Introduction
Osteosarcoma, the most common primary malignant bone
tumor in children and adolescents, arises from cells of mesenchymal
osteoblast origin (1,2). Despite considerable advances in surgery
and multiagent chemotherapy, the cure rate has not significantly
increased over the past 20 years (3,4).
Therefore, the development of novel treatments and drugs for
osteosarcoma treatment is urgently required.
Norcantharidin (NCTD), which is a purified component
from blister beetles, is well-known for its antioxidant, antitumor
and antimetastatic functions. Previous studies have reported that
NCTD exhibits potential antitumor activity in a variety of cancer
types, including oral cancer (5),
colorectal adenocarcinoma (6,7) and urinary bladder carcinoma (8). However, to the best of our knowledge,
the antitumor effect of NCTD in osteosarcoma and the underlying
molecular mechanisms of this effect have never been
investigated.
In the current study, it was demonstrated that NCTD
inhibits proliferation and induces G2/M-phase arrest and cell
apoptosis in human osteosarcoma cells. Furthermore, the possible
mechanisms of NCTD action in Akt/mammalian target of rapamycin
(mTOR) signaling pathways are discussed. Collectively, the current
data suggest that NCTD may be an anticancer agent for the treatment
of osteosarcoma.
Materials and methods
Cells and cell culture
The human osteosarcoma cell lines 143B and SJSA were
purchased from American Type Culture Collection (Manassas, VA, USA)
and cultured in high-glucose Dulbecco's modified Eagle's medium
(HyClone; GE Healthcare, Logan, UT, USA) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare), 100 U/ml penicillin
and 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a humidified incubator at 37°C in 5%
CO2.
Materials and chemicals
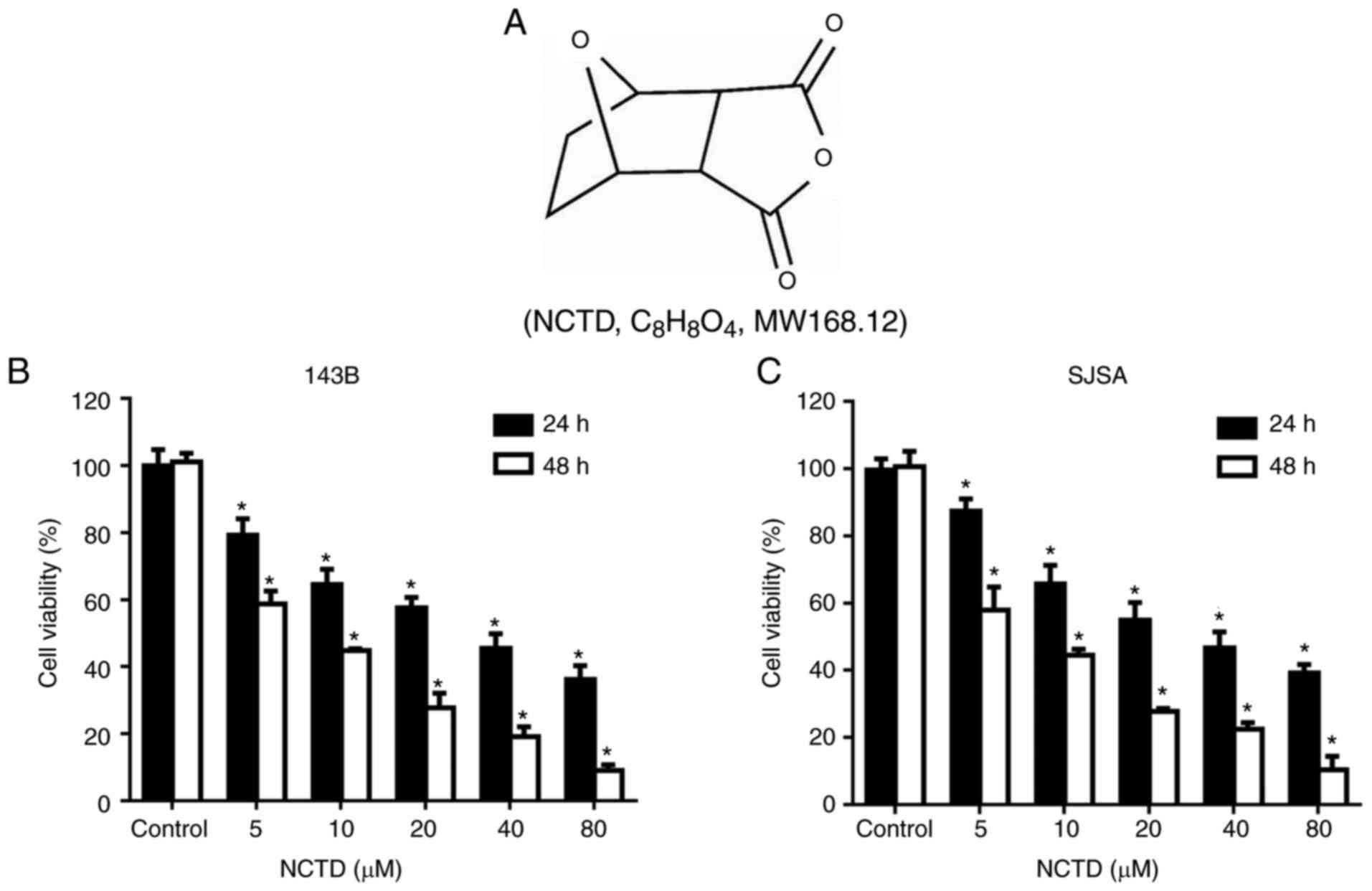
NCTD (Fig. 1A) was
obtained from the National Institutes for Food and Drug Control
(Shandong, China), dissolved in dimethyl sulfoxide (DMSO) as an 80
mmol/l stock solution and stored in the dark at −20°C. Antibodies
against cleaved-caspase-3 (cat. no. 9661), cleaved-poly
(ADP-ribose) polymerase (cleaved-PARP; cat. no. 5625), Akt (cat.
