Introduction
As one of the most common malignant tumors,
incidence of bladder cancer ranks the 9th among all malignant
tumors, among which, 30% of patients have muscular invasive bladder
cancer (1). Bladder cancer has the
characteristics of rapid progress and high degree of malignancy and
high recurrence and mortality rates (2). Radical cystectomy can be used to
effectively remove tumor lesions, but the postoperative recurrence
rate is higher and adjuvant postoperative chemotherapy is usually
needed (3). Postoperative
chemotherapy can inhibit local recurrence and distant metastasis,
so as to improve the overall survival of patients (4). The standard treatment method for bladder
cancer is the clinical first-line chemotherapy GC (gemcitabine plus
cisplatin). However, bladder cancer patients can easily develop
drug resistance. As a result, chemotherapy is often ineffective in
clinic (5). Therefore, new effective
treatment methods are needed.
In recent years, anti-folic acid drugs have been
widely used in treatment of malignant tumors (6). The most commonly used anti-folic acid
drug is methotrexate (MTX), which can inhibit dihydrofolate
reductase (DHFR) to block the synthesis of tetrahydrofolate (FH4).
Blocked FH4 causes restricted transfer of one-carbon group during
the synthesis of glycosides pyrimidine nucleotide and purine
nucleon, and inhibits the synthesis of DNA (7). Single-agent MTX, as a chemotherapeutic
drug, easily leads to the development of acquired drug resistance,
so it is often used in combination with other drugs such as cell
cycle inhibitor (8). Pralatrexate
(PTX), an upgraded product of methotrexate, is a new anti-folic
acid and anti-tumor drug that can effectively inhibit DHFR. It can
also competitively inhibit folylpolyglutamate synthetase to block
the synthesis of thymidine and other biomolecules that rely on
single-carbon transfer, so as to affect the synthesis of DNA and
promote apoptosis of tumor cells (9).
Palbociclib isethionate (PAL) is an FDA-approved highly selective
CDK4/6 and cell cycle inhibitor that can be used as a first-line
drug for the treatment of HER2-negative, ER-positive breast cancer,
colon cancer and lung cancer. It can also prolong the
progression-free survival of tumor patients (10). DHFR is a main drug target for
anti-infective therapy and tumor chemotherapy, due to its close
relationship with multidrug resistance in various tumors (11). Vascular endothelial growth factor
(VEGF) is a serum marker that reflects the malignancy degree of
tumors. VEGF can promote the proliferation of endothelial cells and
neovascularization, thereby promoting the growth and metastasis of
tumors (12).
At present, there is no report on the application of
PTX combined with PAL in the treatment of bladder cancer. In this
study, a retrospective analysis of medical records of 82 bladder
cancer patients admitted to Shengjing Hospital of China Medical
University (Shenyang, China) was performed. Our study investigated
the clinical efficacy and mechanism of PTX combined with PAL in the
treatment of bladder cancer patients.
Materials and methods
General information
A retrospective analysis of medical records of 82
bladder cancer patients admitted to Shengjing Hospital of China
Medical University from February 2015 to February 2018 was
performed. Patients treated with PTX combined with PAL served as
study group (42 cases) and patients with conventional GC
chemotherapy regimen as the control group (40 cases). There were 28
males and 14 females in the study group, with age ranged from 51 to
77 years and a mean age of 66.57±2.58 years, course of disease
range from 2 to 8 years, with a mean course of disease of 4.26±2.47
years. Clinical stage: 24 cases of stage III and 18 cases of stage
IV; tumor distribution location: 11 cases of lateral wall of
bladder, 19 cases of anterior wall and 12 cases of posterior wall.
There were 29 males and 11 females in control group, aged from 53
to 79 years, with a mean age of 67.21±3.27 years, course of disease
ranged from 3 to 7 years, with a mean course of disease of
4.76±3.08 years. Clinical stage: 21 cases of stage III and 19 cases
of stage IV; tumor distribution location: 13 cases of lateral wall
of bladder, 16 cases of anterior wall and 11 cases of posterior
wall.
Inclusion and exclusion criteria
Inclusion criteria: confirmed as bladder cancer by
pelvic CT, cystoscope biopsy and pathology (13); no contraindication to operation and
treatment; no other history of tumor or radiotherapy and
chemotherapy treatment; complete clinical data. This study was
approved by the Ethics Committee of Shengjing Hospital of China
Medical University. All participants signed informed consent.
Exclusion criteria: those with neurological disorders; those with
hematopoietic disorders and immune diseases; those with mental
illness or a history of family psychosis.
Treatment methods
Control group was treated with a conventional GC
chemotherapy regimen (14).
