Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors with a high incidence worldwide (1). According to the International Agency for
Research on Cancer (IARC) GLOBOCAN 2012 monitoring data, there were
an estimated ~1.36 million cases novel cases of CRC globally, and
by 2017 the global novel cases of would rise to 3.54 million cases
(2,3).
CRC has the third highest incidence among all malignant tumors,
surpassed by only lung and breast cancer, and the fifth highest
mortality rate worldwide (4). The
incidence and mortality rates of CRC have declined in recent years
in Western developed countries, but both parameters are steadily
increasing in developing countries (5,6). Due to
the gradually changing eating habits and increased high fat and
protein intake, the incidence of CRC in China is expected to become
one of the fastest rising of all malignant tumors. The occurrence
of CRC is associated with environmental and genetic factors
(7). Typical monogenic disease
accounts for only 1–5% of CRC cases; the occurrence and development
of CRC is a multi-step, multi-stage process, involving multiple
genes, and events at the genome level and genetic polymorphisms
form the basis of genetic susceptibility (8). Genetic research has primarily been
focused on genes associated with cell cycle regulation, apoptosis,
DNA repair and metabolism.
Adipocyte enhancer-binding protein 1 (AEBP1) is a
newly identified type of inflammation-associated regulatory factor
(9) and a previous study demonstrated
that AEBP1 may inhibit the κB inhibitor, IκBα, in order to promote
the activity of the nuclear factor (NF)-κB pathway, which is
involved in a number of biological functions (10). Additionally, evidence suggests that
AEBP1 is likely to be involved in the occurrence of numerous tumors
(11,12). Further study of the key molecular
mechanisms of AEBP1 for novel drug development and clinical
treatment has important social value and clinical significance. At
present, studies regarding AEBP1 expression in CRC cells and the
use of oxaliplatin in chemotherapy sensitivity are
insufficient.
In the present study, CRC tissues were used to
detect the associations among AEBP1 expression, patient survival
and tumor pathology in CRC, in order to investigate chemotherapy
sensitivity in HT-29 cells following AEBP1 depletion, and to study
the role of AEBP1 in the biological functions of CRC, including
infiltration, differentiation and metastasis. AEBP1 is expected to
become a predictor of recurrent CRC metastasis and is a potential
molecular target for treatment.
Patients and methods
Patient tissue samples
A total of 62 randomly selected patients at Weifang
People's Hospital (Weifang, China) underwent CRC tumor resection
between January 2010 and December 2012. Follow-up, ranging between
4 and 63 months (median, 35 months), was available for all 62
patients. Of the group, 42 patients were male and 20 were female
with ages ranging between 28 and 79 years (median, 66 years). The
clinical features of the patients are presented in Table I. The records of all of the patients
contained basic information, including Tumor-Node-Metastasis (TNM)
stage, the degree of differentiation, lymph node metastasis and
distant metastasis, according to the 2002 International Cancer
Alliance TNM staging criteria (13).
Paired non-cancerous tissues were obtained from a segment of the
resected specimens that was >5 cm from the tumor. All the tissue
specimens were placed in liquid nitrogen or were fixed with 4%
formaldehyde for 24 h at room temperature (RT) within half an hour
of the tumor resection. The present clinical study was approved by
the Medical Ethics Committee of Weifang People's Hospital and
written informed consent was obtained from all patients upon
admission.
 | Table I.Association between AEBP1 distribution
and clinicopathological characteristics in colorectal cancer
patients. |
Table I.
Association between AEBP1 distribution
and clinicopathological characteristics in colorectal cancer
patients.