no. 4685), mTOR (cat. no. 2983), phosphorylated (p)-Akt (cat. no.
4060), p-mTOR (cat. no. 5536) and GAPDH (cat. no. 5174) and an
anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody (cat.
no. 7074) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell viability assay
Cells of the 143B and SJSA human osteosarcoma cell
lines were seeded in 96-well plates (2,000 cells/well). After
growing for 24 h in the incubator, the medium was aspirated, and
the cells were then treated with 200 µl of complete medium
containing various concentrations of NCTD (0, 10, 20, 40, 80 and
160 µM). NCTD was dissolved in DMSO, and the concentration of DMSO
was kept at <0.05% in all wells. After 24/48 h of incubation,
Cell Counting Kit-8 (CCK8) solution was added to each well at a
final concentration of 5 mg/ml. Following incubation for an
additional 4 h at 37°C, the supernatants were removed, and 100 ml
DMSO was added to each well. The absorption was measured at 550 nm
by a microplate spectrophotometer. Three independent experiments
were carried out in triplicate. The cell growth inhibition at each
NCTD concentration was measured, and the IC50 values
were calculated using the SPSS 19.0 statistical software package
(IBM Corp., Armonk, NY, USA).
Cell cycle analysis by flow
cytometry
Cells of the 143B and SJSA human osteosarcoma cell
lines were cultured in 6-well plates at a density of
5×105/ml and treated with NCTD at the indicated
concentration for 12 h. After treatment, the cells were harvested,
washed three times with phosphate-buffered saline (PBS) and fixed
with cold 70% ethyl alcohol at 4°C overnight. The cells were then
stained with PI/RNase staining buffer (BD Biosciences, Franklin
Lakes, NJ, USA) for 15 min at room temperature and analyzed by an
Accuri C6 flow cytometer (BD Biosciences). In addition, the data
were analyzed using ModFit LT v3.1 software (FACSCalibur; BD
Biosciences).
Morphological characteristics of
apoptosis
A Hoechst assay was performed to evaluate the
morphological characteristics of apoptotic cells after NCTD
treatment. In brief, 143B human osteosarcoma cells were seeded in
6-well plates at a density of 5×105/ml and exposed to
vehicle or NCTD at the indicated concentration for 12 h. Then, the
cells were fixed with 4% paraformaldehyde for 15 min at room
temperature and washed three times with PBS. Next, the cells were
permeabilized with 0.1% Triton-X-100 and stained with Hoechst 33258
(Beyotime Institute of Biotechnology, Shanghai, China) for 10 min
at room temperature in the dark. The cells were observed under a
fluorescence microscope.
Flow cytometric analysis of
apoptosis
The Annexin V-FITC/propidium iodide (PI) double
staining assay (Beyotime Institute of Biotechnology) was used to
detect cell apoptosis according to the manufacturer's protocol.
Briefly, 143B human osteosarcoma cells were seeded in 6-well plates
at a density of 5×105/ml and exposed to vehicle or NCTD
at the indicated concentration for 24 h. Then, the cells were
harvested, washed three times with cold PBS and stained with
Annexin V-FITC and PI for 15 min in the dark at room temperature.
In addition, the data were analyzed using Accuri C6 software (BD
Biosciences).