Ondansetron (15) (Fuan
Pharmaceutical Group Ningbo Team Pharmaceutical Co., Ltd., Ningbo,
China; batch number: H10960146) was orally administered before
chemotherapy to stop vomiting, 1,000 mg/m2 of
gemcitabine (Harbin Gloria Pharmaceutical Co., Ltd., Harbin, China;
batch number: H20040958) was infused intravenously for 2 h on the
1st, 8th and 15th days of each cycle, and 30 mg/m2 of
cisplatin (Yunnan Gejiu Biological Pharmaceutical Co., Ltd.,
Yunnan, China; batch number: H53021740) was infused intravenously
for 2 h on the 2nd day. A total of 4 cycles of chemotherapy was
performed, 28 days for one cycle. Study group was treated with PTX
combined with PAL. A total of 30 mg/m2 of PTX injection
(Allos Therapeutics Inc., Westminster, CO, USA) was infused
intravenously, 3 to 5 min each time, once a week. Six consecutive
cycles were performed, 28 days for one cycle. After intravenous
injection of PTX, 50 mg/m2 of PAL (Pfizer Pharmaceutical
Co., Ltd., New York, NY, USA) was orally administered in study
group once a week for 3 weeks, then treatment was stopped for 1
week. Six consecutive cycles were performed, 28 days for one cycle.
Changes of neutrophils and platelets in the two groups were
observed. If the neutrophil count was less than
1.0×109·L−1, then recombinant human
granulocyte colony-stimulating factor injection (Harbin
Pharmaceutical Group Biological Engineering Co., Ltd., Harbin,
China; batch number: S20000061) was administered, treatment was
started when blood cells returned to normal. Treatment methods are
shown in Table I.
 | Table I.Treatment methods. |
Table I.
Treatment methods.
| Chemotherapy
methods | Method of
administration |
|---|
| GC chemotherapy | Oral administration
of ondansetron before chemotherapy |
| (Control group) | Intravenous infusion
of gemcitabine (1,000 mg/m2) for 2 h at 1, 8 and 15
days |
|
| Intravenous infusion
of cisplatin (30 mg/m2) for 2 h on the 2nd day |
|
| 28 days for one
cycle, totally 4 cycles |
| PTX+PAL
chemotherapy | Intravenous infusion
of PTX (30 mg/m2) 3–5 min, once a week |
| (Study group) | Oral intake of PAL
(50 mg/m2), once a week for 3 weeks, followed by stop
for 1 week |
|
| 28 days for one
cycle, totally 6 consecutive cycles |
Efficacy evaluation
Based on the evaluation criteria of solid tumor
clinical treatment established by the World Health Organization
(WHO) (16), clinical efficacy of
study group and control group was evaluated and divided into four
categories: complete remission (CR) the target lesion disappeared
for >4 weeks and no new lesion was detected; partial remission
(PR): after treatment, the target lesion gradually decreased in
diameter, the reduction ratio of the diameter was >50% compared
with that before treatment, duration >4 weeks, and no new lesion
was detected; stable disease (SD): target lesion gradually
decreased in diameter, the reduction ratio of the diameter <50%
compared with that before treatment, and no new lesion was
detected; progressive disease (PD): increase ratio of the diameter
of the target lesion was ≥50% compared with that before treatment,
and new lesion produced. CR and PR were clinically effective rates.
Clinical response rate (RR) = (CR + PR)/total number of cases ×
100%. Adverse reactions during treatment in study group and control
group were observed. Main adverse reactions include
thrombocytopenia, leukopenia, nausea and vomiting, and liver
damage. Evaluation criteria for liver injury are based on the
classification criteria for acute and subacute toxicity of
antitumor drugs (17).
Indicator detection
Changes of liver function indexes were observed 1
day before treatment and 1 day after treatment. Venous blood (5 ml)
was taken and placed in a vacuum tube without anticoagulant. After
the blood was coagulated, blood was placed in a centrifuge
(Shanghai Luxiangyi Centrifuge Instrument Co., Ltd., Shanghai,
China) and centrifuged at 670.8 × g at 20–25°C for 10 min to
separate serum. AU5800 automatic biochemical analyzer [Beckman
Coulter Trading (China) Co., Ltd., Shanghai, China] was used to
detect serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), alkaline phosphatase (ALP) and total
bilirubin (TBil). Kits were purchased from Beckman Coulter Trading
(China) Co., Ltd., and the testing procedure was carried out with
reference to the instructions of the manufacturers.
RT-qPCR was used to detect the relative expression
of serum DHFR mRNA and VEGF mRNA in the 2 groups before treatment
and 1 month after treatment. A total of 10 ml fasting venous blood
was extracted and placed in a vacuum tube without anticoagulant.
Blood was centrifuged at 2000 rpm for 10 min to separate the serum.
