Introduction
Leupaxin (LPXN) was cloned from a human macrophage
cDNA library and was identified as a new member of the paxillin
family by Lipsky et al in 1998 (1). The relative molecular mass of LPXN is 43
kDa, and the protein is primarily expressed in the cellular
cytoplasm of leukemia, prostate cancer, breast cancer, melanoma and
other types of tumor cells (2).
Similar to paxillin, LPXN is localized in the focal adhesion plaque
(FAP), serving as a molecular adaptor involved in integrin-mediated
signaling and as a regulator for proliferation, differentiation,
adhesion and migration (3).
A number of systematic and in-depth studies on LPXN
and prostate cancer have been performed. In the prostate cancer
cell lines PC-3, DU 145 and LNCaP, it has been identified that the
expression levels of LPXN were associated with the degree of
malignancy of the cells (4,5). Upregulating LPXN expression has been
revealed to promote invasion and metastasis of prostate cancer
cells, whereas downregulating LPXN expression by RNA interference
(RNAi) has been revealed to stimulate the isolation and spontaneous
apoptosis of the aforementioned cancer cells (6). Similarly, Chen et al (2) identified that increased expression of
LPXN promoted the migration of MDA-MB-231 breast cancer cells to
the extracellular matrix.
The association of LPXN and leukemia has also gained
increasing attention, as Petti et al (7) utilized the thiophene kinase inhibitor
OSI-930 to selectively inhibit tyrosine phosphorylation of LPXN,
p130Cas and focal adhesion kinase (FAK), which led to
apoptosis of the HMC-1 mast cell leukemia line. Tanaka et al
(8) revealed that when LPXN was
expressed in human leukocytic K562 cells, LPXN significantly
suppressed integrin α5β1-mediated cell adhesion to fibronectin and
inhibited tyrosine phosphorylation of paxillin. In addition, Dai
et al (9) observed that LPXN
was fused to runt-related transcription factor 1 in a patient with
acute myeloid leukemia with a t(11;21)(q12; q22) translocation.
Additionally, Abe et al (10)
reported the generation of the ETV6-LPXN fusion transcript by
t(11;12)(q12.1; p13) in a patient with relapsing acute myeloid
leukemia and indicated that ETS variant 6-LPXN serves a crucial
function in leukemia progression. Taken together, the
aforementioned results indicated that the expression and
phosphorylation of LPXN promote proliferation, invasion and
metastasis of leukemia cells, and also an association with the
occurrence and the development of leukemia.
To the best of our knowledge, the present study is
the first attempt to downregulate LPXN expression by RNAi and to
investigate the possible downstream effects and molecular
mechanisms on proliferation and invasion of the human acute
monocytic leukemia SHI-1 cell line in vitro. An increased
protein expression level of LPXN in SHI-1 cells has been reported,
in addition to higher secretion levels of matrix metalloproteinase
(MMP)-2 and MMP-9 gelatinase, where a marked migration ability and
tumorigenicity has been observed in nude mice (11).
Materials and methods
Reagents
Iscove's modified Dulbecco's medium (IMDM),
Opti-MEM® I reduced serum medium and fibronectin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). Matrigel and
Transwell chambers were purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The antibodies used were against LPXN (dilution,
1:500; ab67571; Abcam, Cambridge, UK), β-actin (dilution, 1:3,000;
#4967S; Cell Signaling Technology, Inc. Danvers, MA, USA) and
p-mitogen-activated protein kinase (p-MAPK; dilution, 1:1,000;
#9910; Cell Signaling Technology, Inc.). The small interfering RNA
(siRNA) sequences of fluorescein amidite (FAM)-siRNA, LPXN-siRNA
and luciferase-siRNA were synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China).
Cell culture
The leukemia cell line SHI-1 was gifted by the
Jiangsu Institute of Haematology, First Affiliated Hospital of
Soochow University (Suzhou, China) and was originally derived from
the mononuclear cells of the bone marrow from a patient with acute
monocytic leukemia in relapse. The cells were maintained as a
stable cell line in vitro (11).
SHI-1 cells were incubated in IMDM with 15% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. Cells in the exponential growth phase following
passaging every 3–4 days were used for the subsequent
experiments.
Transfection of SHI-1 cells with
siRNA
Specific siRNAs of the LPXN gene were designed
according to a previously described protocol (12). Transfection of SHI-1 cells was
accomplished using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol with the following different LPXN gene-specific siRNA
duplexes: L1-siRNA, sense, 5′-UAUUCCAACCCAGCUCCUC-3′ and antisense,
5′-GAGGAGCUGGGUUGGAAUA-3′; L2-siRNA, sense,
5′-GGCGCAGCUCGUGUAUACUACCAAU-3′ and antisense,
5′-AUUGGUAGUAUACACGAGCUGCGCC-3′ (Shanghai GenePharma Co., Ltd.).
The negative control group was transfected with siRNA duplex
oligonucleotides against the firefly luciferase gene (N-siRNA)
(Shanghai GenePharma Co., Ltd.) and the positive control group was
transfected with FAM-siRNA in SHI-1 cells.
