Introduction
Hodgkin's lymphoma (HL) is a common lymphatic tumor,
and the incidence rate by year of HL new cases range from three to
four per 100,000 western individuals (1). HL is an malignant tumor derived from B
cells (2). As it is hard to diagnose
advanced HL, and the pathophysiological mechanisms of HL remain
unknown, approximately 20% of the newly diagnosed cases die from HL
(3). Consequently, more effective
treatments to increase HL survival rates need to be developed. At
the same time, understanding of the mechanisms of HL development is
required. Studies of factors associated with HL, such as genes and
miRNAs, are also important.
miRNAs are 19–23 nucleotides in length. As a
subgroup of non-coding miRNAs, they play a role in regulating
post-transcriptionally via targeting mRNA 3′UTR, causing their
degradation or suppressing mRNA translation (4–6). An
increasing number of studies have emerged, providing evidence that
various miRNAs can exert oncogenic or tumor suppressive functions
(7,8).
For example, miR-148a suppresses migration and invasion of breast
cancer (9). miRNA-29b suppresses
tumor angiogenesis, invasion, and metastasis (10). miR-24-3p was proven to be involved in
multiple kinds of tumors, including HL (11), head and neck squamous cell cancer
(12), and bladder carcinoma
(13). However, the function of
miR-24-3p in mediating HL invasion and migration remains largely
elusive.
The death effector domain-containing protein (DEDD)
has a relevance to different kinds of physiological processes,
including cell mitosis, cycle and apoptosis (14). Studies have shown that DEDD may be
used as a potential therapeutic target and prognostic marker to
cure metastasis of carcinoma (15,16).
However, the effects of DEDD on regulating HL have not yet been
reported. To better understand the anti-metastatic functions and
mechanisms of DEDD in HL, the present study investigated the DEDD
expression levels in HL tissues and cell lines. At the same time,
the correlation between miR-24-3p and DEDD in HL was analyzed.
Materials and methods
Cell cultures
HL cell lines [L1236 (CSC-C0538, Creative Bioarray)
and L428 (ws101345, ATCC)] were cultured in RPMI-1640 medium
(Cellgro; Corning, Inc., Corning, NY, USA) in an atmosphere with 5%
CO2 at 37°C. The medium contained penicillin/streptomycin, 5% L428,
10% L1236 as well as 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Germinal center (GC)-B cells were sorted from tonsil
tissue samples of three HL donors aged between 3 and 10 years. Two
of the three GC-B cells were purified >98% from human tonsil
tissues on the basis of the
CD20+lgD−CD38+ expression, as
previously described. The third sample was magnetic-activated cell
sorting purified >95% based on expression of
IgD−CD138−CD3−CD10+.
Written informed consents were obtained for the use of the tonsil
samples from the parents of the children.
Tissue specimens
We collected the HL patients' tonsil tissue samples
and matched normal tissues from the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) between 2015 and 2017. The
obtained tissues were snap-frozen at −80°C. The parents of the HL
patients provided written informed consent. The present study
obtained approval from the Ethics Committee of the First Affiliated
Hospital of Zhengzhou University.
Cell transfection
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) was used to transfect
miR-24-3p mimics or inhibitor as well as overexpression vector of
DEDD into HL cells. Plasmids were transfected into HL cell lines by
X-tremeGENE HP DNA Transfection Reagent (Roche Diagnostics, Basel,
Switzerland).
Reverse transcription-quantitative PCR
(RT-qPCR)
The TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to isolate the total RNA from HL
tissues, adjacent normal tissues and cultured L1236 and L428 cells
respectively according to the manufacturer's protocol. The reverse
transcription reaction was then conducted to synthesize cDNAs using
M-MLV Reverse Transcriptase reagent kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). RT-qPCR was conducted by Bio-Rad CFX96™
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with the SYBR-Green kit (Takara Bio, Inc., Otsu,
Japan) using specific primers (Table
I). The conditions for PCR were as follows: 95°C for 5 min, 40
cycles of denaturation at 95°C (15 sec), 50°C (30 sec) and 72°C (30
sec). The miR-24-3p expression was normalized to U6 while the DEDD
expression was normalized to GAPDH. The 2−ΔΔCq method
(17) was used to calculate the
relative expression levels of genes.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Primers | Sequences |
|---|
| miRNA-24-3p | F
5′-AACACACCTATTCAAGGATTCA-3′ |
|
| R
5′-CCCATTCAGCAGGAACAGAAA-3′ |
| U6 | F
5′-CTCGCTTCGGCAGCACA-3′ |
|
| R
5′-AACGCTTCACGAATTTGCGT-3′ |
| DEDD | F
5′-TCCCCAGCCCTCTAAAACAG-3′ |
|
| R
5′-CCGCAGTCTGATGTCACATG-3′ |
| GAPDH | F
5′-TGCACCACCAACTGCTTAGC-3′ |
|
| R
5′-GGCATGGACTGTGGTCATGAG-3′ |
Western blotting
HL cells were lysed by chilled RIPA lysis buffer
(9803; Cell Signaling Technology, Inc., Danvers, MA, USA) which
contained phenylmethylsulfonyl fluoride protease inhibitor. The
lysates were put onto ice for half an hour, then centrifuged at
14,000 × g at 4°C for 15 min. The BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.) was applied to detect the protein
concentration according to the manufacturer's protocol. A total of
10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) was used to separate the proteins and then the separated
proteins were transferred onto a polyvinylidene difluoride (PVDF)
membrane which was incubated at 4°C overnight with primary
antibodies diluted in 5% milk. The primary antibodies were as
follows: rabbit polyclonal antibodies against DEDD (dilution,
1:1,000; cat. no. ab203655; Abcam, Cambridge, MA, USA) and GAPDH
(dilution, 1:1,000, cat. no. ab37168; Abcam). A secondary
incubation step was performed with appropriate secondary antibody
for 1 h at room temperature. The proteins were detected by a
chemiluminescence method. GAPDH (Abcam) was used as an internal
loading control.
