Introduction
Gastrointestinal cancer is one of the most common
causes of death worldwide. Many patients remain incurable mainly
because the disease is detected late, thus requiring palliative
medical care. Some of the most important factors to be considered
are patients' nutritional status including weight loss, muscle
wasting known as sarcopenia, and inflammation (1). Systemic inflammation that results from
the tumor existence and progression has been shown to play key
roles in these adverse effects, and recent studies have suggested
that some genetic polymorphisms involved in immune or inflammatory
processes may have an influence on patient outcomes, such as weight
loss or survival, through the modulation of these pathways
(2,3).
Our research group has reported the essential roles of interleukin
(IL)-1B, IL-1RN, IL-6, IL-8 and IL-10 in human gastrointestinal
cancers, underscoring the importance of these cytokines in the
etiology of gastrointestinal cancers and subsequent systemic
reactions (4–6). One rural hospital in central Japan, Iga
General Hospital in the town of Ninja, provides palliative care
including nutritional intervention for such patients with
gastrointestinal cancers, and this hospital has accumulated
considerable amounts of clinical data since the establishment of an
outpatient clinic for cancer chemotherapy and palliative care in
2011. Here, we analyzed the body composition of almost all of the
cancer patients who visited this clinic monthly, using the body
composition measurement machine, InBody® (https://inbodyusa.com/). Our aim was to evaluate the
patients' health conditions including nutritional status to provide
more effective palliative care, including nutritional
interventions. In this study, we used clinical data from Iga
General Hospital to examine the influence of genetic cytokine
polymorphisms [IL-1B C-31T, IL-1RN variable number
tandem repeats (VNTR), IL-6 C-634G, IL-8 T-251A,
IL-10 T-819C, and IL-10 A-1082G] on clinical outcomes
of the gastrointestinal cancer patients, to determine how to
establish personalized palliative care for gastrointestinal cancer
patients based on genetic information.
Materials and methods
Patients and samples
Data from 59 patients with gastrointestinal cancers
(consisting of esophageal, gastric, colorectal, biliary, and
pancreatic cancers) who visited the outpatient clinic for cancer
chemotherapy and palliative care at Iga General Hospital (Iga,
Japan) were analyzed. All of the patients underwent palliative
chemotherapy (53 patients after surgery, 6 patients who did not
undergo surgery) together with the body composition measurement
including total body weight, skeletal muscle weight, fat weight,
and water weight each time they visited this clinic, which was
approximately once a month. All of the patients agreed to provide
their clinical data for analyses and their blood for DNA testing
after written informed consent. Patients' weight loss was evaluated
as a weight loss of >5% body weight (WL5) or that of >10%
body weight (WL10) within 6 months after recruitment (which was
equal to the time they underwent surgery or the initial course of
chemotherapy). This study was approved by the Institutional Review
Board of Nagoya University Graduate School of Medicine (Nagoya,
Japan; approval no. 2013-0220-10).
DNA sample preparation and
genotyping
DNA was extracted from the buffy coat using the
Qiagen DNeasy mini kit (Qiagen, Hilden, Germany). Genotyping for
IL-1B C-31T, IL-1RN VNTR, IL-6 C-634G,
IL-8 T-251A, and IL-10 T-819C polymorphisms was
conducted using polymerase chain reaction with confronting two-pair
primers (PCR-CTPP) (7), and that for
the IL-10 A-1082G polymorphism was conducted using the ABI
PRISM 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The sequences of the primers
used are the same as previously described (8–10).
Statistical analysis
The χ2 test was used to compare the
frequencies in the contingency tables. A Kaplan-Meier curve
together with the log-rank test and the Cox proportional hazard
model were used for the evaluation of survival-time analyses. The
unconditional logistic regression model adjusted for sex and age
was used to estimate the risk of weight loss. In addition, to
evaluate the effect of measured genotypes on body composition of
the participating patients, the β-values for the slope of
each body composition element [i.e., the skeletal muscle weight
(kg) and extra-cellular water [in proportion to the total body
weight)] per allele model, which was the additive model, were
calculated using linear regression. All P-values were two-sided,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Patient characteristics are summarized in Table I. A total of 59 patients with
gastrointestinal cancers in palliative care participated in the
study, in whom colorectal cancer patients (67.8%) and patients with
Union for International Cancer Control (UICC) clinical stage IV
(49.2%) predominated, and polymorphisms of IL-1B C-31T,
IL-1RN VNTR, IL-6 C-634G, IL-8 T-251A,
IL-10 T-819C and IL-10 A-1082G were successfully
genotyped for all the patients.
 | Table I.Characteristics of the study
subjects. |
Table I.