Serum was stored in a −80°C refrigerator (Wuxi Guanya Refrigeration
Technology Co., Ltd., Wuxi, China) before use. Serum total RNA was
extracted using TRIzol kit (ABI Corporation, Lee's Summit, MO, USA)
according to manufacturer's instruction. Integrity of total RNA was
determined by 1% agarose gel electrophoresis and RNA concentration
was measured using a UV-Vis spectrophotometer (INESA Analytical
instrument Co., Ltd.; Shanghai, China). Reverse transcription was
performed using M-MLV reverse transcription kit (Beijing
Shengkeboyuan Biotechnology Co., Ltd., Beijing, China). Reaction
conditions: 45°C for 25 min and 80°C for 10 min. The synthesized
cDNA sample was stored at −20°C before use. DHFR mRNA and VEGF mRNA
fluorescent quantitative PCR kits were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA), U6 was used as
an endogenous control. Primers were designed and synthesized by
Invitrogen; Thermo Fisher Scientific, Inc. (Table II). RT-qPCR (20 µl of total volume)
reaction system: 10 µl of SYBRGreen mix, 1 µl of PCR primer mix, 5
µl of cDNA template diluted 10-fold and 4 µl of RNase free water.
PCR reaction conditions: Pre-denaturation at 94°C for 1 min,
followed by 35 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 20 sec and extension at 72°C for 1 min, and final
extension at 72°C for 10 min. PCR reactions were performed on ABI
PRISM 7300 fluorescence quantitative PCR instrument (ABI
Corporation) with U6 as endogenous control. Results were processed
using 2−ΔΔcq method (18).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Upstream | Downstream |
|---|
| DHFR |
5′-TGGTTCGCTAAACTGCATCGT-3′ |
5′-CAGGAATGGAGAACCAGGTCTTC-3′ |
| VEGF |
5′-GAGTATATCTTCAAGCCGTCCTGT-3′ |
5′-ATCTGCATAGTGACGTTGCTCTC-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
Statistical analysis
SPSS 19.0 [Yiyun (Shanghai) Information Technology
Co., Ltd., Shanghai, China] was used for statistical analysis.
Measurement data are expressed using mean ± standard deviation.
t-test was used for comparisons of data between groups, paired
t-test was used for comparison between data before treatment and
after treatment within the same group. Chi-square test was used for
comparison of enumeration data between groups. P<0.05 indicates
the difference is statistically significant.
Results
Baseline data of study group and
control group
There was no significant difference between study
group and control group in general clinical baseline data such as
sex, age, course of disease, histopathological type, pathological
differentiation degree, tumor distribution location, clinical stage
and existence of distant metastasis (P>0.05) (Table III).
 | Table III.Baseline data of study group and
control group [n (%)]/(mean ± SD). |
Table III.
Baseline data of study group and
control group [n (%)]/(mean ± SD).
| Category | Study group
(n=42) | Control group
(n=40) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.329 | 0.636 |
|
Male | 28 (66.67) | 29 (72.50) |
|
|
|
Female | 14 (33.33) | 11 (27.50) |
|
|
| Age (years) | 66.57±2.58 | 67.21±3.27 | 0.986 | 0.326 |
| Course of disease
(years) |
4.26±2.47 |
4.76±3.08 | 0.812 | 0.418 |
| Histopathological
type |
|
| 0.965 | 0.611 |
|
Transitional cell
carcinoma | 37 (88.10) | 36 (90.00) |
|
|
|
Squamous carcinoma | 1 (2.38) | 2 (5.00) |
|
|
|
Adenocarcinoma | 4 (9.52) | 2 (5.00) |
|
|
| Pathological
differentiation degree |
|
| 0.185 | 0.912 |
|
Well-differentiated | 14 (33.33) | 12 (30.00) |
|
|
|
Moderately differentiated | 19 (45.24) | 18 (45.00) |
|
|
| Poorly
differentiated | 9
(21.43) | 10 (25.00) |
|
|
| Tumor distribution
location |
|
| 0.419 | 0.811 |
| Lateral
wall of bladder | 11 (26.19) | 13 (32.50) |
|
|
|
Anterior wall | 19 (45.24) | 16 (40.00) |
|
|
|
Posterior wall | 12 (28.57) | 11 (27.50) |
|
|
| Clinical stage |
|
| 0.178 | 0.825 |
| Stage
III | 24 (57.14) | 21 (52.50) |
|
|
| Stage
IV | 18 (42.86) | 19 (47.50) |
|
|
| Distant
metastasis |
|
| 1.279 | 0.322 |
|
Yes | 9 (21.43) | 13 (32.50) |
|
|
| No | 33 (78.57) | 27 (67.50) |
|
|
| Tumor size |
|
| 0.483 | 0.513 |
| ≥3
cm | 22 (52.38) | 24 (60.00) |
|
|
| <3
cm | 20 (47.62) | 16 (40.00) |
|
|
Clinical efficacy of study group and
control group
After treatment, there were 14 cases of CR (33.33%),
8 cases of PR (19.05%), 11 cases of SD (26.19%), 9 cases of PD
(21.43%) and 22 cases of RR (52.38%) in study group and 9 cases of
CR (22.50%), 8 cases of PR (20.00%), 12 cases of SD (30.00%), 11
cases of PD (27.50%) and 17 cases of RR (42.50%) in control group.