|
|
| AEBP1 |
|
|---|
|
|
|
|
|
|---|
| Variable | Number | High | Low | χ2 | P-value |
|---|
| Total cases | 62 | 39 | 23 |
|
|
| Age, years |
|
|
| 1.986 | 0.159 |
| ≥60 | 36 | 20 | 16 |
|
|
|
<60 | 26 | 19 | 7 |
|
|
| Sex |
|
|
| 0.353 | 0.553 |
| Male | 30 | 20 | 10 |
|
|
|
Female | 32 | 19 | 13 |
|
|
| Tumor size, cm |
|
|
| 1.728 | 0.189 |
| ≥5 | 31 | 22 | 9 |
|
|
|
<5 | 31 | 17 | 14 |
|
|
| TNM stage |
|
|
| 5.939 | 0.015 |
| I+II | 28 | 13 | 15 |
|
|
|
III+IV | 34 | 26 | 8 |
|
|
| Tumor
differentiation |
|
|
| 2.375 | 0.305 |
| WD | 20 | 10 | 10 |
|
|
| MD | 20 | 13 | 7 |
|
|
| PD | 22 | 16 | 6 |
|
|
| Recurrence |
|
|
| 7.281 | 0.007 |
| Yes | 30 | 24 | 6 |
|
|
| No | 32 | 15 | 17 |
|
|
| Metastasis |
|
|
| 8.793 | 0.003 |
|
Yes | 34 | 27 | 7 |
|
|
| No | 28 | 12 | 16 |
|
|
Immunohistochemistry (IHC) and result
interpretation
AEBP1 expression was analyzed by IHC on
paraffin-embedded tissue specimens from the 62 patients with CRC.
The excised tumor samples were fixed with 4% formaldehyde for 18–24
h at RT and embedded in paraffin prior to being prepared into
consecutive 5-µm sections. The sections were dewaxed with xylene
then hydrated through a graded series of ethanol (70, 80, 90, 95
and 100%) for 5 min of each series at RT. Following general
deparaffinization, antigen retrieval was performed at 95°C for 30
sec with an autoclave using 0.01 mol/l sodium citrate buffer (pH
6.0). Hydrogen peroxide (0.3%) was used to block endogenous
peroxidase activity for 30 min at 37°C, and non-specific
immunoglobulin binding sites were blocked using 5% normal goat
serum (cat. no. SP KIT-B1; Fuzhou Maixin Biotech Co., Ltd.) for 30
min at 37°C. The sections were incubated at 4°C overnight with a
purified AEBP1 rabbit polyclonal antibody (Novus Biologicals, LLC,
Littleton, CO, USA; dilution, 1:200). The sections were rinsed 3
times with phosphate-buffered saline (PBS) for 5 min each time
prior to being incubated for 30 min at RT with a biotinylated
anti-mouse/rabbit IgG secondary antibody [dilution, 1:100; cat. no.
KIT-0305; UltraSensitive™ SP (Mouse/Rabbit) IHC Kit, Fuzhou Maixin
Biotech Co., Ltd.]. Following washing, the sections were incubated
for 30 min at RT with streptavidin-biotin conjugated with
horseradish peroxidase [dilution, 1:100; cat. no. KIT-0305;
UltraSensitive™ SP (Mouse/Rabbit) IHC kit], and the slides were
visualized with 3,3′-diaminobenzidine tetrahydrochloride. The
sections were stained with Meyer's hematoxylin for 2 min at RT. As
a negative control, normal rabbit IgG (cat. no. A7016) or mouse IgG
(cat. no. A7028; both dilution, 1:100; both Beyotime Institute of
Biotechnology, Haimen, China) was used as the primary antibody and
incubated for 30 min at RT.