Western blot assay
Cells were washed three times with cold PBS
solution, and protein was extracted using RIPA Lysis Buffer
(Beyotime Institute of Biotechnology) containing protease and
phosphatase inhibitors (Beyotime Institute of Biotechnology). After
quantification with the Standard BCA Protein Assay kit (Beyotime
Institute of Biotechnology), equal amounts of protein (40 µg) were
boiled, electrophoretically separated on 10 or 15% polyacrylamide
gels at 80–120 V and transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA) at 80–120 V for 1–2
h. Then, the membranes were blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 60 min at room
temperature and incubated overnight at 4°C with 1:1,000 dilutions
of the primary antibodies. After incubation, the membranes were
washed three times with PBS containing 0.1% Tween-20 and then
probed with an HRP-conjugated secondary antibody (1:5,000) for 1 h
at room temperature. Antibody binding was visualized with enhanced
chemiluminescence (ECL) using an ECL substrate (EMD Millipore).
Statistical analysis
All data analyses were performed using SPSS software
(version 18.0; SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard deviation. The statistical
differences were calculated by one-way analysis of variance with
Dunnett's test or unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
NCTD inhibits cell viability in 143B
and SJSA human osteosarcoma cells in a time- and dose-dependent
manner
To explore the cytotoxicity of NCTD in human
osteosarcoma cells, the growth of 143B and SJSA cells treated with
NCTD was assessed by CCK8 assay. The cells were treated with
various concentrations of NCTD for 24 and 48 h, followed by
performance of the CCK8 assay. As indicated in Fig. 1B and C, NCTD significantly decreased
osteosarcoma cell viability, compared with the control (P<0.05).
The IC50 values for 143B and SJSA were 28.75 and 36.56
µΜ, respectively, after 24 h of NCTD treatment. After 48 h of NCTD
treatment, the IC50 values for 143B and SJSA were 13.98
and 18.08 µΜ, respectively. These results demonstrated that NCTD
inhibits the proliferation of osteosarcoma cells in a dose- and
time-dependent manner.
NCTD induces cell cycle arrest in 143B
and SJSA human osteosarcoma cells
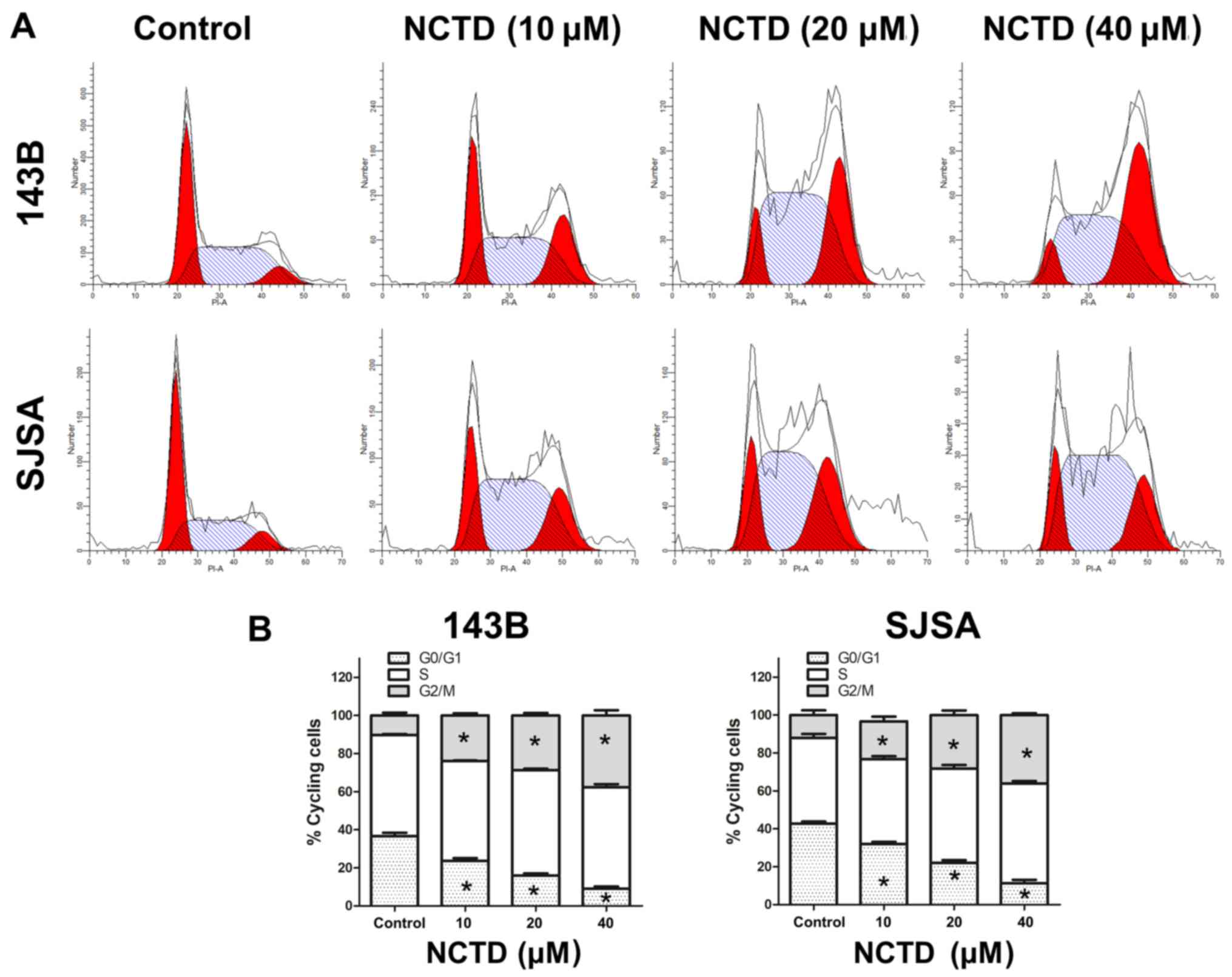
To further explore the mechanism of the
antiproliferative effect of NCTD in osteosarcoma cells, cell cycle
distribution was analyzed by flow cytometry after NCTD treatment.