To improve the transfection efficiency, several
modifications to the transfection protocol were performed. Briefly,
prior to plating the cells in 400 µl Opti-MEM® I in
24-well dishes, 4×105 SHI-1 cells were washed with
serum-free IMDM. Prior to combining the reagents, the siRNA
oligomer and Lipofectamine 2000 were diluted in 50 µl
Opti-MEM® I. The transfection efficiency was assessed
with FAM-siRNA using flow cytometry (FCM), as previously described
by Wang et al (11).
Western blot analysis
According to the manufacturer's protocol of Membrane
and Cytosol Protein Extraction kit (Beyotime Institute of
Biotechnology, Nanjing, China), total cellular extracts were
collected and the protein concentration was determined after
transfection 48 h. Equal amounts of extracted proteins were
subjected to western blotting and the specific experiments were
conducted as previously described (13). Following SDS-PAGE (5% concentration
gel and 10% separation gel) electrophoresis and membrane transfer
to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA), the proteins were blocked with 5% bovine serum albumin
(Beyotime Institute of Biotechnology) at room temperature for 2 h
and then incubated with the primary antibodies against LPXN
(Abcam), phosphorylated (p)-c-Jun, N-terminal kinase (p-JNK), p-p38
MAPK, p-extracellular-signal-regulated kinase (p-ERK) (Phospho-MAPK
Family Antibody Sampler kit; dilution, 1:1,000; cat. no. 9910) and
β-actin (dilution, 1:3,000; cat. no. 4967S) (both from Cell
Signaling Technology, Inc.) at 4°C overnight separately. The
membranes were washed three times in TBS with 0.1% Tween-20 and
subsequently probed with HRP-conjugated goat anti-mouse IgG
(dilution, 1:10,000; cat. no. 7072; Cell Signaling Technology,
Inc.) at room temperature for 2 h. Following incubation, the blots
were washed three times in TBS with 0.1% Tween-20, enhanced
chemiluminescent reagents (Beyotime Institute of Biotechnology)
were added and scanned with an ImageQuant LAS 4000 Mini (GE
Healthcare, Chicago, IL, USA).
CCK-8 assay
A CCK-8 assay was used to determine proliferation
according to the manufacturer's protocol. SHI-1 cells were
transfected with siRNA against LPXN and the luciferase gene,
serving as the negative control, as aforementioned. Following
incubation with the transfection medium at room temperature. for 24
h, cells were harvested and seeded in a 96-well plate at
4×105 cells/well with three wells of each group. Cells
were incubated for an additional 48 h and the spectrophotometric
absorbance of each sample was determined at a wavelength of 450 nm,
following the addition of 10% CCK-8 for 3 h.
Cell invasion assay
A Costar Transwell chamber system was used to
determine cell invasion. Membrane filters with a pore size of 8 µm
and a diameter of 6.5 mm were coated with 50 µl Matrigel and
subsequently placed into the wells forming the upper chamber.
Following transfection for 24 h, the siRNA-treated SHI-1 cells were
suspended in 200 µl serum-free IMDM and were seeded at a final
density of 2.0×105 cells/well in the upper chamber. The
lower chamber of invasion was filled with 800 µl IMDM, supplemented
with 10% human serum, containing 100 ng/ml stromal-cell-derived
factor 1-α as a source of chemoattractants. The chambers were
incubated for 24 h at 37°C with 5% CO2. The cells which
had migrated into the lower compartment were counted by inverted
optical microscope (magnification, ×400; Leica Microsystems GmbH,
Wetzlar, Germany) and the invasion rate was calculated by comparing
the cell number in the lower compartment medium with the total
number of leukemia cells, 2.0×105 cells, loaded into the
upper compartment.
Gelatin zymography
Cell culture supernatants were analyzed for the
presence of the secreted MMP-2 and MMP-9 gelatinases using
zymography. SHI-1 cells were transfected with siRNA against LPXN
and the luciferase gene, serving as the negative control, as
aforementioned. Following incubation with the transfection medium
for 24 h, SHI-1 cells were harvested and seeded in a 96-well plate
at 4×105 cells/well with serum-free IMDM. The
supernatant of each sample was harvested after the 24 h incubation.
The samples were separated by SDS-PAGE (10% gels) copolymerized
with 0.1% gelatin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at 4°C and were incubated at 37°C with developing buffer containing
0.02% Brij-35 (Sigma-Aldrich; Merck KGaA), according to our
previously published protocol (13).
Gelatinase-digested regions were visualized as light bands against
a blue background at 37°C under natural light.