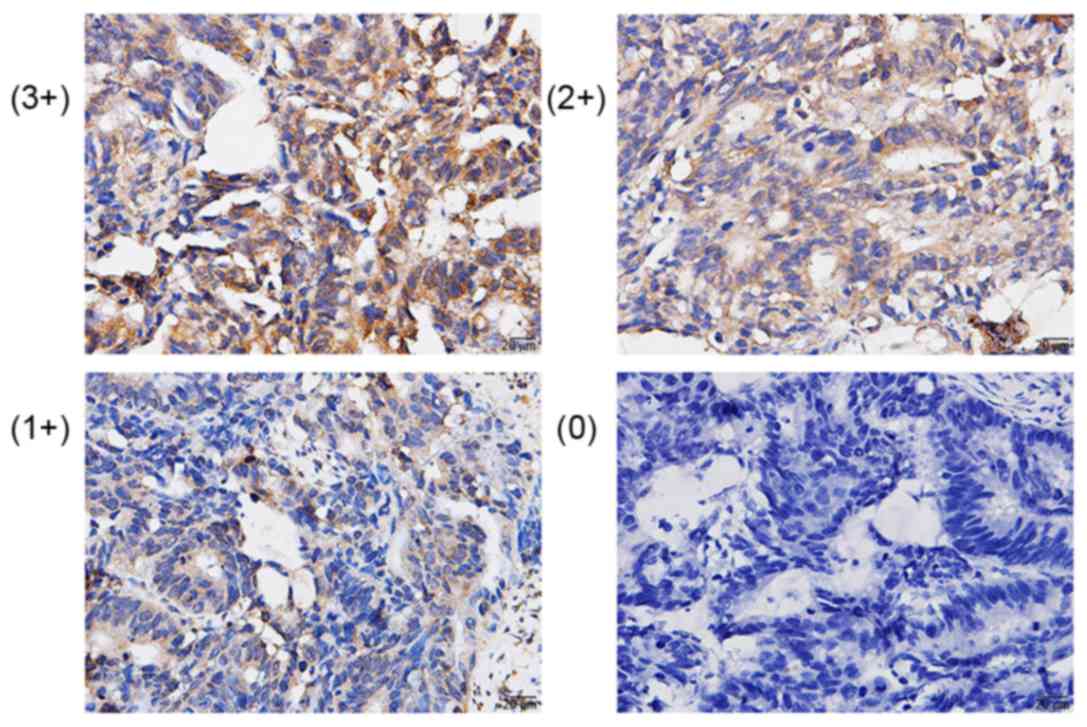
The staining intensity score was defined as 0 points
(negative), 1 point (weakly positive), 2 points (positive) and 3
points (strongly positive). Percent positivity scores were defined
as follows: 1 (<25% cell staining), 2 (25–50% cell staining), 3
(51–75%) and 4 (>75% of cells staining). The final score was
defined as the value of the percent positivity score multiplied by
the staining intensity score and final scores ranged between 0 and
12. The tumors were divided as follows: Negative (−), score 0; low
expression (1+), score 1–4; moderate expression (2+), score 5–8;
and strong expression (3+), score 9–12. The IHC results of AEBP1
were grouped into 2 categories: Low expression (0 and 1+) and high
expression (2+ and 3+).
Cell transfection and cell
proliferation/apoptosis detection
The human CRC HT29 cell line was purchased from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The cells were cultured at
37°C in a 5% CO2 incubator and used in subsequent experiments when
they had grown in cell culture flask to 80–100% confluence. AEBP1
depletion siRNA (Shanghai GenePharma Co., Ltd, Shanghai, China) was
transfected into cells in 6-well plates for 36 h of cell culture
until the cells reached 70–80% confluence. The AEBP1 sequences used
were as follows: Forward, 5′-CATCTACCCACTCACCTGGAA-3′ and reverse,
5′-CACTCCTCGTTCACCACCTT-3′. A microRNA 214 (miR-214) mimic
(transfected with 1 µg/100 µl miR-214 mimic; Nanjing KeyGen BioTech
Co., Ltd, Nanjing, China), negative-scramble control (transfected
with negative mimic, NC) and blank control (non-transfected cells,
BC) were used for transfection. The miR-214 sequences were as
follows: Forward, 5′-AGCATAATACAGCAGGCACAGAC-3 and reverse,
5′-AAAGGTTGTTCTCCACTCTCTCAC-3′. The transfections were performed
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). To begin with, solution A [10 µl 100 pmol/µl AEBP1 siRNA +
250 µl opti-minimum essential medium (MEM; Gibco; Thermo Fisher
Scientific, Inc.)] and solution B (10 µl Lipofectamine 2000 + 250
µl opti-MEM) were prepared. The 2 solutions (A + B) were then mixed
together and incubated for 20 min at RT. Next, 500 µl A + B and 1.5
ml opti-MEM were added dropwise into each well of the 6-well plate.
The transfected cells were cultured at 37°C for 6 h, the medium was
removed, and then 2 ml RPMI-1640 medium with 10% FBS (both Gibco;
Thermo Fisher Scientific, Inc.) was added, followed by incubation
for 36 h prior to further experiments. Each group was set up in 3
replicate wells.