Cell Counting kit-8 (CCK-8)
The cell proliferation ability of HL cells was
observed using CCK-8 (Beyotime Institute of Biotechnology, Haimen,
China). HL cells with different transfections were seeded and
cultured in 96-well plates for 0, 12, 24, 48 and 72 h,
respectively, adding 10 µl CCK-8 reagent into the 96-well plates.
At 2 h after being cultured at 37°C, the absorbance of HL cells in
different groups at 450 nm was detected by a microplate reader
(BioTeke, Winooski, VT, USA).
Transwell assays
The migration and invasion abilities of the treated
HL cell lines were assessed by Transwell assays. For the migration
assay, the treated cells (1×105 cells/well) were seeded
in the top chambers, and RPMI-1640 medium containing 20% FBS was
seeded into the bottom chambers, being incubated at 37°C for 24 h
with 5% CO2. After that, the migratory cells were fixed, stained
and counted. The difference between the migration and invasion
assay was that Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was added into the wells.
Luciferase reporter assays
The treated HL cells were co-transfected with
luciferase reporter plasmids which contained wild-type or mutant
3′UTRs of DEDD and miR-24-3p by Lipofectamine 2000. At 48 h after
transfection, the Dual-Luciferase Reporter Assay kit (Promega
Corp., Madison, WI, USA) was used to perform the luciferase
reporter assays.
Statistical analysis
All the above experiments were performed 3 times.
The statistical analysis was evaluated by the GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA) together with SPSS
18.0 version (SPSS, Inc. Chicago, IL, USA). Students t test and one
way ANOVA followed by Tukeys post hoc test were used to analyze two
or multiple groups, respectively. Statistically significant
difference was set at P<0.05.
Results
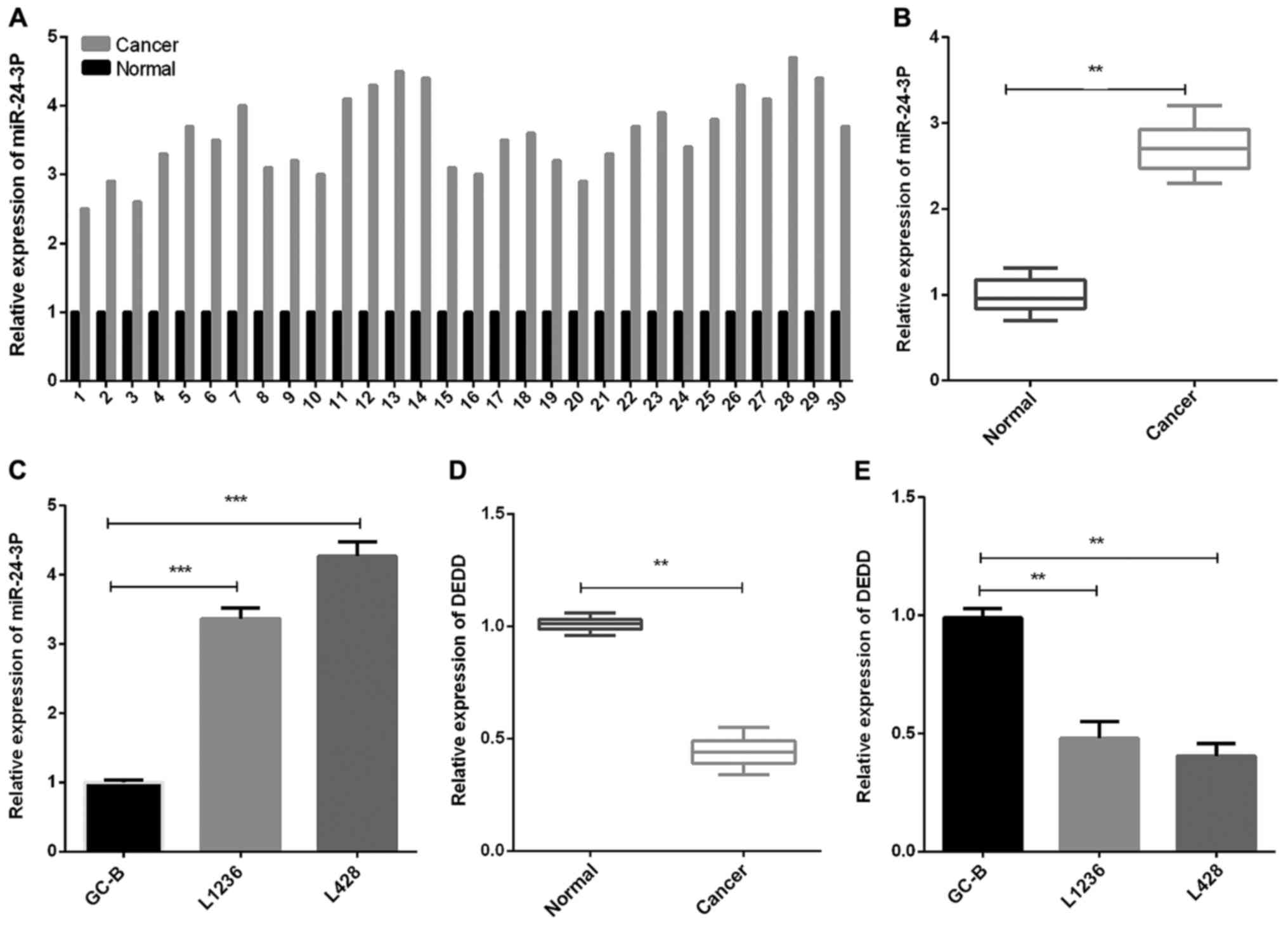
miR-24-3p expression is upregulated
and DEDD expression is downregulated in HL
To evaluate miR-24-3p and DEDD expression levels in
HL, we collected HL tissues and matched normal tissues from 30 HL
patients, and then measured DEDD expression as well as miR-24-3p
expression. The results of RT-qPCR revealed that the miR-24-3p