Characteristics of the study
subjects.
| Variables | n (or years) |
|---|
| Age [years
(sd)] | 69.1 (8.4) |
| Sex [n (%)] |
|
|
Male | 37 (62.7) |
|
Female | 22 (37.3) |
| Cancer type [n
(%)] |
|
|
Esophageal | 2 (3.4) |
|
Stomach | 11 (18.6) |
|
Colorectal | 40 (67.8) |
|
Pancreatic | 5 (8.5) |
|
Billiary | 1 (1.7) |
| UICC stage [n
(%)] |
|
| I | 5 (8.5) |
| II | 10 (16.9) |
|
III | 15 (25.4) |
| IV | 29 (49.2) |
| Genotype
frequency |
|
|
IL-1B C-31T
(rs1143627) |
|
|
C/C | 17 (28.8) |
|
C/T | 30 (50.9) |
|
T/T | 12 (20.3) |
|
IL-1RN VNTR |
|
2/2 | 1 (1.7) |
|
2/5 | 3 (5.1) |
|
5/5 | 53 (89.8) |
|
5/6 | 2 (3.4) |
|
IL-6 C-634G
(rs1800796) |
|
|
C/C | 36 (61.0) |
|
C/G | 20 (33.9) |
|
G/G | 3 (5.1) |
|
IL-8 T-251A
(rs4073) |
|
T/T | 23 (39.0) |
|
A/T | 28 (47.4) |
|
A/A | 8 (13.6) |
|
IL-10 T-819C
(rs3021097) |
|
|
T/T | 26 (44.1) |
|
C/T | 27 (45.7) |
|
C/C | 6 (10.2) |
|
IL-10 A-1082G
(rs1800896) |
|
|
A/A | 52 (88.1) |
|
A/G | 7 (11.9) |
|
G/G | 0 (0.0) |
Correlation between cytokine
polymorphisms and weight loss
Table II shows the
frequency of weight loss stratified by genotype for each cytokine
polymorphism. Although a significant increase in the risk of 10%
weight loss (WL10) was observed in patients with an increased
number of C alleles with the IL-10 T-819C
polymorphism (per-allele OR=3.09, P=0.046, by the sex- and
age-adjusted additive model), none of the other cytokine
polymorphisms showed statistical significance. Additionally,
further analyses of change in the body composition by genotype
revealed a significant reduction in skeletal muscle weight by the
increasing number of the long-repeat (L) alleles of the
IL-1RN VNTR polymorphism (P=0.001). Additionally, there was
a significant increase in the weight proportion of extra-cellular
water (ECW) with an increasing number of C alleles in the
IL-10 T-819C polymorphism (P=0.040; Table III).
 | Table II.Risk of weight loss by cytokine
genotype. |
Table II.
Risk of weight loss by cytokine
genotype.