HT29 cells (2×103) were plated into each well of a
96-well plate and were subsequently transfected with AEBP1
depletion siRNA, a negative scramble control and a BC. Following
cell transfection, 50 µg/ml oxaliplatin was added to the plate at
6, 12, 24, 36, 48, 60 and 72 h prior to the addition of the CCK-8
reagent at 37°C. (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). The optical density value was detected at a wavelength of
450 nm. Each group was set up in 5 replicate wells.
Suspended cells (2×104) were plated into each well
of 6-well plates (with a coverslip) for transfection. The cells
were then treated with oxaliplatin (added dropwise, 100 µg/2 ml)
for 72 h and a FITC Annexin V-PI apoptosis detection kit (Nanjing
KeyGen Biotech Co., Ltd.) was performed. Cells were collected by
trypsin (without ethylenediamime-N,N,N'N'-tetraacetic acid)
digestion. Following washing and centrifuging at 700 × g for 5 min
at 4°C, 5×105 cells were suspended in 500 µl binding buffer
(Nanjing KeyGen Biotech Co., Ltd.), and incubated for 15 min with 5
µl Annexin V-fluorescein isothiocyanate and 5 µl propidium iodide
for 10 min at RT in the dark. The cells were quantitatively
analyzed by a standard Becton-Dickinson FACSAria instrument (BD
FACSAria™III; BD Biosciences, Franklin Lakes, NJ, USA). The data
was acquired and analyzed using the FACS DiVa 4.1 software (BD
Biosciences).
AEBP1 upstream target gene prediction
and dual-luciferase assay
Targetscan (http://www.targetscan.org/), Pictar (http://pictar.mdc-berlin.de/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
predict upstream miRNA target genes. Through bioinformatics
prediction from the three aforementioned websets, the AEBP1
3′-untranslated region (UTR) was revealed to be associated with an
miR-214 binding site and thus, wild-type (WT) and mutant (MUT)
AEBP1 3′-UTR luciferase reporter gene plasmids (Luc-AEBP-3′-UTR;
Shanghai GenePharma Co., Ltd.) were constructed for further study.
Luc-AEBP-3′-UTR (WT) or (MUT) 3′-pGL3 luciferase reporter vectors
were constructed by Shanghai GeneChem Co., Ltd. (Shanghai, China).
293T cells were plated onto 96-well plates at 60% confluence 24 h
prior to transfection. According to Lipofectamine® 2000
Transfection Reagent Instructions (Invitrogen; Thermo Fisher
Scientific, Inc.) for transfection operation. At the time of
transfection, 50 µl RPMI-1640 medium, 50 µl Dual Glo®
Luciferase reagent (premix; Promega Corporation, Madison, WI, USA),
and 100 µl Glo® Stop and Glo® reagent
(Promega Corporation) were added to each well of a LockWell
MaxiSorp test plate (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The Firefly and Renilla luciferase fluorescence values
(Renilla luciferase activity as a reference for transfection
efficiency) were detected using a Dual-Luciferase®
Reporter Assay System (Promega Corporation). The experiment was
repeated 3 times.
Western blot analysis
Total HT-29 cell protein was extracted in lysis
buffer (Pierce; Thermo Fisher Scientific, Inc.) and quantified
using the Bradford method (14).
AEBP1 was detected. Protein (50 µg) was separated using 12%
SDS-PAGE electrophoresis. After the proteins were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA), and the membranes were blocked with 5% skimmed milk in
Tris-buffered saline with 0.1% Tween-20 at RT for 2 h; following
this, the membranes were incubated overnight at 4°C with antibodies
against AEBP1 (cat. no. sc-271374; 1:500), or β-actin (cat. no.
sc-47778; 1:2,000; both Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Following washing with TBS with Tween-20 three times, the
membranes were incubated with rabbit anti-mouse IgG-HRP (cat. no.