expression in HL tissues was significantly increased in contrast
with that in the normal control tonsil tissues (Fig. 1A and B, P<0.01). Moreover, we found
that the miR-24-3p expression level in GC-B cells was decreased
compared with that in HL cells (L1236 and L428) (Fig. 1C, P<0.001). The DEDD expression in
HL tissues was significantly decreased compared to normal tonsil
tissues (Fig. 1D, P<0.01).
Furthermore, similar results were found in GC-B and HL cells (L1236
and L428) (Fig. 1E, P<0.01).
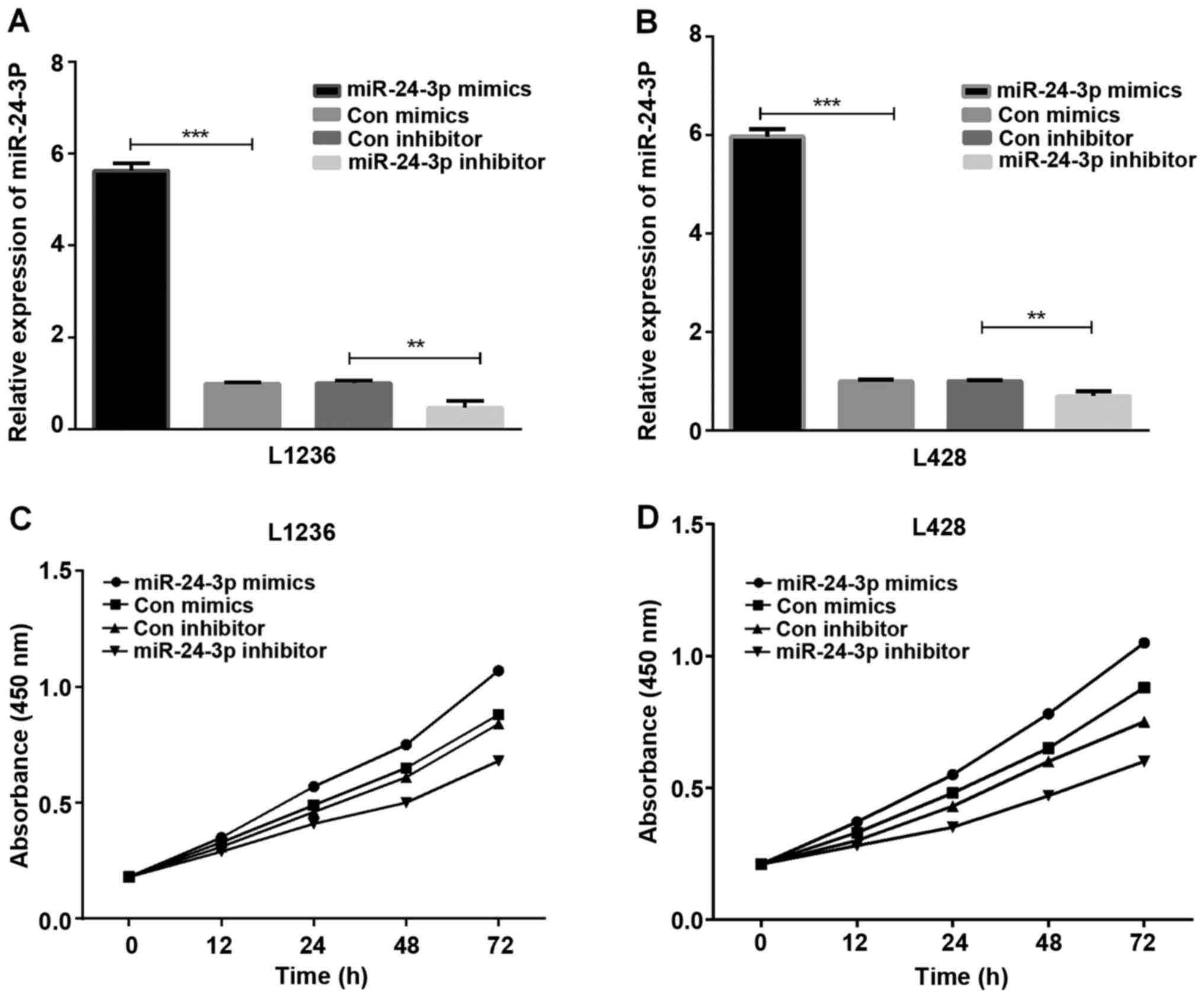
miR-24-3p accelerates HL cell
proliferation
The HL cells (L1236 and L428) transfected with
mimics or inhibitor of miR-24-3p were used for detecting the role
of miR-24-3p in HL cell proliferation. The results of RT-qPCR
assays demonstrated that the miR-24-3p mimic expression was high in
L1236 and L428 cells (Fig. 2A and B,
P<0.001 and <0.01). Subsequently, the CCK-8 assay was
conducted to observe HL cell proliferation ability, and the results
demonstrated that miR-24-3p promoted L1236 cell (Fig. 2C) and L428 cell (Fig. 2D) proliferation.
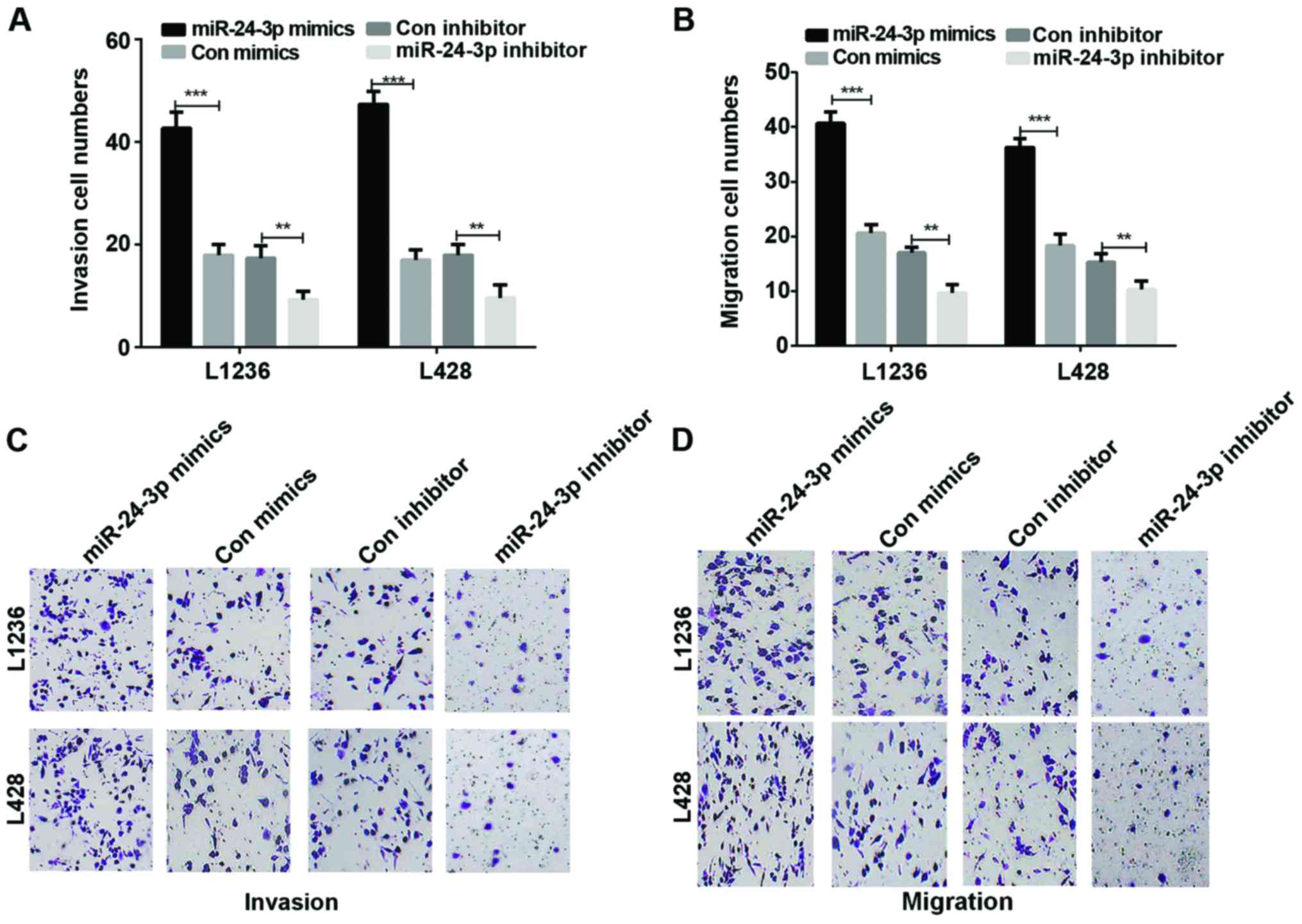