| Polymorphism | WL5 | WL5 (−) | Crude OR | aOR | WL10 | WL10 (−) | Crude OR | aOR |
|---|
| IL-1B C-31T
(rs1143627) |
|
C/C | 11 | 4 | 1 | 1 | 4 | 11 | 1 | 1 |
|
C/T | 17 | 9 | 0.69 | 0.76 | 11 | 15 | 2.02 | 2.07 |
|
|
|
| (0.17–2.79) | (0.18–3.19) |
|
| (0.51–8.05) | (0.50–8.53) |
|
T/T | 4 | 6 | 0.24 | 0.20 | 4 | 6 | 1.83 | 1.73 |
|
|
|
| (0.04–1.33) | (0.03–1.19) |
|
|
(0.33–10.10) | (0.31–9.78) |
| IL-1RN
VNTR |
|
L/L | 31 | 17 | 1 | 1 | 18 | 30 | 1 | 1 |
|
L/2+2/2a | 1 | 2 | 0.27 | 0.27 | 1 | 2 | 0.83 | 0.87 |
|
|
|
| (0.02–3.25) | (0.02–3.29) |
|
| (0.07–9.86) |
(0.07–10.45) |
| IL-6 C-634G
(rs1800796) |
|
C/C | 19 | 12 | 1 | 1 | 12 | 19 | 1 | 1 |
|
C/G | 11 | 6 | 1.16 | 1.12 | 5 | 12 | 0.66 | 0.69 |
|
|
|
| (0.34–3.96) | (0.32–3.97) |
|
| (0.19–2.35) | (0.19–2.54) |
|
G/G | 2 | 1 | 1.26 | 1.11 | 2 | 1 | 3.17 | 3.32 |
|
|
|
|
(0.10–15.49) |
(0.08–14.89) |
|
|
(0.26–38.84) |
(0.25–44.45) |
| IL-8 T-251A
(rs4073) |
|
T/T | 15 | 5 | 1 | 1 | 10 | 10 | 1 | 1 |
|
A/T | 14 | 11 | 0.42 | 0.40 | 7 | 18 | 0.39 | 0.30 |
|
|
|
| (0.12–1.53) | (0.10–1.60) |
|
| (0.11–1.34) | (0.08–1.20) |
|
A/A | 3 | 3 | 0.33 | 0.33 | 2 | 4 | 0.50 | 0.46 |
|
|
|
| (0.05–2.21) | (0.05–2.26) |
|
| (0.07–3.38) | (0.06–3.29) |
| IL-10 T-819C
(rs3021097) |
|
T/T | 11 | 10 | 1 | 1 | 5 | 16 | 1 | 1 |
|
C/T | 19 | 8 | 2.16 | 2.48 | 12 | 15 | 2.56 | 3.24 |
|
|
|
| (0.66–7.10) | (0.71–8.69) |
|
| (0.73–9.01) |
(0.84–12.43) |
|
C/C | 2 | 1 | 1.82 | 2.08 | 2 | 1 | 6.40 | 8.73 |
|
|
|
|
(0.14–23.25) |
(0.15–28.05) |
|
|
(0.47–86.34) |
(0.60–127.38) P for
trend=0.046 |
|
C/T+C/C | 21 | 9 | 2.12 | 2.44 | 14 | 16 | 2.80 | 3.55 |
|
|
|
| (0.67–6.76) | (0.72–8.30) |
|
| (0.82–9.62) | (0.94–13.35)
P=0.061 |
| IL-10
A-1082G (rs1800896) |
|
A/A | 30 | 15 | 1 | 1 | 17 | 28 | 1 | 1 |
|
A/G | 2 | 4 | 0.25 | 0.22 | 2 | 4 | 0.82 | 0.75 |
|
|
|
| (0.04–1.52) | (0.04–1.43) |
|
| (0.14–4.99) | (0.12–4.65) |
 | Table III.Change in body composition by
genotypes. |
Table III.
Change in body composition by
genotypes.
| Polymorphism | β for the