ab6728; 1:5,000; Abcam, Cambridge, MA, USA) for 2 h at RT. The
enhanced chemiluminescence chromogenic system (EMD Millipore) was
used prior to imaging, and the experiment was repeated in
triplicate.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows
was used for all statistical analyses. The χ2 test was used to
evaluate the associations between AEBP1 expression and various
clinicopathological parameters. The differences between the means
of the two groups were determined by one-way analysis of variance
(ANOVA) with post-hoc Dunnett's comparison. The Kaplan-Meier method
was used to estimate patient survival rates and these results were
compared using the log-rank test. Cox regression was performed for
univariate and multivariate analysis of prognostic variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AEBP1-positive expression and the
association between AEBP1 and CRC clinicopathological
parameters
AEBP1 protein expression in the CRC tissues and
their paired non-cancerous tissues from the 62 patients were
examined by IHC and AEBP1 was primarily expressed in the nucleus
and the cytoplasm (Fig. 1), with a
high expression in 39/62 of the CRC samples. There was no
expression in normal colonic mucosal tissues. The χ2 test results
confirmed that there was a significant positive association between
AEBP1 protein expression and the presence of lymph node metastasis
(P=0.003), with a positive expression rate of 54.8% (34/62;
χ2=8.793). The AEBP1 protein level was also significantly
associated with TNM staging. The AEBP1-positive expression rate at
tumor stages III and IV was 76.5% (26/34), significantly higher
than that at stages I and II at 46.4% (13/28; χ2=5.939; P=0.015).
The AEBP1-positive expression rate was 80.0% (24/30) in the
recurrence group, significantly higher than that in the
non-metastasis group at 46.9% (15/32) (χ2=7.281; P=0.007). However,
no association was observed between AEBP1 protein expression and
age or sex (Table I).
Impact of AEBP1 expression on overall
survival (OS) and (DFS) in CRC
Upon univariate analysis, age, sex, tumor size and