Statistical analyses
All experiments were repeated at least three times
and the data were expressed as the mean ± standard deviation. The
image processing software Photoshop CS3 (Adobe Systems Inc., San
Jose, CA, USA) and statistical software SPSS (version 15.0; SPSS,
Inc., Chicago, IL, USA) was used for analysis. Student's t-test was
used to compare the means of two groups and one-way analysis of
variance was used to compare means of multiple samples followed by
the Least-Significant-Difference and Dunnett's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FAM-siRNA transfection of SHI-1
cells
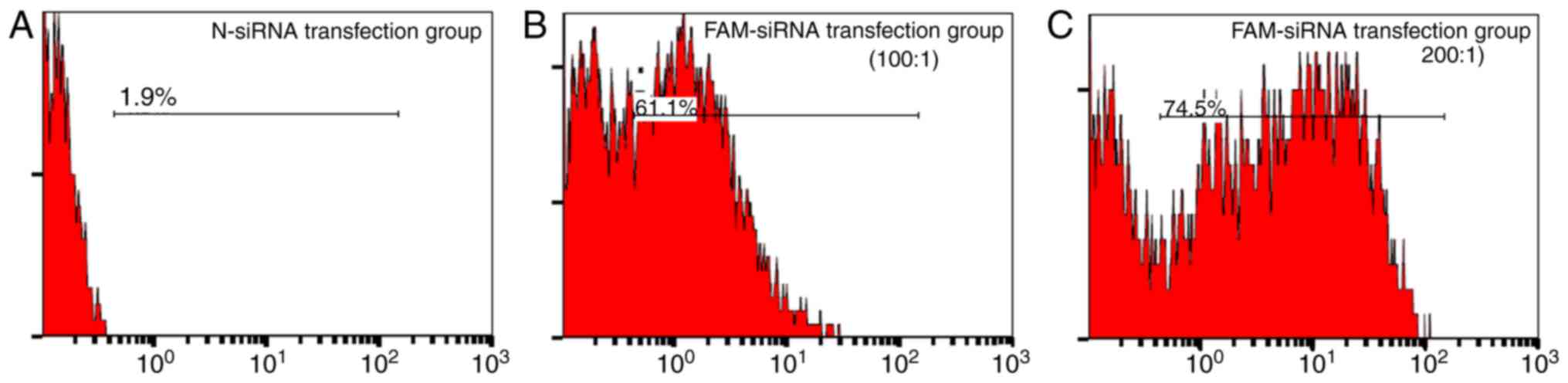
Among the repeated experiments, the highest
transfection efficiency reached 74.5%, when the SHI-1 cells were
seeded at a density of 4×105 cells/ml and the mixing
ratio of FAM-siRNA/Lipofectamine 2000 was 200 pmol/1 µl (Fig. 1). Therefore, these conditions were
used for transfection of LPXN siRNA in SHI-1 cells.
L2-siRNA downregulates LPXN expression
in SHI-1 cells
After 48 h of transient transfection of L1-siRNA and
L2-siRNA, the protein expression of LPXN was detected using western
blot analysis. As indicated in lane 1 (200 pmol/1 µl) and lane 2
(100 pmol/1 µl) in Fig. 2,
transfection of L1-siRNA had no effect on the expression of the
LPXN protein. Similarly, lane 3 (200 pmol/1 µl) and lane 4 (100
pmol/1 µl) of the N-siRNA, negative control group, did not change
LPXN expression levels. In contrast, transfection of L2-siRNA,
indicated in lanes 5 (200 pmol/1 µl) and 6 (100 pmol/1 µl),
downregulated LPXN expression in SHI-1 cells compared with the
negative control group. In particular, when the transfection ratio
of L2-siRNA/Lipofectamine 2000 was 200 pmol/1 µl, LPXN expression
was effectively downregulated, as indicated in lane 5. Therefore,
in subsequent experiments, L2-siRNA was transfected at the optimal
ratio of 200 pmol L2-siRNA/1 µl Lipofectamine 2000 to investigate
the effect of LPXN on the proliferation and invasion of SHI-1
cells.
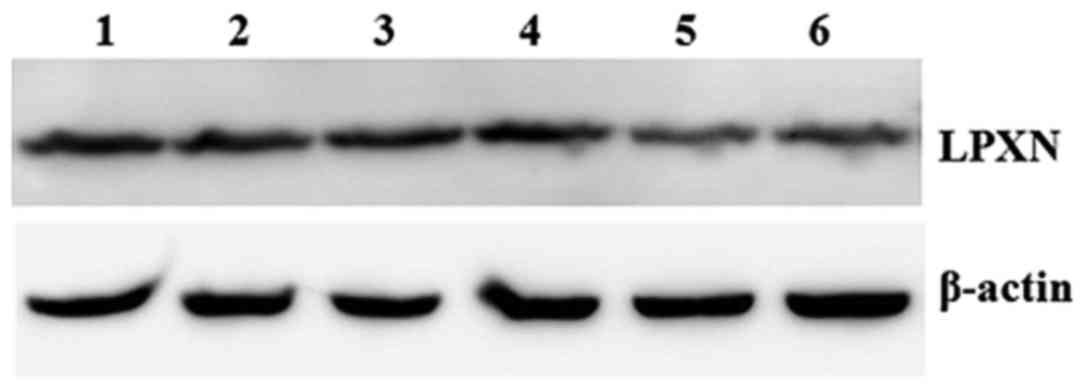 | Figure 2.Effects of two different siRNAs,
L1-siRNA and L2-siRNA, on LPXN expression in SHI-1 cells detected
by western blot analysis. LPXN expression was downregulated when
the transfection ratio of L2-siRNA/Lipofectamine 2000 was 200
pmol/1 µl. Lane 1, siRNA/Lipofectamine 2000 at 200 pmol/1 µl; lane
2, L1-siRNA/Lipofectamine 2000 at 100 pmol/1 µl; lane 3,
N-siRNA/Lipofectamine 2000 at 200 pmol/1 µl; lane 4,
N-siRNA/Lipofectamine 2000 at 100 pmol/1 µl; lane 5,
L2-siRNA/Lipofectamine 2000 at 200 pmol/1 µl; lane 6,
L2-siRNA/Lipofectamine 2000 at 100 pmol/1 µl. siRNA, small
interfering RNA; LPXN, leupaxin. |
Downregulating LPXN expression
decreases SHI-1 proliferation and transmembrane invasion
The effective L2-siRNA and N-siRNA negative control
were transfected into SHI-1 cells at a ratio of 200 pmol siRNA/1 µl
Lipofectamine 2000. The proliferation of SHI-1 cells was determined
using the CCK-8 assay followed by transient transfection for 48 h.