slope of skeletal musclea,b | P | β for the
slope of ECWa,b | P |
|---|
| IL-1B C-31T
(rs1143627) | 0.172 (−0.100,
0.445) | 0.211 | 0.0077 (−0.0963,
0.1116) | 0.883 |
| IL-1RN
VNTR | −0.681 (−1.064,
−0.299) | 0.001 | 0.0208 (−0.1385,
0.1801) | 0.795 |
| IL-6 C-634G
(rs1800796) | −0.040 (−0.367,
0.288) | 0.809 | 0.0007 (−0.0051,
0.0019) | 0.245 |
| IL-8 T-251A
(rs4073) | −0.175 (−0.458,
0.107) | 0.219 | 0.0059 (−0.1019,
0.1136) | 0.913 |
| IL-10 T-819C
(rs3021097) | −0.117 (−0.410,
0.176) | 0.427 | 0.1119 (−0.0051,
0.2187) | 0.040 |
| IL-10
A-1082G (rs1800896) | −0.267 (−0.858,
0.325) | 0.370 | 0.0036 (−0.2204,
0.2277) | 0.974 |
Correlation between cytokine
polymorphisms and patients' survival
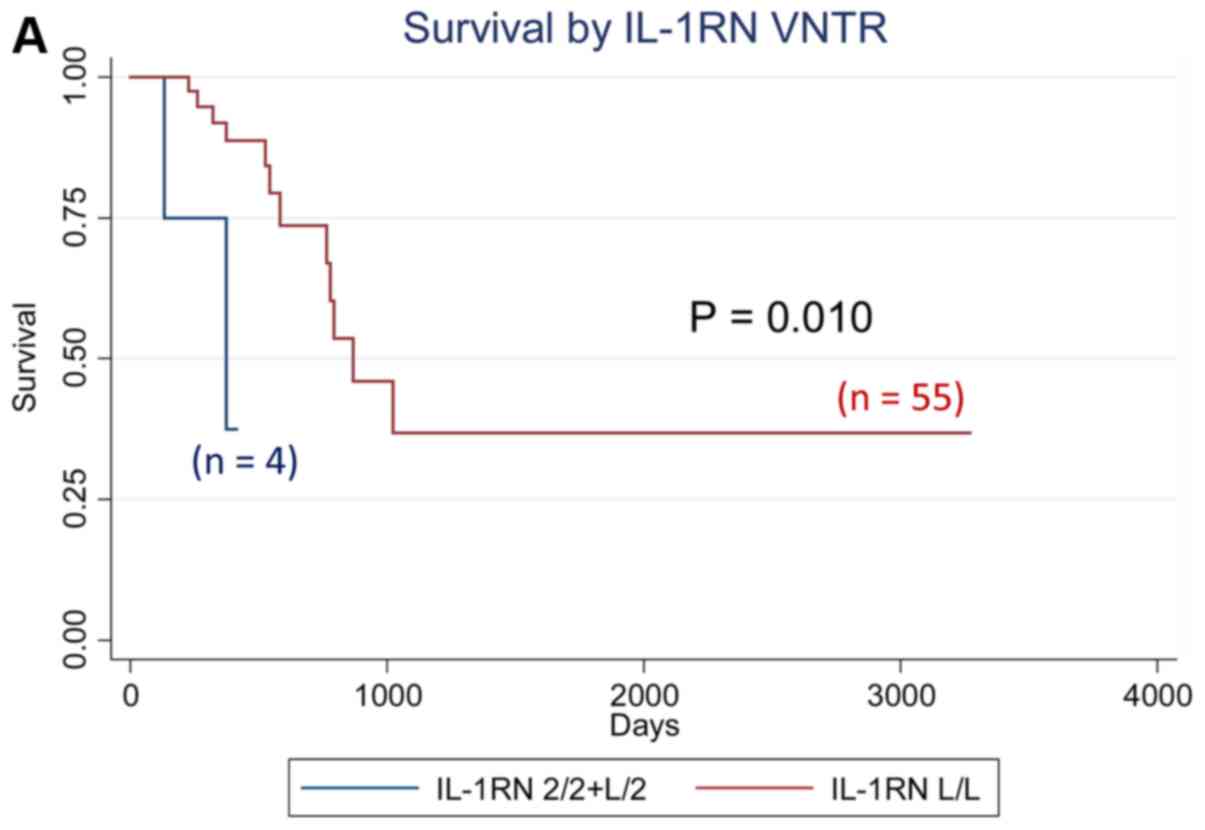
We analyzed the effect of all cytokine polymorphisms
examined on patient survival using the Kaplan-Meier method. Those
with at least one IL-1RN VNTR 2 allele showed
significantly worse survival (P=0.010, logrank test), whereas those
with the IL-6−634 G/G genotype showed significantly
worse survival (P=0.0001), as shown in Fig. 1. Further multivariate analyses using
the Cox proportional hazard model revealed that the
IL-10−1082 A/G genotype was an independent prognostic factor
for overall survival in patients with gastrointestinal cancer, with
the adjusted hazard ratio (aHR) of 6.48 (95% CI: 1.03–40.54,
P=0.046) when adjusted for sex, age, and clinical stage, whereas
the aHR for the IL-1 RN VNTR [aHR=8.84 (95% CI: 1.48–52.85,
P=0.017) for subjects with 2 allele vs. L/L genotype]
and that for the IL-6 C-634G [aHR=39.15 (4.25–360.67,
P=0.001) for subjects with G/G genotype vs. others] also had
significantly elevated aHRs (Table
IV). The effects of the IL-1RN VNTR, IL-6 C-634G,
and IL-10 A-1082G polymorphisms on the survival of patients
with colorectal cancer, which is the most frequent type of cancer,
were in the same direction as those of all the patients in this
study (data not shown).
 | Table IV.Patient prognosis by cytokine
genotypes. |
Table IV.