There was no significant difference in treatment RR between study
group and control group (P>0.05) (Table IV).
 | Table IV.Comparison of results of clinical
efficacy between study group and control group [n (%)]. |
Table IV.
Comparison of results of clinical
efficacy between study group and control group [n (%)].
| Category | Study group
(n=42) | Control group
(n=40) | χ2
value | P-value |
|---|
| CR | 14 (33.33) | 9
(22.50) | – | – |
| PR | 8
(19.05) | 8
(20.00) | – | – |
| SD | 11 (26.19) | 12 (30.00) | – | – |
| PD | 9
(21.43) | 11 (27.50) | – | – |
| RR | 22 (52.38) | 17 (42.50) | 0.802 | 0.387 |
Changes in liver function indexes
before and after treatment in study group and control group
Serum concentrations of ALT, AST, ALP and TBil in
study group were not significantly different from those in control
group (P>0.05). After treatment, serum ALT concentration of
study group and control group was significantly higher than that
before treatment (t=11.300, P<0.001; t=14.570, P<0.001), and
serum AST concentration (t=20.220, P<0.001; t=22.510,
P<0.001), serum ALP concentration (t=15.68, P<0.001;
t=19.190, P<0.001), and serum TBil concentration (t=12.720,
P<0.001; t=20.080, P<0.001) were significantly higher than
before treatment. After treatment, serum ALT, AST, ALP, and TBil
concentrations in study group were significantly lower than those
in control group (t=3.536, P<0.001; t=3.541, P<0.001;
t=7.276, P<0.001) (Table V).
 | Table V.Changes in liver function indexes
before and after treatment in the study group and the control group
(mean ± SD). |
Table V.
Changes in liver function indexes
before and after treatment in the study group and the control group
(mean ± SD).
|
| Study group
(n=42) |
|
| Control group
(n=40) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Indexes | Before
treatment | After
treatment | t value | P-value | Before
treatment | After
treatment | t value | P-value |
|---|
| ALT (U/l) | 23.17±5.03 |
35.91±5.18a,b | 11.300 | <0.001 | 22.89±5.28 |
40.03±5.37c | 14.570 | <0.001 |
| AST (U/l) | 20.13±2.17 |
35.48±4.39a,b | 20.220 | <0.001 | 19.87±2.23 |
38.98±4.56c | 22.510 | <0.001 |
| ALP (U/l) | 66.29±9.37 |
110.13±15.37a,b | 15.68 | <0.001 | 66.47±9.03 |
119.51±14.97c | 19.190 | <0.001 |
| TBil (µmol/l) | 10.58±1.33 |
14.25±1.28a,b | 12.720 | <0.001 | 10.34±1.24 |
16.47±1.48c | 20.080 | <0.001 |
Treatment of adverse reactions in
study group and control group
Common adverse reactions in study group and control
group were thrombocytopenia, leukopenia, nausea and vomiting and
liver function impairment. Study group had 6 cases of
thrombocytopenia (14.29%), 19 cases of leucopenia (45.24%), 15
cases of nausea and vomiting (35.71%) and 3 cases of liver function
impairment (7.14%). The control group showed 4 cases of
thrombocytopenia (10.00%), 16 cases of leukopenia (40.00%), 22
cases of nausea and vomiting (55.00%) and 10 cases of liver
function impairment (25.00%). There was no significant difference
in the rate of thrombocytopenia and leukopenia during treatment
between study group and control group (P>0.05). The rates of
nausea and vomiting and liver function impairment during treatment
in study group were significantly lower than those in control group
(χ2=4.843, P=0.044; χ2=4.897, P=0.035) (Table VI).
 | Table VI.Comparison of results of treatment of
adverse reactions between study group and control group [n
(%)]. |
Table VI.
Comparison of results of treatment of
adverse reactions between study group and control group [n
(%)].
| Category | Study group
(n=42) | Control group
(n=40) | χ2
value | P-value |
|---|
|
Thrombocytopenia | 6 (14.29) | 4 (10.00) | 0.799 | 0.738 |
| Leukopenia | 19 (45.24) | 16 (40.00) | 0.230 | 0.661 |
| Nausea and
vomiting | 15 (35.71) | 22 (55.00) | 4.843 | 0.044 |
| Liver function
impairment | 3 (7.14) | 10 (25.00) | 4.897 | 0.035 |
Changes of expression of serum DHFR
mRNA and VEGF mRNA before and after treatment in study group and
control group
Relative expression levels of serum DHFR mRNA and
VEGF mRNA before treatment in study group were (3.86±1.12) and
(13.36±4.69), respectively, and those after treatment were
(2.05±0.52) and (5.41±2.57), respectively. Those before treatment
in control group were (3.73±1.31) and (13.57±4.73), respectively,
and those after treatment were (2.94±0.49) and (7.15±2.63),
respectively. There was no significant difference in the before
treatment between study group and control group (P>0.05). In
study group and control group, relative expression level of serum
DHFR mRNA after treatment was significantly lower than that before
treatment (t=9.499, P<0.001; t=3.572, P<0.001), and serum
VEGF mRNA level after treatment was significantly lower than that
before treatment (t=9.634, P<0.001; t=7.503, P<0.001).