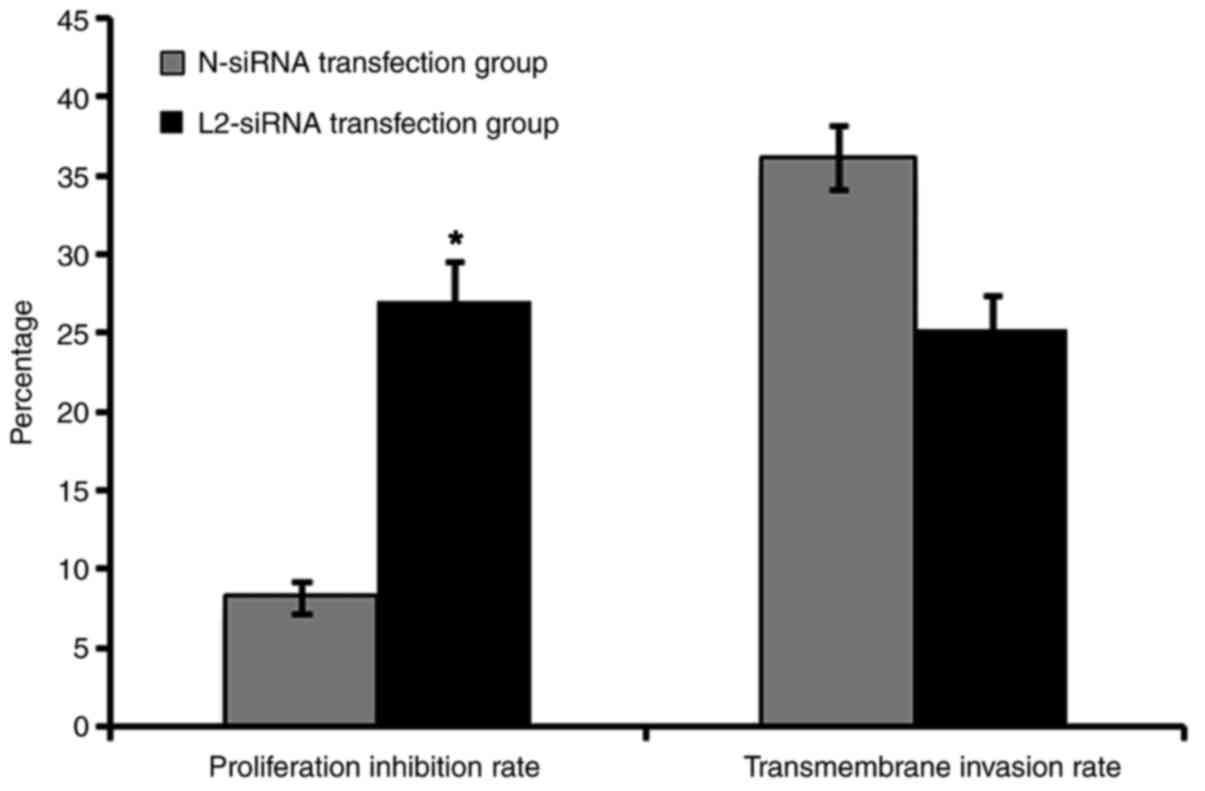
The inhibition proliferation rate of the L2-siRNA transfection
group was 27.043±2.051%, compared with that of the negative control
group of 8.247±1.003% (Fig. 3). In
addition, there was a significant difference between these two
groups (P<0.05), which indicated that transfection with L2-siRNA
decreased the proliferation rate in SHI-1 cells by downregulating
LPXN expression.