Patient prognosis by cytokine
genotypes.
| Polymorphism | Comparison
groups | Log-rank P | Model | Crude HR | aHR-1 | aHR-2 |
|---|
| IL-1B C-31T
(rs1143627) | All 3
genotypes | 0.916 | Additive | 1.03 | 0.89 | 0.74 |
|
|
|
|
| (0.53–2.00) | (0.39–2.03) | (0.33–1.65) |
|
| vt.
hetero+vt. homo vs. wt. homo | 0.792 | Dominant | 1.16 | 1.02 | 0.93 |
|
|
|
|
| (0.39–3.44) | (0.29–3.54) | (0.27–3.21) |
|
| vt. homo vs.
others | 0.889 | Recessive | 0.91 | 0.65 | 0.34 |
|
|
|
|
| (0.25–3.30) | (0.14–3.04) | (0.06–1.83) |
| IL-1RN
VNTRa | All 3
genotypes | <0.0001 | Additive | 8.29 | 8.28 | 8.88 |
|
|
|
|
| (2.05–33.47) | (2.05–33.51) | (2.07–38.14) |
|
| vt.
hetero+vt. homo vs. wt. homo | 0.010 | Dominant | 6.83 | 6.61 | 8.84 |
|
|
|
|
| (1.25–37.36) | (1.19–36.71) | (1.48–52.85) |
|
| vt. homo vs.
others | <0.0001 | Recessive | – | – | – |
| IL-6 C-634G
(rs1800796) | All 3
genotypes | 0.0005 | Additive | 2.61 | 2.71 | 3.05 |
|
|
|
|
| (1.03–6.58) | (0.99–7.40) | (1.07–8.67) |
|
| vt.
hetero+vt. homo vs. wt. homo | 0.206 | Dominant | 1.96 | 1.92 | 1.97 |
|
|
|
|
| (0.68–5.65) | (0.64–5.73) | (0.66–5.90) |
|
| vt. homo vs.
others | 0.0001 | Recessive | 13.31 | 15.01 | 39.15 |
|
|
|
|
| (2.40–73.84) | (2.22–101.41) | (4.25–360.67) |
| IL-8 T-251A
(rs4073) | All 3
genotypes | 0.869 | Additive | 0.84 | 0.77 | 0.97 |
|
|
|
|
| (0.37–1.87) | (0.34–1.77) | (0.40–2.39) |
|
| vt.
hetero+vt. homo vs. wt. homo | 0.798 | Dominant | 0.87 | 0.83 | 0.94 |
|
|
|
|
| (0.30–2.50) | (0.29–2.43) | (0.32–2.78) |
|
| vt. homo vs.
others | 0.601 | Recessive | 0.58 | 0.43 | 1.07 |
|
|
|
|
| (0.08–4.49) | (0.05–3.77) | (0.11–10.23) |
| IL-10 T-819C
(rs3021097) | All 3
genotypes | 0.541 | Additive | 1.10 | 1.07 | 1.06 |
|
|
|
|
| (0.52–2.36) | (0.49–2.34) | (0.45–2.50) |
|
| vt.
hetero+vt. homo vs. wt. homo | 0.478 | Dominant | 1.49 | 1.50 | 1.24 |
|
|
|
|
| (0.49–4.47) | (0.48–4.66) | (0.39–4.00) |
|
| vt. homo vs.
others | 0.578 | Recessive | 0.57 | 0.50 | 0.74 |
|
|
|
|
| (0.07–4.34) | (0.06–3.96) | (0.09–5.87) |
| IL-10
A-1082G | A/G vs.
A/A | 0.198 | – | 2.66 | 2.98 | 6.48 |
| (rs1800896) |
|
|
| (0.57–12.50) | (0.60–14.73) | (1.03–40.54) |
Discussion
The present study examined the influence of several
cytokine polymorphisms on the clinical outcome of gastrointestinal
cancer patients, and revealed that polymorphisms in IL-1RN
and IL-6 genes have a significant impact on patient
survival, based on data obtained from a single institution. The
findings obtained were consistent with the previous reports
(11,12), which underscored the potential
clinical importance of sequence variations of these cytokines in
gastrointestinal cancer patients' medical care. IL-1RN
encodes the antagonist against IL-1α and IL-1β, and is well-known
to play crucial roles in controlling the balance of various
proinflammatory processes such as in central nervous system events
in the human brain, in the pathogenesis of skin psoriasis, or in
the risk of gastrointestinal cancer (13–15). IL-6
is also known to play key roles in tumor-induced inflammation and
various subsequent responses including the acute phase responses
leading to elevated CRP levels and tumor growth in gastrointestinal
cancer (16).
