Olawaiye AB and
Krivak TC: Preoperative intensity modulated radiation therapy and
chemotherapy for locally advanced vulvar carcinoma: Analysis of
pattern of relapse. Int J Radiat Oncol Biol Phys. 85:1269–1274.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bui QC, Lieber M, Withers HR, Corson K,
van Rijnsoever M and Elsaleh H: The efficacy of hyperbaric oxygen
therapy in the treatment of radiation-induced late side effects.
Int J Radiat Oncol Biol Phys. 60:871–878. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
García-Carbonero R, van Cutsem E, Rivera
F, Jassem J, Gore I Jr, Tebbutt N, Braiteh F, Argiles G, Wainberg
ZA, Funke R, et al: Randomized phase II trial of parsatuzumab
(anti-EGFL7) or placebo in combination with FOLFOX and bevacizumab
for first-line metastatic colorectal cancer. Oncologist.
22:375–e30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahman MA, Matsumura Y, Yano S and Ochiai
B: pH-responsive charge-conversional and hemolytic activities of
magnetic nanocomposite particles for cell-targeted hyperthermia.
ACS Omega. 3:961–972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quinto CA, Mohindra P, Tong S and Bao G:
Multifunctional superparamagnetic iron oxide nanoparticles for
combined chemotherapy and hyperthermia cancer treatment. Nanoscale.
7:12728–12736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan Y, Wang X, Zheng N and Shi J: Clinical
research of selective bronchial artery infusion and chemotherapy of
lung cancer. Zhongguo Fei Ai Za Zhi. 6:378–380. 2003.(In Chinese).
PubMed/NCBI
|
|
20
|
Yao X, Niu X, Ma K, Huang P, Grothe J,
Kaskel S and Zhu Y: Graphene quantum dots-capped magnetic
mesoporous silica nanoparticles as a multifunctional platform for
controlled drug delivery, magnetic hyperthermia, and photothermal
therapy. Small. 13:16022252017. View Article : Google Scholar
|
|
21
|
Datta NR, Ordóñez SG, Gaipl US, Paulides
MM, Crezee H, Gellermann J, Marder D, Puric E and Bodis S: Local
hyperthermia combined with radiotherapy and-/or chemotherapy:
Recent advances and promises for the future. Cancer Treat Rev.
41:742–753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Ruan L, Liu H, Yang H and Feng Y:
ABCB1 C3435T polymorphism is associated with leukemia
susceptibility: Evidence from a meta-analysis. Onco Targets Ther.
8:1009–1015. 2015.PubMed/NCBI
|
|
23
|
Gao LZ, Gao EM, Bai YF, Su HL, Zhang F, Ge
MQ, Liu DL and Huang YK: Hyperthermic intraperitoneal chemotherapy
plus high-frequency diathermic therapy followed by intravenous
chemotherapy versus intravenous chemotherapy alone for
postoperative adjuvant treatment of gastrointestinal cancer: A
comparative research study. J BUON. 21:1510–1517. 2016.PubMed/NCBI
|
|
24
|
Narayan P, Crocker I, Elder E and Olson
JJ: Safety and efficacy of concurrent interstitial radiation and
hyperthermia in the treatment of progressive malignant brain
tumors. Oncol Rep. 11:97–103. 2004.PubMed/NCBI
|
|
25
|
Chi MS, Yang KL, Chang YC, Ko HL, Lin YH,
Huang SC, Huang YY, Liao KW, Kondo M and Chi KH: Comparing the
effectiveness of combined external beam radiation and hyperthermia
versus external beam radiation alone in treating patients with
painful bony metastases: A phase 3 prospective, randomized,
controlled trial. Int J Radiat Oncol Biol Phys. 100:78–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gani C, Schroeder C, Heinrich V, Spillner
P, Lamprecht U, Berger B and Zips D: Long-term local control and
survival after preoperative radiochemotherapy in combination with
deep regional hyperthermia in locally advanced rectal cancer. Int J
Hyperthermia. 32:187–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen K, Zhu S, Xiang G, Duan X, He J and
Chen G: Ablation effects of noninvasive radiofrequency
field-induced hyperthermia on liver cancer cells. Saudi Pharm J.
24:329–332. 2016. View Article : Google Scholar : PubMed/NCBI
|