Relative expression levels of serum DHFR mRNA and VEGF mRNA after
treatment in study group were significantly lower than those in
control group (t=7.968, P<0.001; t=3.030, P=0.003) (Fig. 1).
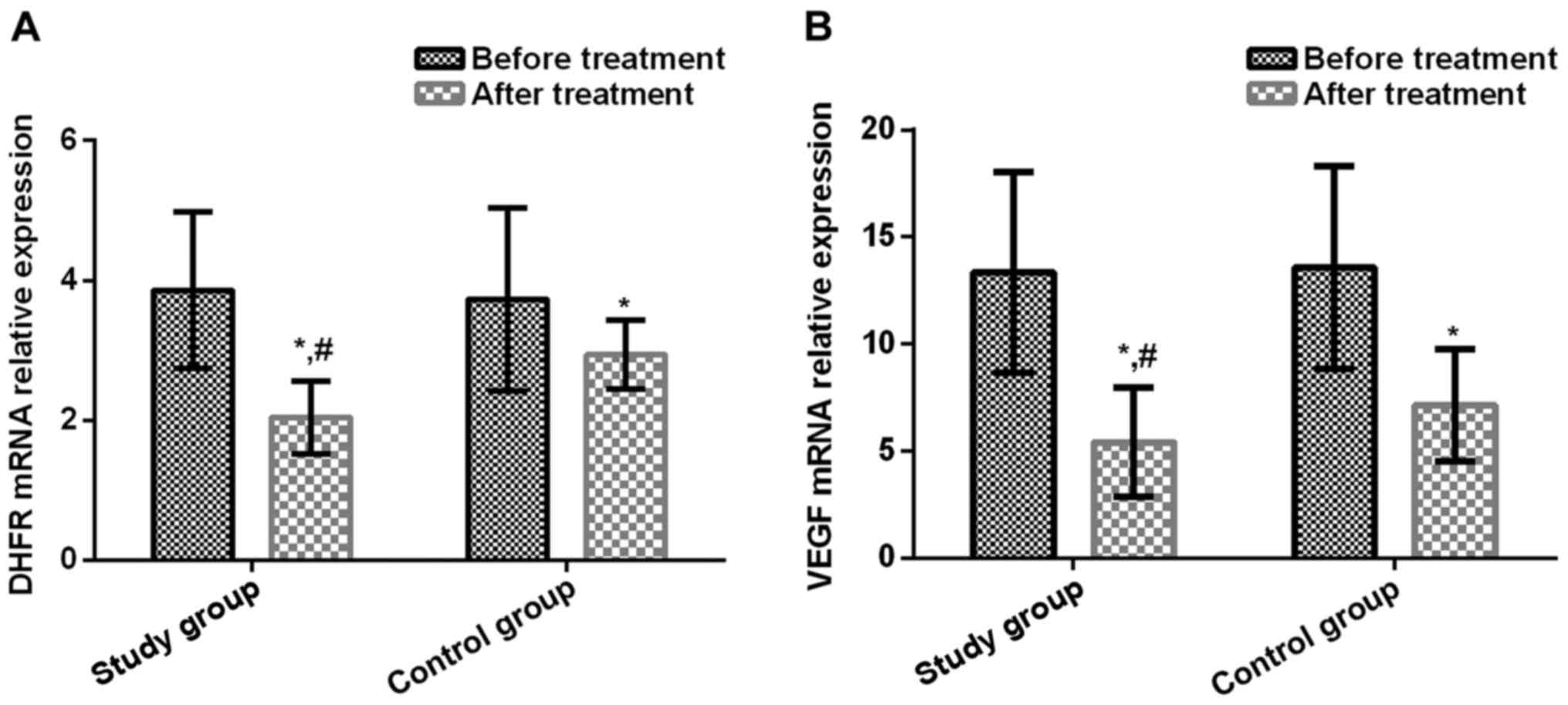 | Figure 1.(A) Comparison of relative expression
levels of serum DHFR mRNA before and after treatment in study group
and control group. Results of RT-qPCR showed that there was no
significant difference in the relative expression levels of serum
DHFR mRNA before treatment between study group and control group
(P>0.05). In study group and control group, serum DHFR mRNA
levels after treatment were significantly lower than those before
treatment (t=9.499, P<0.001; t=3.572, P<0.001). After
treatment, serum DHFR mRNA levels in study group were significantly
lower than in control group (t=7.968, P<0.001). (B) Comparison
of relative expression levels of serum VEGF mRNA before and after
treatment in study group and control group. Results of RT-qPCR
showed that there was no significant difference in the relative
expression of serum VEGF mRNA before treatment between study group
and control group (P>0.05). In study group and control group,
serum VEGF mRNA levels after treatment were significantly lower
than those before treatment (t=9.634, P<0.001; t=7.503,
P<0.001). After treatment, serum VEGF mRNA levels were
significantly lower in study group than in control group (t=3.030,
P=0.003). *P<0.001, compared with before treatment level;
#P<0.001, compared with after treatment level in
control group. |
Discussion
The basic treatment method for bladder cancer is
radical cystectomy, which can prolong the overall survival of
bladder cancer patients. However, surgical operations cause trauma.