miR-24-3p accelerates HL cell invasion
and migration
We studied cell invasion and migration abilities in
HL cells which were transfected with mimics or inhibitor of
miR-24-3p. The results showed that there was a significant rise in
the invasion of L136 and L428 cells transfected with mimics of
miR-24-3p in contrast with the HL cells transfected with control
mimics (P<0.001). On the contrary, the Transwell results
demonstrated a significant decrease in the invasion of L136 and
L428 cells transfected with inhibitor of miR-24-3p compared to the
control group (Fig. 3A and C,
P<0.001 and <0.01). In addition, according to the Transwell
assays, the results also demonstrated that miR-24-3p promoted HL
migration ability (Fig. 3B and D,
P<0.001 and <0.01).
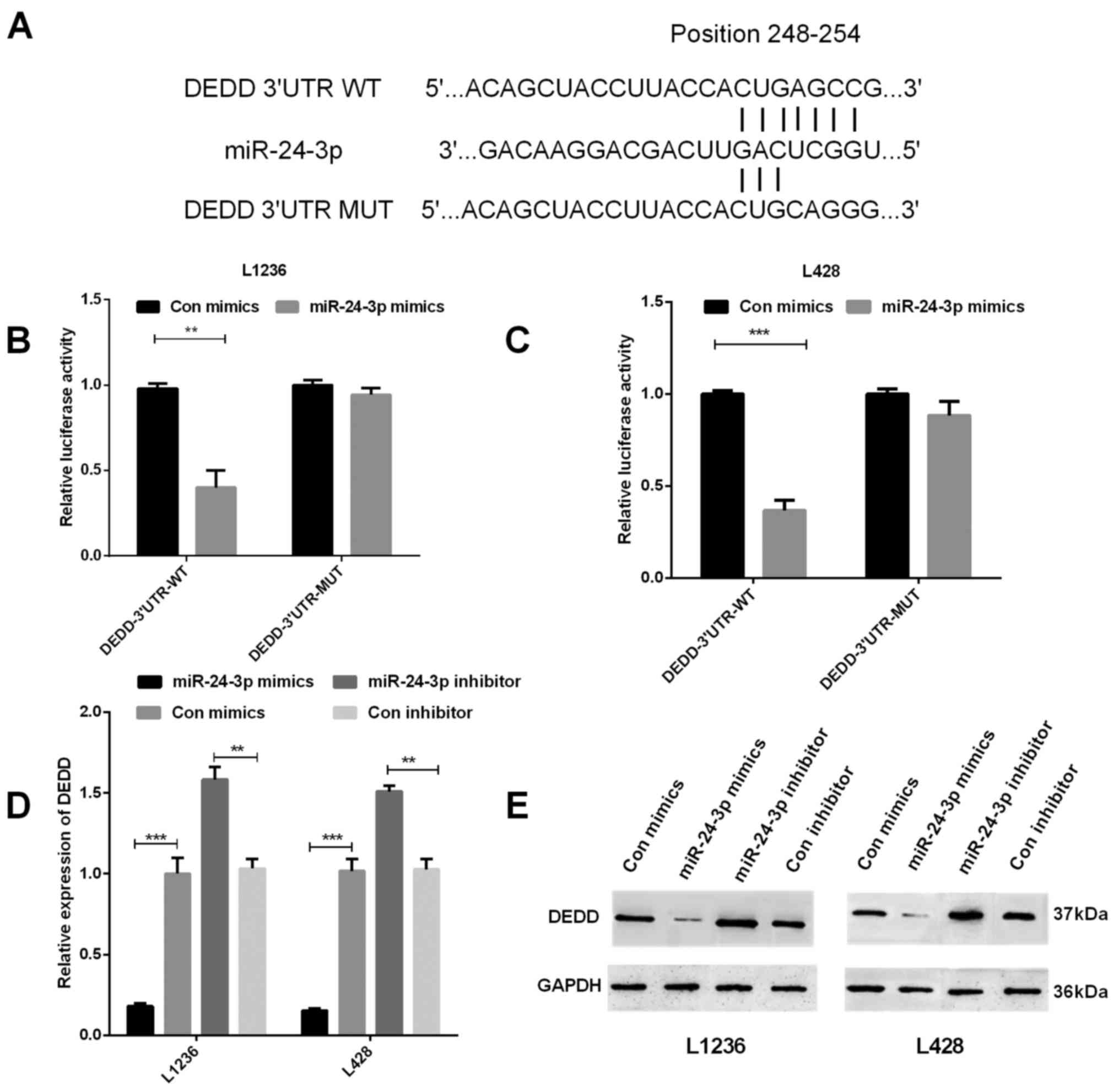
miR-24-3p suppresses DEDD gene
transcription in HL by targeting its 3′UTR
To investigate whether DEDD expression had relevance
to miR-24-3p expression and to better understand the function of
miR-24-3p in HL, TargetScan was used to find the target sites in
the DEDD sequence of miR-24-3p (Fig.