Furthermore, following transfection with L2-siRNA
and N-siRNA for 24 h, the SHI-1 cells were collected and plated in
Transwell chambers to determine the transmembrane invasion rate of
each group. The transmembrane invasion rate of the L2-siRNA
transfection group was 25.270±2.145 and the invasion rate of the
N-siRNA control group was 36.112±2.103 (Fig. 3). The transfection efficiency of siRNA
in suspension cells was not high; however, the results of the
present study indicated that downregulating the expression of LPXN
by L2-siRNA decreased the invasion of SHI-1 cells.
Downregulating the expression of LPXN
decreases the gelatinase levels of MMP-2 and MMP-9 in the culture
supernatant of SHI-1 cells
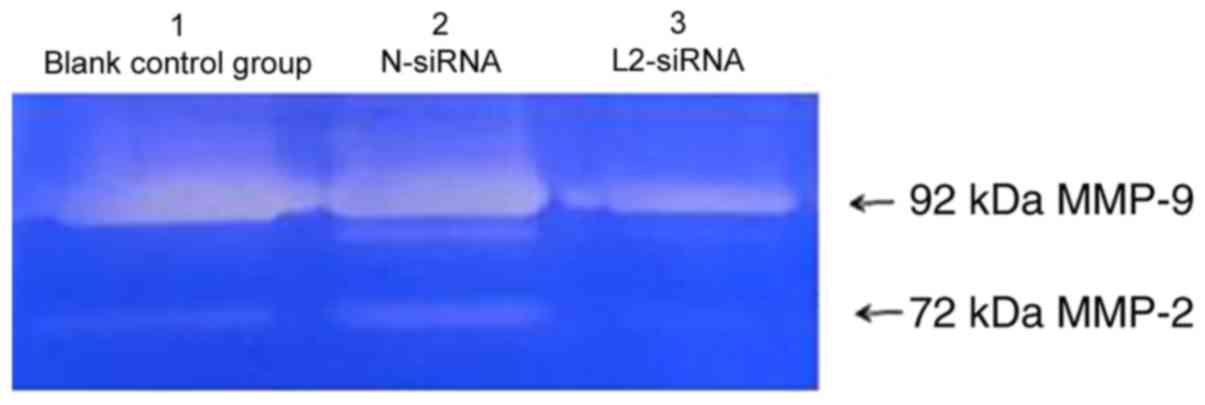
To investigate further the effect of LPXN on
invasion, the culture supernatant of SHI-1 cells transfected with
L2-siRNA and N-siRNA was collected, and the gelatinase level was
analyzed by gelatin zymography. There were two white bands in the
gelatin, which were identified as MMP-2 (72 kDa) and MMP-9 (92 kDa)
(Fig. 4). The gelatinase levels of
MMP-2 and MMP-9 between the negative control group in lane 2,
transfected with N-siRNA, and the blank control group in lane 1,
without any siRNA, remained relatively unchanged (Fig. 4). However, the gelatinase levels in
the supernatant of the L2-siRNA transfection group in lane 3 were
decreased compared with the aforementioned control groups (Fig. 4). Previous studies have indicated that
MMP-2 and MMP-9 serve important functions in the invasion process
of various tumors (11,13). Therefore, these results indicated that
downregulating LPXN expression by L2-siRNA potentially decreased
the secretion of MMP-2 and MMP-9, weakening the invasion of SHI-1
cells.
Downregulating LPXN expression
activates p-JNK and p-p38 MAPK
MAPKs are serine/threonine protein kinases that have
been reported to serve an important function in extrinsic and
intrinsic signal transduction pathways, and regulate proliferation,
apoptosis and invasion (13). In the
present study, the protein expression of p-ERK, p-JNK and p-p38
MAPK were detected by western blotting after 48 h of transient
transfection with L2-siRNA and N-siRNA. In the blots presented in
Fig. 5A, lane 1 was the blank control
group without any siRNA, lane 2 was the negative control group with
N-siRNA transfection and lane 3 was the L2-siRNA transfection
group. The expression level of p-ERK in these three groups remained
unchanged; however, the expression level of p-p38 MAPK and p-JNK
was increased in the L2-siRNA transfection group (lane 3) compared
with the aforementioned control groups (lanes 1 and 2).