As indicated in Fig. 2, the
percentage of osteosarcoma cells in G2/M phase was increased after
24 h treatment with increasing concentrations of NCTD. A
corresponding decrease was noted in the percentage of cells in
G1 phase. These data indicate that NCTD induces G2/M
cell cycle arrest in 143B and SJSA human osteosarcoma cells.
NCTD induces cell apoptosis in human
osteosarcoma cells
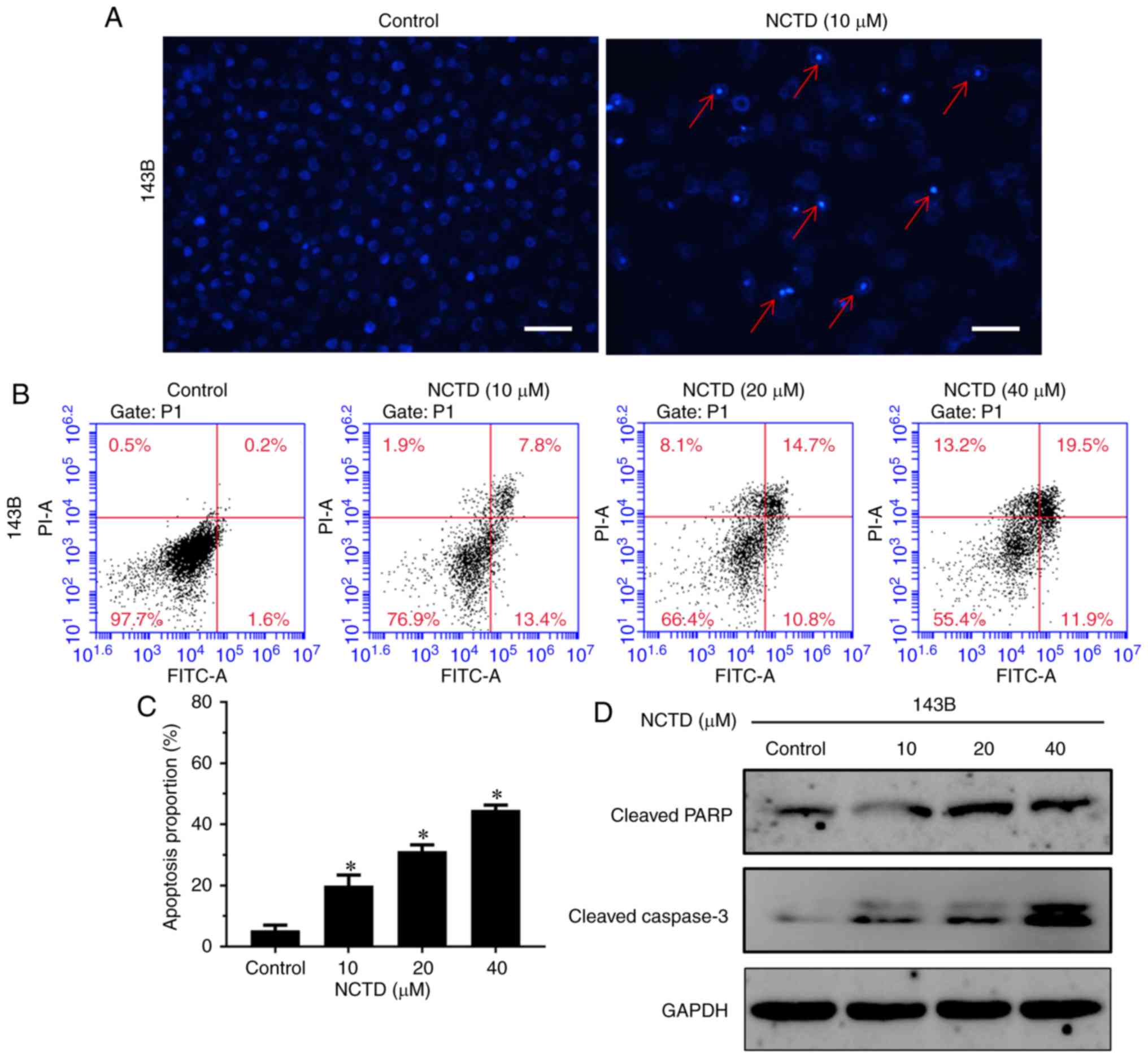
Next, NCTD-induced apoptotic cell death in
osteosarcoma cells was explored. Hoechst staining was used to
evaluate NCTD treatment-altered cell morphology. As indicated in
Fig. 3A, cell shrinkage, nuclear
fragmentation and chromatin condensation were observed after
treatment with 10 µM NCTD for 24 h. To further quantify apoptosis,
Annexin V-FITC/PI double staining was used. The results
demonstrated that treatment with NCTD significantly increased the
percentage of both early and late apoptotic cells, compared with
the control (P<0.05; Fig. 3B and
C). After treatment with 40 µM NCTD for 24 h, 44.6% of cells
were dead (11.9% of cells were early apoptotic, 19.5% of cells were
late apoptotic and 13.2% of cells were necrotic). In addition, the
western blot assay indicated that NCTD notably increased the
activation of cleaved PARP and cleaved caspase-3, compared with the
control (Fig. 3D). These data
indicated that NCTD induced dose-dependent cell apoptosis in
osteosarcoma cells.
NCTD blocks the Akt/mTOR signaling
pathway in human osteosarcoma cells
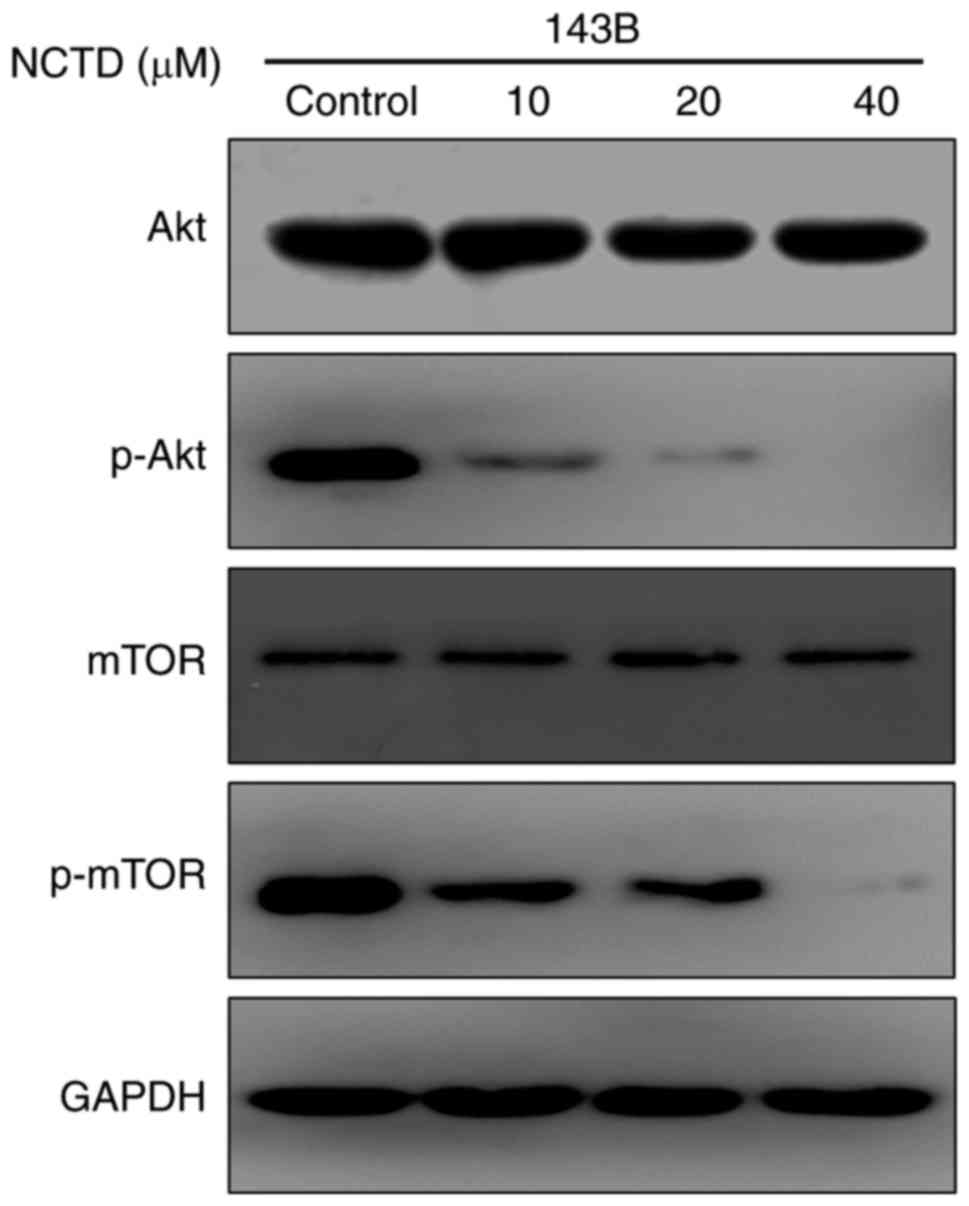
To further investigate the mechanisms underlying the
antitumor activity of NCTD, a western blot assay was used to detect
the effect of NCTD on the Akt/mTOR signaling pathway. As indicated
in Fig. 4, NCTD markedly decreased
the phosphorylation of Akt and mTOR in 143B cells. Previous studies