There is an increasing amount of evidence that
genetic factors play major roles in the regulation of cytokine
production, such as for IL-1RN and IL-6 (9,17,18). Carriers of the IL-1RN 2
polymorphism have been shown to have higher IL-1RN circulating
levels than non-carriers (19,20), while
some other experimental reports suggest the association of the
IL-1RN polymorphism with the amount of IL-1B production, but
the directions of the effect seem inconsistent (7). The IL-1RN VNTR polymorphism is
consisted of two to six tandem repeats of 86-bp conserved sequence,
which is located in the putative protein binding sites and thus may
influence gene expression (15,21).
Considering that the genes for IL-1RN and IL-1B are both located on
chromosome 2q14 within a 360-kb region, it is possible that the
genetic variation of IL-1RN VNTR may affect IL-1B levels
through linkage disequilibrium on the genome. The present study
could not reproduce the previously reported association of
IL-1RN VNTR 2 allele with a better prognosis in
gastrointestinal cancer patients, which might be explained by the
modulation of the IL-1B levels that results from the IL-1RN
VNTR polymorphism, or by random error because of the relatively
small sample size and disease heterogeneity. The significant trend
of reduced skeletal muscle weight associated with a higher number
of IL-1RN VNTR L alleles, however, is consistent with
a previous report (11), considering
the lower IL-1RN circulating levels in L allele carriers and
the subsequent exacerbated inflammation induced by IL-1B. For the
IL-6 polymorphism, the IL-6−634 G allele is
reported to result in higher plasma IL-6 levels, and is associated
with a higher risk of cachexia (12).
The finding of a worse prognosis in those with the IL-6−634
G/G genotype is consistent with a previous report (12), and is regarded as biologically
plausible considering that higher IL-6 levels that result
from the IL-6−634 G/G genotype may exacerbate
systemic inflammation, thereby leading to the worse prognosis in
gastrointestinal cancer patients under palliative care. The
association of the presence of the IL-10−819 C allele
with advanced stages of colorectal cancers, and with possible
weight loss and an increase in ECW, may suggest possible important
roles of this variation in the IL-10 gene in the etiology of
colorectal cancer patients. Previous studies have shown that IL-10
exerts its anti-inflammatory effects independent of IL-10R, PIK3,
or p70S6 kinase (22). Considering
that the T allele of the IL-10 T-819C polymorphism
was associated with a higher IL-10 expression, these associations
of the IL-10−819 C allele with possible weight loss
in gastrointestinal cancer patients might be explained by the
reduced IL-10 expression as a result of this polymorphism (23). The G allele of IL-10
A-1082G polymorphism was reportedly associated with worse prognosis
in patients with gastrointestinal cancers (24). The IL-10 A-1082G polymorphism
lies within the promoter region of the IL-10 gene, where the
G/G genotype of this polymorphism was shown to be associated
with increased levels of IL-10 production (25). Our results in this study verified the
clinical impact of the IL-10−1082 G allele on
gastrointestinal cancer patient prognosis, suggesting the potential
prognostic role of this IL-10 SNP in gastrointestinal cancer
patients receiving palliative care. There also exist several other
genetic polymorphisms reportedly associated with the risk of
cachexia or weight loss in cancer patients to date, such as those
in P-selectin (SELP), leptin receptor (LEPR),
tumor necrosis factor (TNF), lymphotoxin alpha
(LTA) or tumor necrosis factor receptor superfamily
member 1A (TNFRSF1A) genes, which might possibly have
biological interactions with the genes investigated in the present
study, and would become potential candidates for future
investigations as well (26,27).
With regard to technical aspects, a strength of this
study design is the frequent monitoring of the patients with
detailed data, such as body composition, body weight, and
laboratory data, which was conducted at least once to twice per
month. The limitations, however, would be the smaller sample size,
which should be increased in follow-up studies. The present study
result may also suggest that genetic testing of gastrointestinal
patients may be useful for predicting patients' need for supportive
care such as nutritional intervention or possible molecular
therapies, such as recombinant IL-1RN (11). It has been reported that elevated
resting energy expenditure in several types of cancer patients is
associated with the presence of a systemic inflammatory response,
which may lead to a worse clinical outcomes in patients (25). Nutritional interventions such as
administration of eicosapentaenoic acid (EPA) have been shown to be
effective in partially controlling the systemic inflammation in
these cancer patients (28). To
examine this possibility, further studies taking into consideration
the efficacy of nutritional interventions based on the genetic
information are possible future research directions.