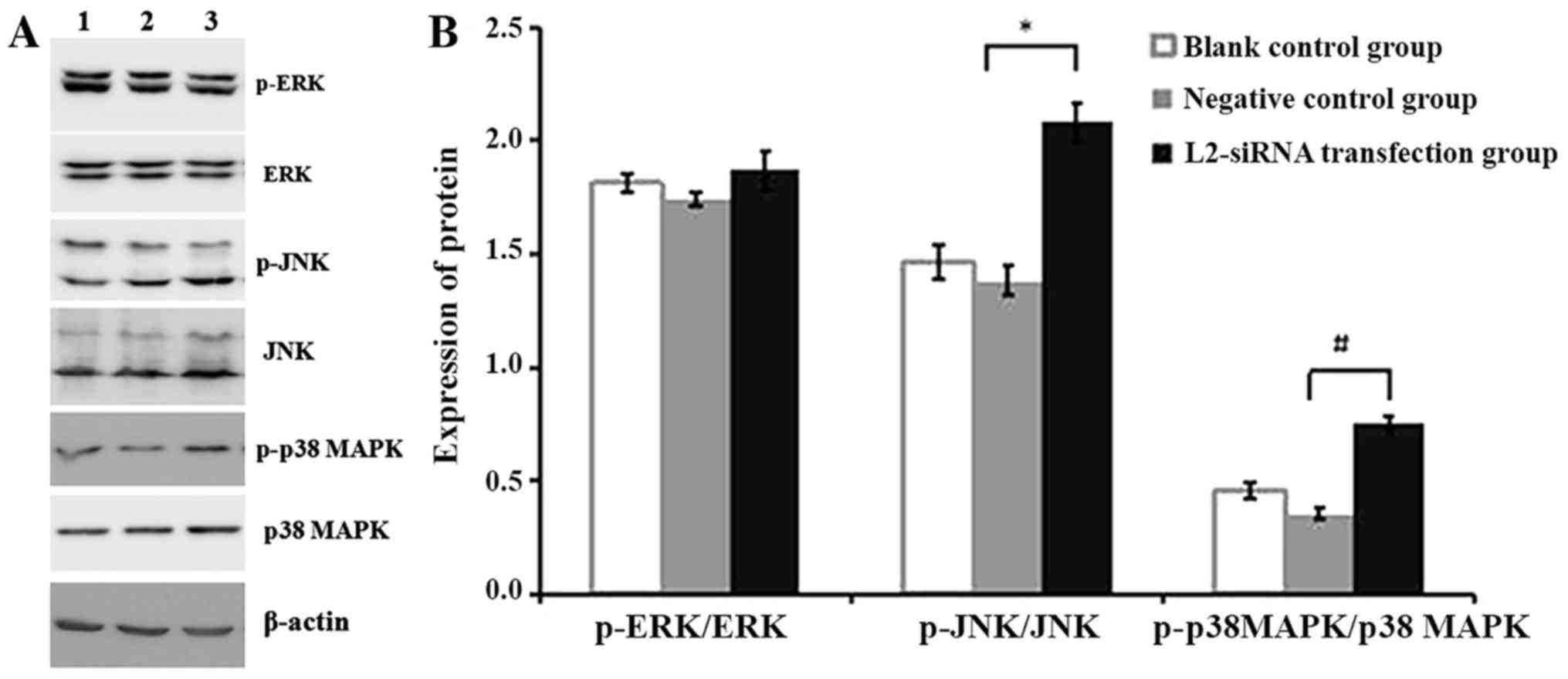 | Figure 5.(A) Expression levels of p-ERK, ERK,
p-JNK, JNK, p38 MAPK and p-p38 MAPK protein detected by western
blot analysis following transfection of SHI-1 cells with L2-siRNA
and N-siRNA. Lane 1, blank control group of SHI-1 cells without
siRNA; lane 2, negative control group N-siRNA-transfected SHI-1
cells; lane 3, L2-siRNA transfection group of SHI-1 cells. (B)
Expression levels of p-ERK, p-JNK and p-p38 MAPK were analyzed by
Photoshop CS3, following transfection of SHI-1 cells with L2-siRNA
and N-siRNA. The data are expressed as the mean ± standard
deviation of three independent experiments. *P<0.05 vs. the
negative control group; #P<0.01 vs. the negative
control group. siRNA, small interfering RNA; N, negative control;
p, phosphorylated; JNK, c-Jun N-terminal kinase; p38 MAPK,
mitogen-activated protein kinase 38; ERK, mitogen-activated protein
kinase 1. |
In addition, Photoshop CS3 and SPSS software were
used to analyze the results for the expression level of p-MAPK. No
difference in the expression levels of ERK, JNK and p38 MAPK
between the negative group and the blank control group was
identified. However, there were significant differences in the
expression level of p-JNK and p-p38 MAPK between the negative group
and the L2-siRNA transfection group (P<0.05; Fig. 5B). The increase in the p-JNK and p-p38
MAPK expression level may not be apparent, due to the fact that the
transfection efficiency of siRNA in SHI-1 cell suspensions was not
high; however, the changes remained statistically significant.
Therefore, it was hypothesized that downregulating the expression
of LPXN by L2-siRNA activated p-JNK and p-p38 MAPK to inhibit SHI-1
proliferation and invasion.
Discussion
LPXN is an important cytoskeletal protein that has
been identified to be involved in integrin-mediated signal
transduction (14,15). When integrin binds to its
intracellular ligand, the cytoskeletal proteins, involving
paxillin, LPXN, talin and vinculin, aggregate to form FAPs and
activate FAK. Activated FAK can subsequently trigger downstream
signal transduction pathways, such as Ras/MAPK, phosphoinositide
3-kinase (PI3K)/protein kinase B (Akt) and signal transducer and
activator of transcription 1 (STAT1), to promote tumor cell
proliferation, invasion and to inhibit tumor cell apoptosis
(16–19).