have demonstrated that the Akt/mTOR signaling pathway serves a key
role in regulating cell growth and apoptosis (9). These results demonstrated that NCTD
blocks the Akt/mTOR signaling pathway, an action that may be
associated with the effect of NCTD on cell growth and
apoptosis.
Discussion
Growing evidence suggests that traditional Chinese
medicines contain anticancer ingredients (10,11). NCTD,
the demethylated form of cantharidin, has exhibited anticancer
potential in multiple cancer cell types (8,12,13). However, to the best of our knowledge,
the ability of NCTD to suppress the growth of human osteosarcoma
cells and the possible underlying mechanisms have never been
investigated. In the present study, the antitumor effects of NCTD
in osteosarcoma were investigated. The results demonstrated that
NCTD could inhibit cell proliferation and induce G2/M cell cycle
arrest and apoptosis, which implies that NCTD may be a novel
antitumor agent for osteosarcoma treatment.
In the current study, two osteosarcoma cell lines
were used to test the antiproliferative effect of NCTD. It was
identified that NCTD could inhibit the growth of both osteosarcoma
cell lines in a dose- and time-dependent manner. The effect of NCTD
on human fibroblasts was also evaluated. Interestingly, human
fibroblasts exhibited resistance to NCTD and the IC50
value for 24 h was 58.82 µΜ (data not shown). Cell cycle imbalance
is an essential mechanism involved in the proliferation of
malignant tumor cells (14–16). Certain studies have supported the
hypothesis that the induction of cell cycle arrest may be an
effective method for controlling tumor proliferation (17–19). The
current results demonstrated that NCTD induced G2/M cell cycle
arrest in both osteosarcoma cell lines in a dose-dependent manner.