4A). We measured the DEDD 3′UTR luciferase activities by
performing luciferase reporter gene assays. We co-transfected
mimics of miR-24-3p and wild-type DEDD 3′UTR vector or mutant DEDD
3′UTR vector into HL cells to observe whether DEDD was the target
of miR-24-3p, and then detected the role of miR-24-3p in regulating
the mRNA and protein expression of DEDD. The results showed that
there was a significant decrease of fluorescence activity in both
L1236 (P<0.01) and L428 (P<0.001) cells co-transfected with
the wild-type DEDD 3′UTR vector and miR-24-3p in contrast with the
control group, however, between cells co-transfected with the
mutant DEDD 3′UTR vector and miR-24-3p and the control group, there
was no significant difference (Fig. 4B
and C). The results of RT-qPCR and western blotting both
demonstrated that miR-24-3p could inhibit DEDD expression in L1236
and L428 cells (Fig. 4D and E,
P<0.001 and <0.01).
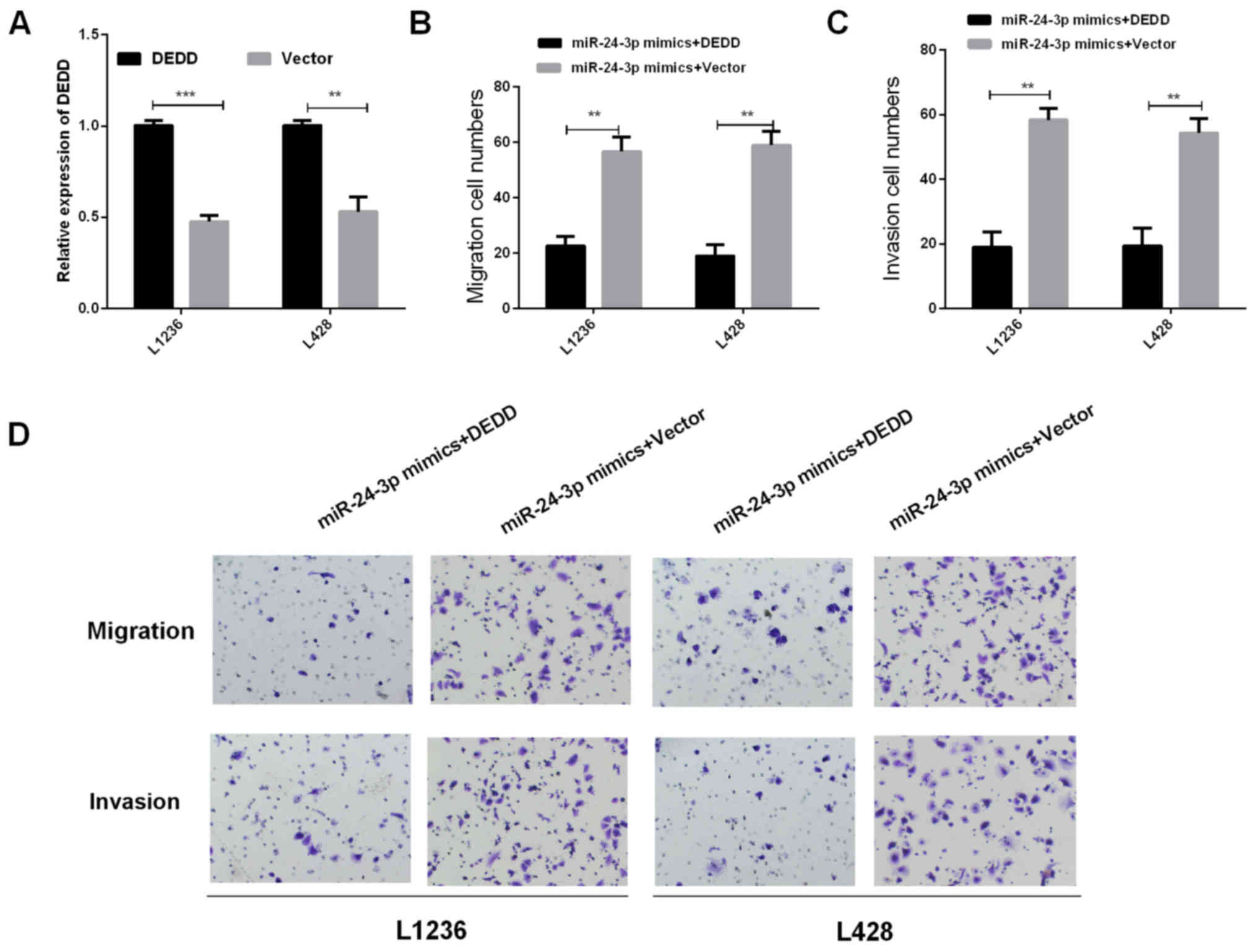
The role of DEDD in regulating
miR-24-3p effects in HL cell migration and invasion
We investigated whether DEDD was required in
regulating miR-24-3p functions of promoting HL cell invasion and
migration. Overexpression vector of DEDD and miR-24-3p mimics were
co-transfected into L1236 and L428 cells using RT-qPCR to detect
the DEDD mRNA expression, the results are shown in Fig. 5A. Then, we detected the migration and
invasion in HL cells which were co-transfected with overexpression
vector of DEDD and miR-24-3p mimics using Transwell assay. The
results revealed that the overexpression of DEDD markedly reversed
miR-24-3p-mediated promotion of cell migration and invasion in HL
cells (Fig. 5B-D, P<0.01).
Collectively, these data suggested that DEDD may reverse partial
function of miR-24-3p in HL cells.
Discussion
HL is a particular B-cell malignancy, accounting for
a large percentage of lymphomas (18). Despite significant advances in the
treatment of HL, existing treatments fail to cure 10–20% of HL
patients. Similarly, 10–20% of HL patients may be over-treated. So,
reliable predictive biomarkers for HL patients who need intensive
therapy remain a challenge. Such biomarkers could also provide
insight into the biology of HL.
Several studies have indicated that different kinds
of miRNAs are involved in different kinds of biological activities,
for instance, cell proliferation, migration, metastasis and
inflammation (19,20). Multiple studies have found that many
different miRNAs play an important role in the development of HL.
For example, miR-124a methylation is associated with aggressive HL
disease (21); miR-374b can suppress
cell proliferation and promote cell apoptosis by inhibiting AKT1