In conclusion, this is the first study that showed
genetic polymorphisms can be used to predict gastrointestinal
cancer patient survival. We also confirmed the previously reported
roles of IL-RN VNTR and IL-6 C-634G polymorphisms in
predicting survival, suggesting the potential feasibility of
genetic testing in gastrointestinal cancer patients with palliative
care. The sample size of the present study is limited, and the
findings should be verified with larger number of patients, to
confirm the possible establishment of personalized palliative care
for gastrointestinal cancer patients.
Acknowledgements
The authors would like to thank Ms. Yoko Mitsuda and
Ms. Keiko Shibata (Nagoya University, Nagoya, Japan) for their
technical assistance.
Funding
The present study was supported in part by
Grants-in-Aid for Scientific Research from the Japanese Ministry of
Education, Culture, Sports, Science and Technology, JSPS KAKENHI
Grant Number JP (grant no. 25460745).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AH, YO, YS and CM contributed to the entire study
design, data collection and analyses, and reviewed the final
version of the paper critically. YM, KO, KT, RN, YI, HS, HU and MT
provided the data from the outpatient clinic to the analysts,
provided clinical interpretation of the results, and reviewed the
paper critically. SM and AO contributed to the collection of
clinical data and genotyping of the patient samples, and reviewed
the paper. YT and DCM provided an interpretation of the results
from the viewpoint of expert surgical scientists and reviewed the
final version of the paper.
Ethical approval and consent to
participate
All of the patients agreed to provide their clinical
data for analyses and their blood for DNA testing after written
informed consent. This study was approved by the Institutional
Review Board of Nagoya University Graduate School of Medicine
(approval no. 2013-0220-10).
Patient consent for publication
All study participants approved the publication of
the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toiyama Y, Miki C, Inoue Y, Tanaka K,
Mohri Y and Kusunoki M: Evaluation of an inflammation-based
prognostic score for the identification of patients requiring
postoperative adjuvant chemotherapy for stage II colorectal cancer.
Exp Ther Med. 2:95–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan BH and Fearon KC: Cytokine gene
polymorphisms and susceptibility to cachexia. Curr Opin Support
Palliat Care. 4:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan BH, Ross JA, Kaasa S, Skorpen F and
Fearon KC: European Palliative Care Research Collaborative:
Identification of possible genetic polymorphisms involved in cancer
cachexia: A systematic review. J Genet. 90:165–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okugawa Y, Miki C, Toiyama Y, Yasuda H,
Yokoe T, Saigusa S, Hiro J, Tanaka K, Inoue Y and Kusunoki M: Loss
of tumoral expression of soluble IL-6 receptor is associated with
disease progression in colorectal cancer. Br J Cancer. 103:787–795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toiyama Y, Miki C, Inoue Y, Minobe S,
Urano H and Kusunoki M: Loss of tissue expression of interleukin-10
promotes the disease progression of colorectal carcinoma. Surg
Today. 40:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konishi N, Miki C, Yoshida T, Tanaka K,
Toiyama Y and Kusunoki M: Interleukin-1 receptor antagonist
inhibits the expression of vascular endothelial growth factor in
colorectal carcinoma. Oncology. 68:138–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamajima N, Saito T, Matsuo K, Kozaki K,
Takahashi T and Tajima K: Polymerase chain reaction with
confronting two-pair primers for polymorphism genotyping. Jpn J
Cancer Res. 91:865–868. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamajima N, Matsuo K, Saito T, Tajima K,
Okuma K, Yamao K and Tominaga S: Interleukin 1 polymorphisms,
lifestyle factors and Helicobacter pylori infection. Jpn J Cancer
Res. 92:383–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suma S, Naito M, Wakai K, Sasakabe T,
Hattori Y, Okada R, Kawai S, Hishida A, Morita E, Nakagawa H, et
al: Effects of IL6 C-634G polymorphism on tooth loss and their
interaction with smoking habits. Oral Dis. 21:807–813. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamajima N, Katsuda N, Matsuo K, Saito T,
Hirose K, Inoue M, Zaki TT, Tajima K and Tominaga S: High
anti-Helicobacter pylori antibody seropositivity associated with
the combination of IL-8-251TT and IL-10-819TT genotypes.