Similar results have indicated that NCTD is an inhibitor of cell
cycle progression in human urinary bladder carcinoma (20) and cervical carcinoma (21). The current results indicated that NCTD
could inhibit cell proliferation by inducing G2/M cell cycle
arrest.
Apoptosis, a self-destructive process counteracting
tumor growth, is characterized by specific morphological and
molecular protein changes in dying cells, including cell membrane
blebbing, nuclear condensation and apoptotic body formation
(22–24). There are two major pathways involved
in the regulation of apoptosis: The mitochondria-mediated intrinsic
pathway and the caspase-dependent extrinsic pathway (25). In the caspase-dependent extrinsic
pathway, caspase-3, −8 and −9 are activated, which subsequently
leads to the cleavage of PARP (26).
The current results demonstrated that NCTD induced cell shrinkage,
nuclear fragmentation and chromatin condensation in osteosarcoma
cells. Ultimately, NCTD led to the upregulation of cleaved
caspase-3 and an increase in the downstream target cleaved PARP,
which suggested that the extrinsic pathway may also be involved in
NCTD-induced cell apoptosis.
Previous studies have reported that the Akt/mTOR
signaling pathway is involved in multiple biological behaviors of
tumors, including growth, metastasis and apoptosis (27,28).
Numerous chemotherapeutic agents inhibit tumor cell growth and
induce apoptosis by impacting the Akt/mTOR signaling pathway
(29). In the current study, the
phosphorylation of Akt and mTOR was observed to be inhibited in
osteosarcoma cells following treatment with NCTD. Together with the
aforementioned results, this observation suggested that NCTD
suppresses the Akt/mTOR signaling pathway, an action that may be
involved in NCTD-induced growth inhibition and cell apoptosis.