In addition, recurrence rate of some high-risk patients can reach
80% after surgery, thereby affecting the prognosis of patients
(19). Postoperative metastasis and
recurrence are major causes of poor treatment outcomes of bladder
cancer. Thus, chemical drug treatment after surgery is usually
needed (20). Chemotherapy drugs kill
tumor cells, but they also damage the body's normal immune
functions. In addition, development of drug-resistance will also
reduce the efficacy of chemotherapy drugs (21). At present, the most widely used
treatment method is GC chemotherapy regimen. With cytotoxic
effects, gemcitabine and cisplatin can cause tumor cell death to
control local tumor proliferation. As a result, the median survival
and relapse-free survival rate of tumor patients are improved
(22). Cognetti et al
(23) showed that using GC
chemotherapy regimen to treat invasive bladder cancer could improve
the 5-year survival rate and disease-free survival rate. Clinical
anti-folic acid drug chemotherapy regimen is used in treatment of
various malignant tumors, its resistance rate of single traditional
anti-folic acid drug MTX is high, so it is often used in
combination with other drugs such as cell cycle inhibitor (24). PTX, a small molecule anti-folic acid
drug with a high affinity for reductive folic acid type I carrier
protein, can increase tyrosine multimerization. It can also
increase the drug uptake rate of cells and prolong the action time
of drugs in tumor cells, so as to increase the drug concentration
in them (25).
PTX is a targeted folic acid chemical for the
treatment of chemotherapy-resistant or recurrent peripheral T-cell
lymphoma that preferentially aggregates in tumor cells (26). CDK4/6, as an important regulatory
protein involved in cell division cycle, can induce cell
transformation from G1 phase to S phase. Inhibiting these two
enzymes may block cell division (27). PAL, as a highly selective CDK4/6
inhibitor, can inhibit cells entering the S phase, cell growth and
DNA replication (28). Results of
this study showed that there was no significant difference in RR
between study group and control group. After treatment, the
concentration of ALT, AST, ALP, TBil in serum of the study group
and the control group was significantly higher than those before
treatment (P<0.05). The concentration of ALT, AST, ALP, TBil in
the study group was significantly lower than that in the control
group after treatment (P<0.05). The rates of nausea and vomiting
and liver function impairment during treatment in study group were
significantly lower than those in control group, suggesting that
PTX combined with PAL has a better clinical efficacy in the
treatment of bladder cancer patients. All the patients had some
liver damage after chemotherapy, and PTX combined with PAL can kill
cancer cells and reduce adverse reactions during treatment. During
the progression of bladder cancer, tumor cells can synthesize
various biological molecules and participate in the occurrence and
development of bladder cancer (29).
As a dihydrofolate (FH2) reductase, DHFR is capable of reducing
dihydrofolateI level in the body. FH4, a carrier of one-carbon
group, is involved in the synthesis of DNA (30).
Studies have shown that DHFR is highly expressed in
drug-resistant tumor cells such as leukemia drug-resistant cells,
breast cancer drug-resistant cells and osteosarcoma drug-resistant
cells, and plays a role in multidrug resistance. With the increase
of drug resistance in cells, DHFR expression significantly
increases (31,32). VEGF can induce vascular endothelial
chemotaxis in vitro or in vivo, and is important for
increasing the permeability of the vascular wall and maintaining
the integrity of blood vessels. It can induce tumor
neovascularization, which is conducive to the growth and
infiltration of tumor cells (33).
Hirata et al (34) showed that
low-dose MTX can inhibit the proliferation of vascular endothelial
cells in vitro and the neovascularization in vivo,
suggesting that anti-folic acid drugs have a certain inhibitory
effect on neovascularization in vivo. Results of this study
showed that relative expression levels of serum DHFR mRNA and VEGF
mRNA after treatment in study group and control group were
significantly lower than those before treatment. After treatment,
levels of serum DHFR mRNA and VEGF mRNA were significantly lower in
study group than those in control group, suggesting that the
mechanism of the actions of PTX combined with PAL in the treatment
of bladder cancer patients is likely related to the suppressed
tumor neovascularization, which is achieved by inhibiting the
expression of DHFR and VEGF. Similar findings have been reported by
Jocham et al (35). By
regulating the expression of DHFR protein, PAL combined with PTX or
MTX has a good effect on mantle cell lymphoma with or without p53
deficiency in blocking tumor cells in G1/S phase.