Helicobacter. 8:105–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graziano F, Ruzzo A, Santini D, Humar B,
Tonini G, Catalano V, Berardi R, Pizzagalli F, Arduini F, Bearzi I,
et al: Prognostic role of interleukin-1beta gene and interleukin-1
receptor antagonist gene polymorphisms in patients with advanced
gastric cancer. J Clin Oncol. 23:2339–2345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang D, Zhou Y, Wu L, Wang S, Zheng H, Yu
B and Li J: Association of IL-6 gene polymorphisms with cachexia
susceptibility and survival time of patients with pancreatic
cancer. Ann Clin Lab Sci. 38:113–119. 2008.PubMed/NCBI
|
|
13
|
Carter DB, Deibel MR Jr, Dunn CJ, Tomich
CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF,
McEwan RN, et al: Purification, cloning, expression and biological
characterization of an interleukin-1 receptor antagonist protein.
Nature. 344:633–638. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arend WP: Interleukin 1 receptor
antagonist. A new member of the interleukin 1 family. J Clin
Invest. 88:1445–1451. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue H, Lin B, Ni P, Xu H and Huang G:
Interleukin-1B and interleukin-1 RN polymorphisms and gastric
carcinoma risk: A meta-analysis. J Gastroenterol Hepatol.
25:1604–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fearon KC, Glass DJ and Guttridge DC:
Cancer cachexia: Mediators, signaling and metabolic pathways. Cell
Metab. 16:153–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furuta T, El-Omar EM, Xiao F, Shirai N,
Takashima M and Sugimura H: Interleukin 1beta polymorphisms
increase risk of hypochlorhydria and atrophic gastritis and reduce
risk of duodenal ulcer recurrence in Japan. Gastroenterology.
123:92–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrari SL, Ahn-Luong L, Garnero P,
Humphries SE and Greenspan SL: Two promoter polymorphisms
regulating interleukin-6 gene expression are associated with
circulating levels of C-reactive protein and markers of bone
resorption in postmenopausal women. J Clin Endocrinol Metab.
88:255–259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hurme M and Santtila S: IL-1 receptor
antagonist (IL-1Ra) plasma levels are co-ordinately regulated by
both IL-1Ra and IL-1beta genes. Eur J Immunol. 28:2598–2602. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vamvakopoulos J, Green C and Metcalfe S:
Genetic control of IL-1beta bioactivity through differential
regulation of the IL-1 receptor antagonist. Eur J Immunol.
32:2988–2996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vamvakopoulos JE, Taylor CJ, Morris-Stiff
GJ, Green C and Metcalfe S: The interleukin-1 receptor antagonist
gene: A single-copy variant of the intron 2 variable number tandem
repeat (VNTR) polymorphism. Eur J Immunogenet. 29:337–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamajima N, Katsuda N, Matsuo K, Saito T,
Hirose K, Inoue M, Zaki TT, Tajima K and Tominaga S: High
anti-Helicobacter pylori antibody seropositivity associated with
the combination of IL-8-251TT and IL-10-819TT genotypes.
Helicobacter. 8:105–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crawley JB, Williams LM, Mander T, Brennan
FM and Foxwell BM: Interleukin-10 stimulation of
phosphatidylinositol 3-kinase and p70 S6 kinase is required for the
proliferative but not the antiinflammatory effects of the cytokine.
J Biol Chem. 271:16357–16362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deans DA, Tan BH, Ross JA, Rose-Zerilli M,
Wigmore SJ, Howell WM, Grimble RF and Fearon KC: Cancer cachexia is
associated with the IL10-1082 gene promoter polymorphism in
patients with gastroesophageal malignancy. Am J Clin Nutr.
89:1164–1172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan BH, Ross JA, Kaasa S, Skorpen F and
Fearon KC: European Palliative Care Research Collaborative:
Identification of possible genetic polymorphisms involved in cancer
cachexia: A systematic review. J Genet. 90:165–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan BH, Fladvad T, Braun TP, Vigano A,
Strasser F, Deans DA, Skipworth RJ, Solheim TS, Damaraju S, Ross
JA, et al: P-selectin genotype is associated with the development
of cancer cachexia. EMBO Mol Med. 4:462–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ries A, Trottenberg P, Elsner F, Stiel S,
Haugen D, Kaasa S and Radbruch L: A systematic review on the role
of fish oil for the treatment of cachexia in advanced cancer: An
EPCRC cachexia guidelines project. Palliat Med. 26:294–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|