histopathological differentiation were not predictive values for OS
or DFS (Table II; P>0.05).
However, AEBP1 expression, TNM stage, metastasis and recurrence
were revealed to be independent prognostic factors for OS and DFS.
(Table II; P<0.05). Multivariate
analysis of the aforementioned prognostic parameters also revealed
that AEBP1 expression (HR, 1.675; 95% CI, 1.142–2.242; P=0.008),
TNM stage, metastasis and recurrence were independent prognostic
indicators for the OS of patients with CRC (Table II; P<0.05). Additionally, AEBP1
expression (HR, 1.611; 95% CI, 1.143–2.333; P=0.006), TNM stage,
metastasis and recurrence were independent prognostic indicators
for DFS in patients with CRC (Table
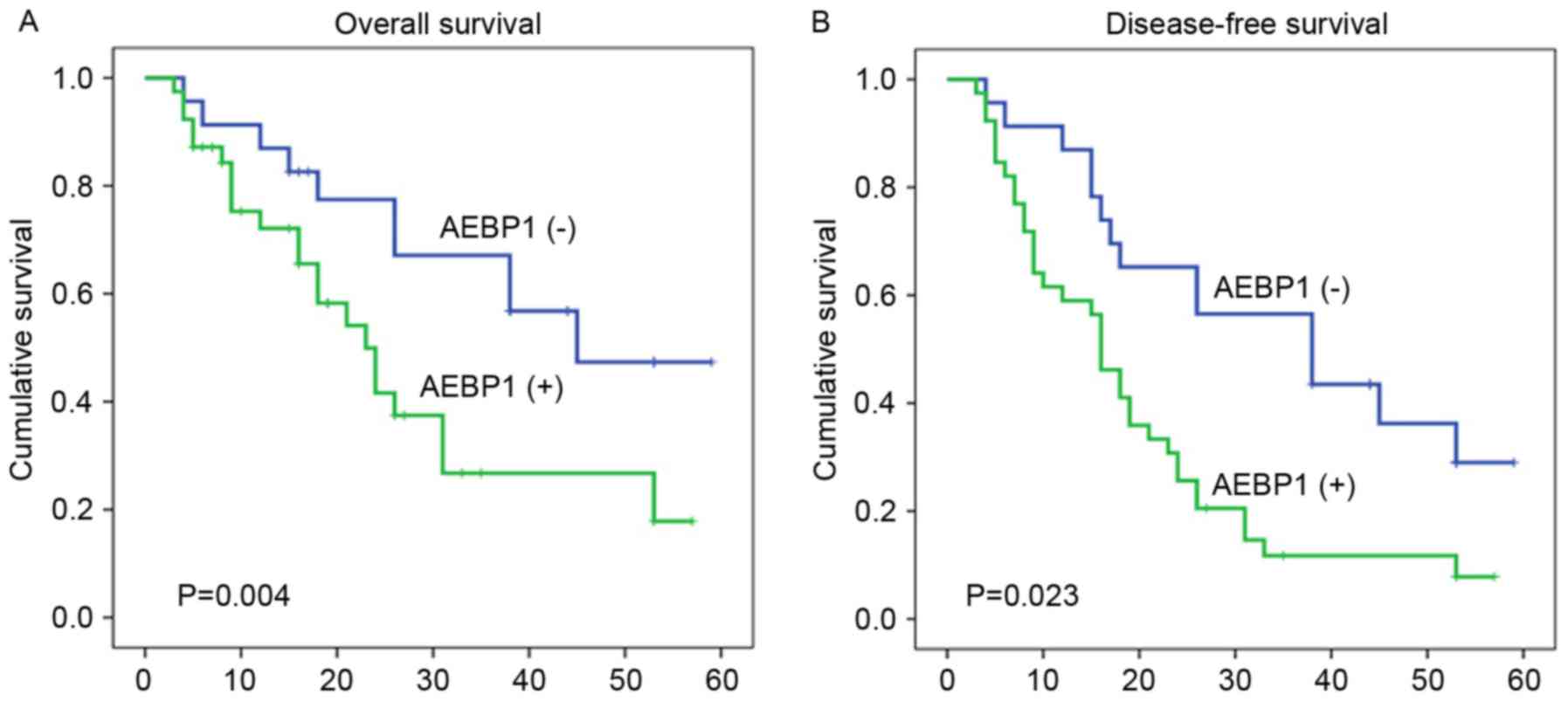
III; P<0.05). Using the Kaplan-Meier method and the log-rank
test, CRC samples with a higher expression of AEBP1 were revealed
to have a shorter OS or DFS (Fig. 2A and
B; log-rank values, 8.286 and 5.177, respectively; P=0.004 and
P=0.023, respectively).
 | Table II.Univariate survival analyses of
individual parameters and associations with OS and DFS using the
Cox proportional hazards model. |
Table II.
Univariate survival analyses of
individual parameters and associations with OS and DFS using the
Cox proportional hazards model.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| AEBP1
expression | 1.994 | 1.322–2.839 | 0.006a | 1.661 | 1.178–2.341 | 0.004a |
| Age | 1.211 | 0.476–1.118 | 0.473 | 1.321 | 0.544–1.008 | 0.532 |
| Sex | 1.107 | 0.797–1.543 | 0.633 | 1.009 | 0.808–1.431 | 0.562 |
| TNM stage | 1.865 | 1.315–2.865 | 0.004a | 1.930 | 1.455–2.744 | 0.003a |
| Tumor size | 1.205 | 0.901–1.578 | 0.173 | 1.327 | 0.879–1.666 | 0.099 |
| Tumor
differentiation | 1.110 | 0.821–1.581 | 0.544 | 1.132 | 0.769–1.415 | 0.650 |
| Recurrence | 1.644 | 1.202–2.887 | 0.017a | 1.600 | 1.173–2.591 | 0.011a |
| Metastasis | 1.555 | 1.039–2.303 | 0.020a | 1.471 | 1.119–2.552 | 0.014a |
 | Table III.Multivariate survival analysis of
individual parameters and associations with OS and DFS using the
Cox proportional hazards model. |
Table III.