and Wnt-16 in T-cell lymphoblastic lymphoma (22); miR-9 methylation is a common event in
HL and participated in HL pathogenesis (23); overexpression of miR-155 and
inhibition of its target NIAM enhance cell growth in HL (24).
miR-24-3p is one of the most studied miRNAs in
different kinds of tumors. Over the past decade, increasing
evidence has indicated that dysregulation of miRNAs makes
contributions to various aspects of tumorigenesis process.
miR-24-3p is a master regulator in gene regulation and is related
to diverse human disease (25).
Several other studies also revealed an oncogenic role for miR-24-3p
in other types of cancer, for instance, miR-24-3p has been proved
to promote glioma cell proliferation by targeting MXI1 (26); miR-24-3p represses small cell lung
carcinoma VP16-DDP chemoresistance through ATG4A (27). However, as far as we know, there have
been no previous studies which report the mechanism of miR-24-3p in
HL. To explore the functional relevance of miR-24-3p in HL, we
compared its expression level in HL tissues to that in
paracarcinoma tissues. The results demonstrated that the miR-24-3p
expression level in HL tissues was higher than that in
paracarcinoma tissues. In addition, it also indicated that
miR-24-3p could promote HL cell proliferation, invasion and
migration through DEDD.
The present study provides evidence that miR-24-3p
is involved in regulating HL cell proliferation, invasion and
migration. At the same time, miR-24-3p expression was increased and
that of DEDD expression was decreased in HL tissues. Moreover,
miR-24-3p was able to suppress the gene transcription of DEDD.
Hence, the present study demonstrated that miR-24-3p promoted HL
progression via suppressing DEDD. Thus, it is suggested that
miR-24-3p may be a key potential therapeutic target for the
treatment of HL.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW drafted the manuscript. JW, KY and XLv were
mainly devoted to cell culture. JW, QY and MS performed RT-qPCR.
XLi and HS were responsible for western blotting. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China). Signed informed consent was obtained from the parents of
the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gibcus JH, Tan LP, Harms G, Schakel RN, de
Jong D, Blokzijl T, Möller P, Poppema S, Kroesen BJ and van den
Berg A: Hodgkin lymphoma cell lines are characterized by a specific
miRNA expression profile. Neoplasia. 11:167–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vardiman JW: The World Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paydas S, Acikalin A, Ergin M, Celik H,
Yavuz B and Tanriverdi K: Micro-RNA (miRNA) profile in Hodgkin
lymphoma: Association between clinical and pathological variables.