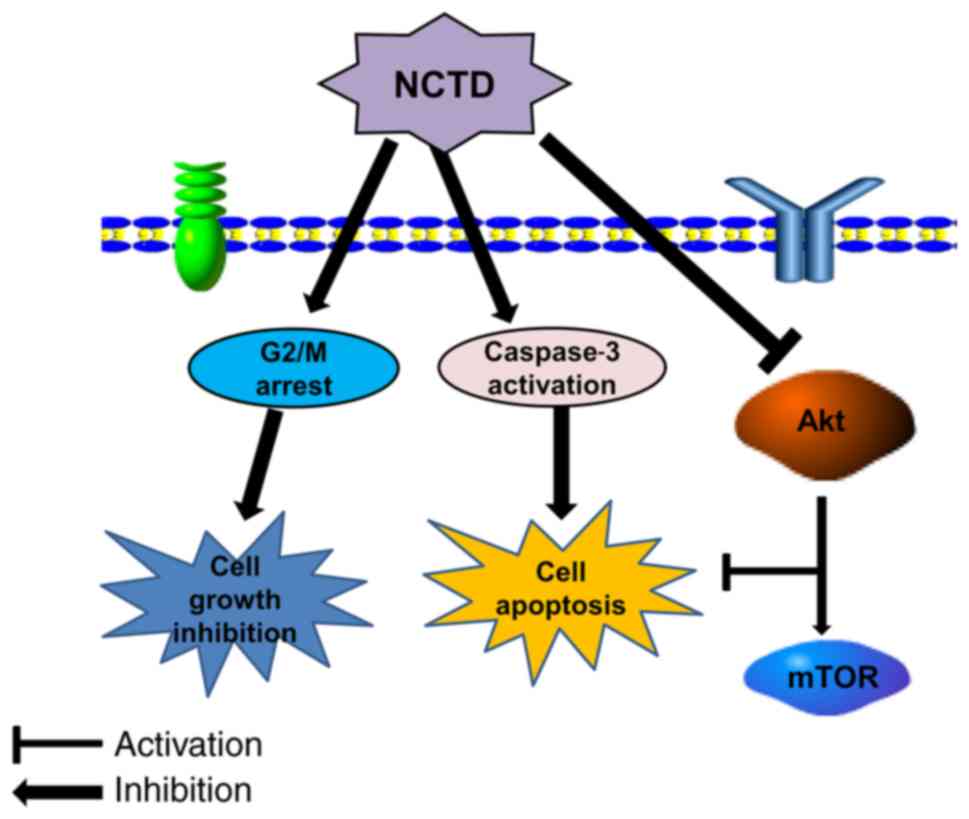
In conclusion, the current study demonstrated for
the first time that NCTD could effectively inhibit cell growth and
induce cell cycle arrest and apoptosis in osteosarcoma cells. The
current research indicates that NCTD inhibits the Akt/mTOR
signaling pathway, an action that may be associated with
NCTD-induced growth inhibition and cell apoptosis (Fig. 5). These findings suggest that NCTD may
be used for the treatment of patients with osteosarcoma in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ and XJ designed the experiment. ZW and YM
performed the experiment. YZ analyzed the data and wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sergi C and Zwerschke W: Osteogenic
sarcoma (osteosarcoma) in the elderly: Tumor delineation and
predisposing conditions. Exp Gerontol. 43:1039–1043. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Denduluri SK, Wang Z, Yan Z, Wang J, Wei
Q, Mohammed MK, Haydon RC, Luu HH and He TC: Molecular pathogenesis
and therapeutic strategies of human osteosarcoma. J Biomed Res.
30:5–18. 2016.
|
|
3
|
Pruksakorn D, Phanphaisarn A, Pongnikorn
D, Daoprasert K, Teeyakasem P, Chaiyawat P, Katruang N and
Settakorn J: AgeStandardized incidence rates and survival of
osteosarcoma in northern thailand. Asian Pac J Cancer Prev.
17:3455–3458. 2016.PubMed/NCBI
|
|
4
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ,
Lou IU, Lee MS, Hsiao M and Lin SK: Comparisons of norcantharidin
cytotoxic effects on oral cancer cells and normal buccal
keratinocytes. Oral Oncol. 39:19–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu
Y, Mao YQ, Kan B, Lei S, Wang GS, et al: Induction of apoptosis by
norcantharidin in human colorectal carcinoma cell lines:
Involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol.
128:223–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang PY, Chen MF, Tsai CH, Hu DN, Chang FR
and Wu YC: Involvement of caspase and MAPK activities in
norcantharidin-induced colorectal cancer cell apoptosis. Toxicol In
Vitro. 24:766–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan YZ, Zhao ZM, Fu JY, Chen CQ and Sun W:
Norcantharidin inhibits growth of human gallbladder carcinoma
xenografted tumors in nude mice by inducing apoptosis and blocking
the cell cycle in vivo. Hepatobiliary Pancreat Dis Int. 9:414–422.