Previous research on the function of LPXN in tumors
has been primarily focused on prostate cancer. Previous studies,
such as Sahu et al (20) and
Kaulfuss et al (12),
identified that LPXN is highly expressed in PC-3 and DU 145
prostate cancer cells with marked invasiveness, whereas its
expression level is relatively decreased in non-invasive prostate
cancer LNCaP cells.
It has been demonstrated that downregulating LPXN
expression by RNAi in LNCaP cells promotes cancer cell separation
and spontaneous apoptosis (12). In
addition, it has been identified that interference or knockout of
the LPXN gene in the prostate cancer PC-3 and DU 145 cell lines is
able to decrease the invasiveness of the aforementioned tumor cell
types (21). The specific underlying
molecular mechanism in these prostate cancer cells has been
reported as follows: LPXN, actin, paxillin and other focal adhesion
proteins accumulate in FAPs and interact with FAK, protein tyrosine
kinase 2β, c-Src protein kinases and the protein tyrosine
phosphatase non-receptor to activate the downstream signaling
pathway involving Ras/MAPK, PI3K/Akt, STAT1, p53 and GTPase, which
ultimately increase proliferation, adhesion and invasion in
prostate cancer cells (22,23). Similarly, Petti et al (7) revealed that the OSI-930 thiophene
inhibitor weakened the activation of Ras/Raf/ERK, PI3K and
STAT3/5/6. In addition, it promoted apoptosis in cells and
inhibited cellular proliferation, migration and invasion in HMC-1
mast cell leukemia cells by downregulating the phosphorylation of
paxillin, LPXN, FAK and Lyn proto-oncogene Src family tyrosine
kinase (7).
In the present study, the siRNA sequence that
downregulated the high expression of the LPXN gene in SHI-1 cells
was selected, and the effects on proliferation and invasion were
observed. Using FAM-siRNA and repeatedly transfecting it into SHI-1
cells, it was observed that the optimal transfection conditions
included a cell density of 4×105 cells/ml and a
siRNA/Lipofectamine 2000 ratio of 200 pmol/1 µl. The highest
transfection efficiency was 74.5%. In addition, the effect of
L2-siRNA was examined by western blotting and it was indicated that
L2-siRNA decreased the protein expression of LPXN in SHI-1 cells.
The present study determined that downregulating LPXN expression by
L2-siRNA in SHI-1 cells could inhibit the secretion of MMP-2 and
MMP-9, activate JNK and p38 MAPK, and weaken the malignant
proliferation and the transmembrane invasive ability of SHI-1 cells
in vitro.
Chen et al (24) identified that by increasing the
expression of paxillin, proliferation and migration of AGS gastric
cancer cells could be upregulated, whereas decreasing the
expression of paxillin inhibited the proliferation and migration of
SGC7901 gastric cancer cells. In addition, Chew and Lam (25) identified that overexpression of LPXN
inhibited the activation of JNK and p38 MAPK in mouse A20 B
lymphocytes, thereby attenuating the secretion of interleukin-2 and
the function of B cells. The results of the aforementioned studies
were consistent with those of the present study, as downregulation
of LPXN expression also weakened the proliferation and the
transmembrane invasion of leukemic SHI-1 cells, potentially by
activating JNK and p38 MAPK. Transfection conditions of FAM-siRNA
were optimized using FCM and the effects of L2-siRNA on LPXN
expression in SHI-1 cells were screened by western blot analysis.
The results of the present study suggested that members of the
paxillin family may affect the proliferation and invasion of tumor
cells by regulating the activity of MAPK.
Acknowledgements
The authors would like to thank the First Affiliated
Hospital of Soochow University, who provided the SHI-1 cell
lines.
Funding
The present study was supported by the National
Natural Science Foundation of China, Beijing, China (grant no.
81000222) and the Natural Science Foundation for the Youth of
Jiangsu, Nanjing, China (grant no. BK20161052).
Availability of data and materials
The datasets and analyze during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
QS and GHZ designed the experiments and worked on
the manuscript. HPD, QZ and GHZ performed the experiments. HPD and
QS assisted in the data analysis. All authors discussed the results
and commented on the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lipsky BP, Beals CR and Staunton DE:
Leupaxin is a novel LIM domain protein that forms a complex with
PYK2. J Biol Chem. 273:11709–22713. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen PW and Kroog GS: Leupaxin is similar
to paxillin in focal adhesion targeting and tyrosine
phosphorylation but has distinct roles in cell adhesion and
spreading. Cell Adh Migr. 4:527–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deakin NO, Pignatelli J and Turner CE:
Diverse roles for the paxillin family of proteins in cancer. Genes
Cancer. 3:362–370. 2015. View Article : Google Scholar
|
|
4
|
Kaulfuss S, von Hardenberg S, Schweyer S,
Herr AM, Laccone F, Wolf S and Burfeind P: Leupaxin acts as a
mediator in prostate carcinoma progression through deregulation of
p120catenin expression. Oncogene. 28:3971–3982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe N, Amano N, Ishizuka H and
Mashima K: Leupaxin binds to PEST domain tyrosine phosphatase PEP.