Med Oncol. 33:342016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lima CR, Gomes CC and Santos MF: Role of
microRNAs in endocrine cancer metastasis. Mol Cell Endocrinol.
456:62–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hemmatzadeh M, Mohammadi H, Karimi M,
Musavishenas MH and Baradaran B: Differential role of microRNAs in
the pathogenesis and treatment of esophageal cancer. Biomed
Pharmacother. 82:509–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Q, He M, Ma MT, Wu HZ, Yu ZJ, Guan
S, Jiang LY, Wang Y, Zheng DD, Jin F, et al: MicroRNA-148a inhibits
breast cancer migration and invasion by directly targeting WNT-1.
Oncol Rep. 35:1425–1432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y,
Huang X, Zhang JP, Guan XY and Zhuang SM: MicroRNA-29b suppresses
tumor angiogenesis, invasion, and metastasis by regulating matrix
metalloproteinase 2 expression. Hepatology. 54:1729–1740. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan Y, Kluiver J, Koerts J, de Jong D,
Rutgers B, Abdul Razak FR, Terpstra M, Plaat BE, Nolte IM, Diepstra
A, et al: miR-24-3p is overexpressed in Hodgkin lymphoma and
protects Hodgkin and Reed-Sternberg cells from apoptosis. Am J
Pathol. 187:1343–1355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun X, Xiao D, Xu T and Yuan Y:
miRNA-24-3p promotes cell proliferation and regulates
chemosensitivity in head and neck squamous cell carcinoma by
targeting CHD5. Future Oncol. 12:2701–2712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu G, Jia Z and Dou Z: miR-24-3p regulates
bladder cancer cell proliferation, migration, invasion and
autophagy by targeting DEDD. Oncol Rep. 37:1123–1131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hua F, Xue JF, Lü XX and Hu ZW: DEDD
decreases Smad3 activity, promotes tumor cell apoptosis and
inhibits proliferation. Yao Xue Xue Bao. 48:680–685. 2013.(In
Chinese). PubMed/NCBI
|
|
15
|
Carstens JL, Lovisa S and Kalluri R:
Microenvironment-dependent cues trigger miRNA-regulated feedback
loop to facilitate the EMT/MET switch. J Clin Invest.
124:1458–1460. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv Q, Hua F and Hu ZW: DEDD, a novel tumor
repressor, reverses epithelial-mesenchymal transition by activating
selective autophagy. Autophagy. 8:1675–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Xie X, Yang X, Jiang G and Gu J:
The influence of marital status on the survival of patients with
Hodgkin lymphoma. Oncotarget. 8:51016–51023. 2017.PubMed/NCBI
|
|
19
|
Wang J, Paris PL, Chen J, Ngo V, Yao H,
Frazier ML, Killary AM, Liu CG, Liang H, Mathy C, et al: Next
generation sequencing of pancreatic cyst fluid microRNAs from low
grade-benign and high grade-invasive lesions. Cancer Lett. 356:(2
Pt B). 404–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Leva G and Croce CM: The role of
microRNAs in the tumorigenesis of ovarian cancer. Front Oncol.
3:1532013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ben Dhiab M, Ziadi S, Ksiaa F, Louhichi T,
Ben Gacem R, Ben Zineb A, Amara K, Hachana M and Trimeche M:
Methylation of miR124a-1, miR124a-2, and miR124a-3 in Hodgkin
lymphoma. Tumour Biol. 36:1963–1971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian D, Chen K, Deng H, Rao H, Huang H,
Liao Y, Sun X, Lu S, Yuan Z, Xie D, et al: MicroRNA-374b suppresses
proliferation and promotes apoptosis in T-cell lymphoblastic
lymphoma by repressing AKT1 and Wnt-16. Clin Cancer Res.
21:4881–4891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ben Dhiab M, Ziadi S, Louhichi T, Ben
Gacem R, Ksiaa F and Trimeche M: Investigation of miR9-1, miR9-2
and miR9-3 methylation in Hodgkin lymphoma. Pathobiology.
82:195–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slezak-Prochazka I, Kluiver J, de Jong D,
Smigielska-Czepiel K, Kortman G, Winkle M, Rutgers B, Koerts J,
Visser L, Diepstra A, et al: Inhibition of the miR-155 target NIAM
phenocopies the growth promoting effect of miR-155 in B-cell
lymphoma. Oncotarget. 7:2391–2400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chhabra R, Dubey R and Saini N:
Cooperative and individualistic functions of the microRNAs in the
miR-23a~27a~24-2 cluster and its implication in human diseases. Mol
Cancer. 9:2322010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K,
Xu H and Jiang S: miR-24-3p and miR-27a-3p promote cell
proliferation in glioma cells via cooperative regulation of MXI1.
Int J Oncol. 42:757–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan B, Chen Y, Song H, Xu Y, Wang R and
Chen L: Mir-24-3p downregulation contributes to VP16-DDP resistance
in small-cell lung cancer by targeting ATG4A. Oncotarget.
6:317–331. 2015. View Article : Google Scholar : PubMed/NCBI
|