In this study, subjects were screened in strict
accordance with the inclusion and exclusion criteria. There was no
significant difference between study group and control group in
general clinical baseline data such as sex, age, course of disease,
histopathological type, pathological differentiation degree, tumor
distribution location, clinical stage and the existence of distant
metastasis, indicating the high reliability of this study. However,
in this study, bladder cancer patients were included, so the
regulatory mechanism of PTX combined with PAL on DHFRV, VEGF was
not clarified. The clinicopathological parameters of bladder cancer
patients are different from those of other malignant tumors, and
there are some limitations on whether PTX combined with PAL is
applicable to other malignant tumors. In future investigations, it
is necessary to extend the research time, expand the category of
malignant tumors, and carry out in vitro experiments to
explore the mechanism of PTX combined with PAL in malignant
tumors.
In conclusion, PTX combined with PAL can reduce
adverse reactions of nausea and vomiting and liver function
impairment during treatment. The mechanism of its actions may be
related to the suppressed tumor neovascularization, which is
achieved by inhibiting expression of DHFR and VEGF. PTX combined
with PAL may become a new method for the treatment of bladder
cancer patients. DHFRN and VEGF is expected to be a new biological
therapy target for bladder cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW collected the general information of patients. XW
and HW were responsible for clinical efficacy evaluation. YS
detected and analyzed the indicators. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shengjing Hospital of China Medical University (Shenyang, China).
Patients, who participated in this research, had complete clinical
data. Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: a
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arcangeli G, Strigari L and Arcangeli S:
Radical cystectomy versus organ-sparing trimodality treatment in
muscle-invasive bladder cancer: A systematic review of clinical
trials. Crit Rev Oncol Hematol. 95:387–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tekin A, Aki FT and Ozen H: Radical
cystectomy versus alternative treatments for muscle-confined
bladder cancer. Int Urol Nephrol. 33:357–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HS, Jeong CW, Kwak C, Kim HH and Ku
JH: Adjuvant chemotherapy for muscle-invasive bladder cancer: A
systematic review and network meta-analysis of randomized clinical
trials. Oncotarget. 8:81204–81214. 2017.PubMed/NCBI
|
|
6
|
Tanino R, Tsubata Y, Harashima N, Harada M
and Isobe T: Novel drug-resistance mechanisms of pemetrexed-treated
non-small cell lung cancer. Oncotarget. 9:16807–16821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhushan B, Ahuja D, Verma S, Saluja S,
Siddiqui S and Kapur S: Relation of cell viability and apoptosis
with clinical remission following induction chemotherapy in ALL and
AML. J Exp Clin Cancer Res. 26:313–321. 2007.PubMed/NCBI
|
|
8
|
Depau L, Brunetti J, Falciani C, Scali S,
Riolo G, Mandarini E, Pini A and Bracci L: Coupling to a
cancer-selective heparan-sulfate-targeted branched peptide can
by-pass breast cancer cell resistance to methotrexate. Oncotarget.
8:76141–76152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchi E, Mangone M, Zullo K and O'Connor
OA: Pralatrexate pharmacology and clinical development. Clin Cancer
Res. 19:6657–6661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nemoto A, Saida S, Kato I, Kikuchi J,
Furukawa Y, Maeda Y, Akahane K, Honna-Oshiro H, Goi K, Kagami K, et
al: Specific antileukemic activity of PD0332991, a CDK4/6
inhibitor, against Philadelphia chromosome-positive lymphoid
leukemia. Mol Cancer Ther. 15:94–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fawal MA, Jungas T, Kischel A, Audouard C,
Iacovoni JS and Davy A: Cross talk between one-carbon metabolism,
Eph signaling, and histone methylation promotes neural stem cell
differentiation. Cell Rep. 23:2864–2873.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raghunathachar Sahana K, Akila P, Prashant
V, Sharath Chandra B and Nataraj Suma M: Quantitation of vascular
endothelial growth factor and interleukin-6 in different stages of
breast cancer. Rep Biochem Mol Biol. 6:33–39. 2017.PubMed/NCBI
|
|
13
|
Chang SS, Boorjian SA, Chou R, Clark PE,
Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD,
et al: Diagnosis and treatment of non-muscle invasive bladder
cancer: AUA/SUO guideline. J Urol. 196:1021–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazza P, Moran GW, Li G, Robins DJ,
Matulay JT, Herr HW, Decastro GJ, McKiernan JM and Anderson CB:
Conservative management following clinical complete response to
neoadjuvant chemotherapy of muscle invasive bladder cancer:
Contemporary outcomes of a multi-institutional cohort study. J
Urol. May 19–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bell GC, Caudle KE, Whirl-Carrillo M,
Gordon RJ, Hikino K, Prows CA, Gaedigk A, Agundez J, Sadhasivam S,
Klein TE, et al: Clinical Pharmacogenetics Implementation
Consortium (CPIC) guideline for CYP2D6 genotype and use of
ondansetron and tropisetron. Clin Pharmacol Ther. 102:213–218.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al; OAK Study Group, . Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: Validity and reliability of the US National
Cancer Institute's patient-reported outcomes version of the common
terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol.
1:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perkins JR, Dawes JM, McMahon SB, Bennett
DL, Orengo C and and Kohl M: ReadqPCR and NormqPCR: R packages for
the reading, quality checking and normalisation of RT-qPCR
quantification cycle (Cq) data. BMC Genomics. 13:2962012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mak RH, Hunt D, Shipley WU, Efstathiou JA,
Tester WJ, Hagan MP, Kaufman DS, Heney NM and Zietman AL: Long-term
outcomes in patients with muscle-invasive bladder cancer after
selective bladder-preserving combined-modality therapy: A pooled
analysis of Radiation Therapy Oncology Group protocols 8802, 8903,
9506, 9706, 9906, and 0233. J Clin Oncol. 32:3801–3809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soave A, Riethdorf S, Dahlem R, von
Amsberg G, Minner S, Weisbach L, Engel O, Fisch M, Pantel K and
Rink M: A nonrandomized, prospective, clinical study on the impact
of circulating tumor cells on outcomes of urothelial carcinoma of
the bladder patients treated with radical cystectomy with or
without adjuvant chemotherapy. Int J Cancer. 140:381–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi X, Wong BL, Lau SH, Ng KT, Kwok SY,
Kin-Wai Sun C, Tzang FC, Shao Y, Li CX, Geng W, et al: A
hemoglobin-based oxygen carrier sensitized Cisplatin based
chemotherapy in hepatocellular carcinoma. Oncotarget.
8:85311–85325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Narayan V, Mamtani R, Keefe S, Guzzo T,
Malkowicz SB and Vaughn DJ: Cisplatin, gemcitabine, and lapatinib
as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer
Res Treat. 48:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cognetti F, Ruggeri EM, Felici A, Gallucci
M, Muto G, Pollera CF, Massidda B, Rubagotti A, Giannarelli D and
Boccardo F; Study Group, . Adjuvant chemotherapy with cisplatin and
gemcitabine versus chemotherapy at relapse in patients with
muscle-invasive bladder cancer submitted to radical cystectomy: An
Italian, multicenter, randomized phase III trial. Ann Oncol.
23:695–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cipolleschi MG, Marzi I, Rovida E,
Olivotto M and Dello Sbarba P: Low-dose methotrexate enhances
cycling of highly anaplastic cancer cells. Cell Cycle. 16:280–285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta SC, Sung B, Prasad S, Webb LJ and
Aggarwal BB: Cancer drug discovery by repurposing: Teaching new
tricks to old dogs. Trends Pharmacol Sci. 34:508–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Connor OA, Hamlin PA, Portlock C,
Moskowitz CH, Noy A, Straus DJ, Macgregor-Cortelli B, Neylon E,
Sarasohn D, Dumetrescu O, et al: Pralatrexate, a novel class of
antifol with high affinity for the reduced folate carrier-type 1,
produces marked complete and durable remissions in a diversity of
chemotherapy refractory cases of T-cell lymphoma. Br J Haematol.
139:425–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toogood PL, Harvey PJ, Repine JT, Sheehan
DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T,
et al: Discovery of a potent and selective inhibitor of
cyclin-dependent kinase 4/6. J Med Chem. 48:2388–2406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen MJ, Shimada T, Moulton AD, Cline A,
Humphries RK, Maizel J and Nienhuis AW: The functional human
dihydrofolate reductase gene. J Biol Chem. 259:3933–3943.
1984.PubMed/NCBI
|
|
31
|
Banerjee D, Mayer-Kuckuk P, Capiaux G,
Budak-Alpdogan T, Gorlick R and Bertino JR: Novel aspects of
resistance to drugs targeted to dihydrofolate reductase and
thymidylate synthase. Biochim Biophys Acta. 1587:164–173. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poyet C, Thomas L, Benoit TM, Delmo DA,
Luberto L, Banzola I, Günthart MS, Sais G, Eberli D, Sulser T, et
al: Implication of vascular endothelial growth factor A and C in
revealing diagnostic lymphangiogenic markers in node-positive
bladder cancer. Oncotarget. 8:21871–21883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirata S, Matsubara T, Saura R, Tateishi H
and Hirohata K: Inhibition of in vitro vascular endothelial cell
proliferation and in vivo neovascularization by low-dose
methotrexate. Arthritis Rheum. 32:1065–1073. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jocham D, Witjes F, Wagner S, Zeylemaker
B, van Moorselaar J, Grimm MO, Muschter R, Popken G, König F and
Knüchel R: Improved detection and treatment of bladder cancer using
hexaminolevulinate imaging: A prospective, phase III multicenter
study. J Urol. 174:862–866. 2005. View Article : Google Scholar : PubMed/NCBI
|