Mol Cell Biochem. 269:13–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundberg-Smith LJ, DiMichele LA, Sayers
RL, Mack CP and Taylor JM: The LIM protein leupaxin is enriched in
smooth muscle and functions as an serum response factor cofactor to
induce smooth muscle cell gene transcription. Circ Res.
102:1502–1511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petti F, Thelemann A, Kahler J, McCormack
S, Castaldo L, Hunt T, Nuwaysir L, Zeiske L, Haack H, Sullivan L,
et al: Temporal quantitation of mutant kit tyrosine kinase
signaling attenuated by a novel thiophene kinase inhibitor OSI-930.
Mol Cancer Ther. 4:1186–1197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka T, Moriwaki K, Murata S and
Miyasaka M: LIM domain-containing adaptor, leupaxin, localizes in
focal adhesion and suppresses the integrin-induced tyrosine
phosphorylation of paxillin. Cancer Sci. 101:363–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai HP, Xue YQ, Zhou JW, Li AP, Wu YF, Pan
JL, Wang Y and Zhang J: LPXN, a member of the paxillin superfamily,
is fused to RUNX1 in an acute myeloid leukemia patient with a
t(11;21)(q12;q22) translocation. Genes Chromosomes Cancer.
48:1027–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abe A, Yamamoto Y, Iba S, Kanie T, Okamoto
A, Tokuda M, Inaguma Y, Yanada M, Morishima S, Mizuta S, et al:
ETV6-LPXN fusion transcript generated by t(11;12) (q12.1;p13) in a
patient with relapsing acute myeloid leukemia with NUP98-HOXA9.
Genes Chromosomes Cancer. 55:242–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang CL, Chen ZX, Li ZJ and Cen J: The
essential roles of matrix metalloproteinase-2, membrane type 1
metalloproteinase and tissue inhibitor of metalloproteinase-2 in
the invasive capacity of acute monocytic leukemia SHI-1 cells. Leuk
Res. 34:1083–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaulfuss S, Grzmil M, Hemmerlein B, Thelen
P, Schweyer S, Neesen J, Bubendorf L, Glass AG, Jarry H, Auber B,
et al: Leupaxin, a novel coactivator of the androgen receptor, is
expressed in prostate cancer and plays a role in adhesion and
invasion of prostate carcinoma cells. Mol Endocrinol. 22:1606–1621.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q and
Zhai YL: Curcumin induces apoptosis and suppresses invasion through
MAPK and MMP signaling in human monocytic leukemia SHI-1 cells.
Pharm Biol. 54:1303–1311. 2016.PubMed/NCBI
|
|
14
|
Schaller MD: Paxillin: A focal
adhesion-associated adapter protein. Oncogene. 20:6459–6472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Nimwegen MJ and van de Water B: Focal
adhesion kinase: A potential target in cancer therapy. Biochem
Pharmacol. 73:597–609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calderwood DA, Shattil SJ and Ginsberg MH:
Integrins and actin filaments: Reciprocal regulation of cell
adhesion and signaling. J Biol Chem. 275:22607–22610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omari S: Focal adhesion kinase: A key
mediator of cancer pathogenesis. HealthMED. 5:807–818. 2011.
|
|
18
|
Sahu SN, Nunez S, Bai G and Gupta A:
Interaction of Pyk2 and PTP-PEST with leupaxin in prostate cancer
cells. Am J Physiol Cell Physiol. 292:C2288–C2296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Wu WJ, Hu G, Yu X, Yu GS, Lu H,
Yang ML, Liu B and Wu ZX: Regulation of β-catenin transcription
activity by leupaxin in hepatocellular carcinoma. Tumour Biol.
37:2313–2320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahu SN, Khadeer MA, Robertson BW, Núñez
SM, Bai G and Gupta A: Association of leupaxin with Src in
osteoclasts. Am J Physiol Cell Physiol. 292:C581–C590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaulfuss S, Herr AM, Büchner A, Hemmerlein
B, Günthert AR and Burfeind P: Leupaxin is expressed in mammary
carcinoma and acts as a transcriptional activator of the estrogen
receptor α. Int J Oncol. 47:106–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanarotti MS, Finkelstein DB, Guibao CD,
Nourse A, Miller DJ and Zheng JJ: Structural basis for the
interaction between Pyk2-FAT domain and leupaxin LD repeats.
Biochemistry. 55:1332–1345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dierks S, von Hardenberg S, Schmidt T,
Bremmer F, Burfeind P and Kaulfuss S: Leupaxin stimulates adhesion
and migration of prostate cancer cells through modulation of the
phosphorylation status of the actin-binding protein caldesmon.
Oncotarget. 6:13591–13606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS,
Luo HY, Wang F, Qiu MZ, Bai L, Zhang DS, et al: Abnormal expression
of paxillin correlates with tumor progression and poor survival in
patients with gastric cancer. J Transl Med. 11:2772013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chew V and Lam KP: Leupaxin negatively
regulates B cell receptor signaling. J Biol Chem. 282:27181–27191.
2007. View Article : Google Scholar : PubMed/NCBI
|