2010.PubMed/NCBI
|
|
9
|
Fulda S: Synthetic lethality by
co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling.
Mitochondrion. 19A:85–87. 2014. View Article : Google Scholar
|
|
10
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radic Biol Med. 106:24–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Li S, Li J, Yin F, Hua Y, Wang Z,
Lin B, Wang H, Zou D, Zhou Z, et al: Natural product
pectolinarigenin inhibits osteosarcoma growth and metastasis via
SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis.
7:e24212016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang D, Yang C, Wang Z, Yang Y, Li D, Ding
X, Xu W and Zheng Q: Norcantharidin combined with Coix seed oil
synergistically induces apoptosis and inhibits hepatocellular
carcinoma growth by downregulating regulatory T cells accumulation.
Sci Rep. 7:93732017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Z, Li B, Wang J, Zhang X, Li Z, Dai L,
Cao M and Jiang J: Norcantharidin inhibits SK-N-SH neuroblastoma
cell growth by induction of autophagy and apoptosis. Technol Cancer
Res Treat. 16:33–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hainaut P and Plymoth A: Targeting the
hallmarks of cancer: Towards a rational approach to next-generation
cancer therapy. Curr Opin Oncol. 25:50–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mills CC, Kolb EA and Sampson VB: Recent
advances of cell-cycle inhibitor therapies for pediatric cancer.
Cancer Res. 77:6489–6498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terzuoli E, Nannelli G, Frosini M,
Giachetti A, Ziche M and Donnini S: Inhibition of cell cycle
progression by the hydroxytyrosol-cetuximab combination yields
enhanced chemotherapeutic efficacy in colon cancer cells.
Oncotarget. 8:83207–83224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu P, Xia X, Yang Z, Tian Y, Di J and Guo
M: Silencing of TCTN1 inhibits proliferation, induces cell cycle
arrest and apoptosis in human thyroid cancer. Exp Ther Med.
14:3720–3726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KM, Lee K, Choi YK, Choi YJ, Seo HS
and Ko SG: SH003induced G1 phase cell cycle arrest induces
apoptosis in HeLa cervical cancer cells. Mol Med Rep. 16:8237–8244.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Yan Y, Jiang Y, Qian J, Jiang L,
Hu G, Lu Y and Luo C: Knockdown of SALL4 expression using RNA
interference induces cell cycle arrest, enhances early apoptosis,
inhibits invasion and increases chemosensitivity to temozolomide in
U251 glioma cells. Oncol Lett. 14:4263–4269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu CC, Ko FY, Yu CS, Lin CC, Huang YP,
Yang JS, Lin JP and Chung JG: Norcantharidin triggers cell death
and DNA damage through S-phase arrest and ROS-modulated apoptotic
pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J
Oncol. 41:1050–1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong X, Li JC, Jiang YY, Xia MY, Tashiro
S, Onodera S and Ikejima T: p38-NF-κB-promoted
mitochondria-associated apoptosis and G2/M cell cycle arrest in
norcantharidin-treated HeLa cells. J Asian Nat Prod Res.
14:1008–1019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tiwari M, Prasad S, Tripathi A, Pandey AN,
Ali I, Singh AK, Shrivastav TG and Chaube SK: Apoptosis in
mammalian oocytes: A review. Apoptosis. 20:1019–1025. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao Z, Shan J, Li C, Luo L, Lu J, Li S,
Long D and Li Y: Mechanisms of cyclosporine-induced renal cell
apoptosis: A systematic review. Am J Nephrol. 37:30–40. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lopez-Beltran A, Maclennan GT, de la
Haba-Rodriguez J, Montironi R and Cheng L: Research advances in
apoptosis-mediated cancer therapy: A review. Anal Quant Cytol
Histol. 29:71–78. 2007.PubMed/NCBI
|
|
27
|
Daneshmanesh AH, Hojjat-Farsangi M,
Moshfegh A, Khan AS, Mikaelsson E, Österborg A and Mellstedt H: The
PI3K/AKT/mTOR pathway is involved in direct apoptosis of CLL cells
induced by ROR1 monoclonal antibodies. Br J Haematol. 169:455–458.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu W, Sun H, Zha W, Cui W, Xu L, Min Q and
Wu J: Apigenin attenuates adriamycin-induced cardiomyocyte
apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement
Alternat Med. 2017:25906762017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
So KS, Rho JK, Choi YJ, Kim SY, Choi CM,
Chun YJ and Lee JC: AKT/mTOR down-regulation by CX-4945, a CK2
inhibitor, promotes apoptosis in chemorefractory non-small cell
lung cancer cells. Anticancer Res. 35:1537–1542. 2015.PubMed/NCBI
|