Multivariate survival analysis of
individual parameters and associations with OS and DFS using the
Cox proportional hazards model.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| AEBP1
expression | 1.675 | 1.142–2.242 | 0.008a | 1.611 | 1.143–2.333 | 0.006a |
| TNM stage | 1.654 | 1.153–2.737 | 0.007a | 1.720 | 1.211–2.554 | 0.009a |
| Recurrence | 1.426 | 1.104–2.665 | 0.021a | 1.459 | 1.060–2.408 | 0.020a |
| Metastasis | 1.344 | 1.102–2.208 | 0.028a | 1.394 | 1.110–2.446 | 0.023a |
Effects of AEBP1 depletion combined
with oxaliplatin treatment on cell proliferation and apoptosis of
HT-29 cells
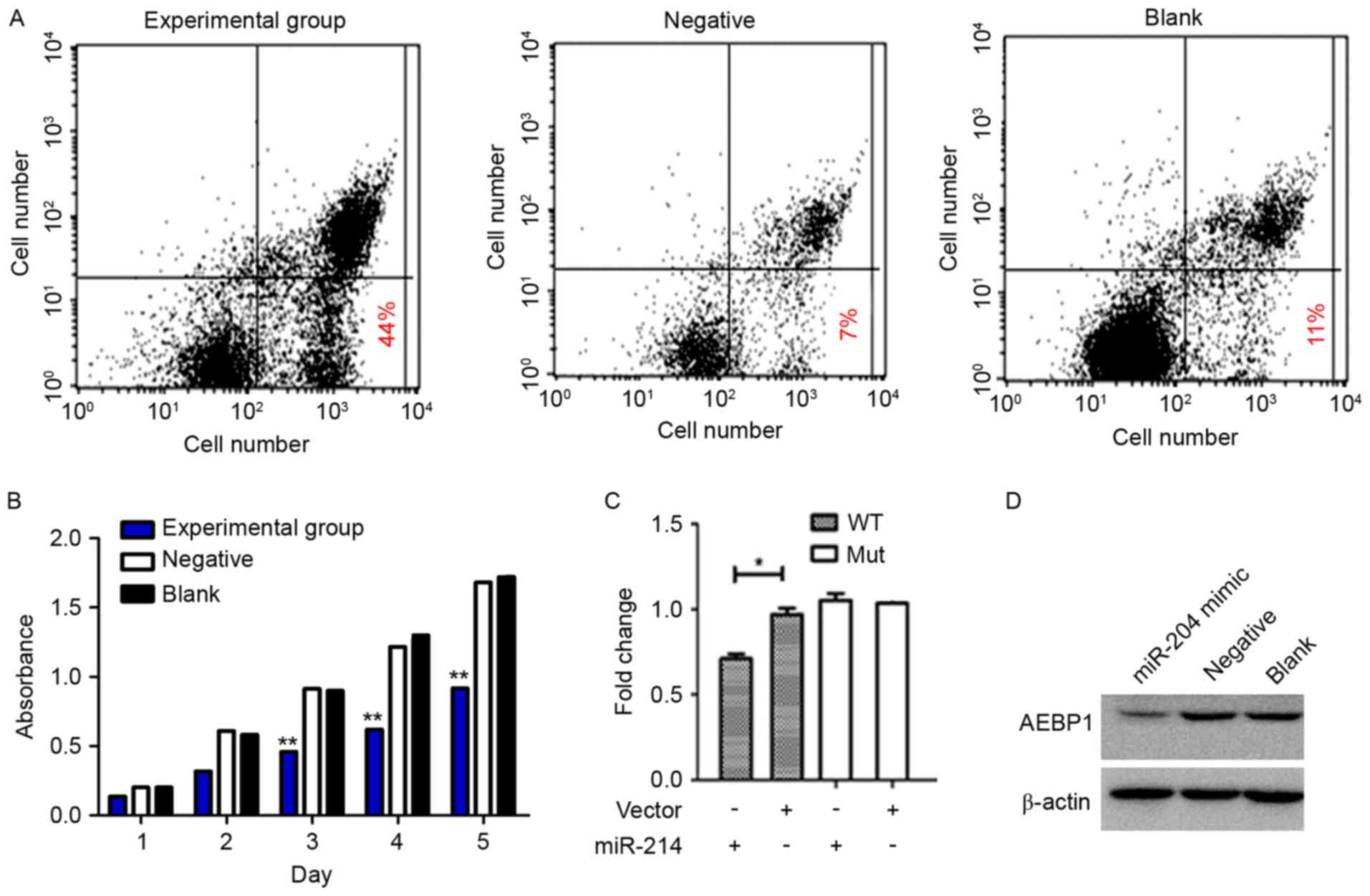
Apoptosis detection revealed that the apoptosis rate
of the experimental group was significantly higher, compared with
the negative control and BC group (Fig.
3A; P<0.05), and no statistically significant differences
were observed between the two control groups (P>0.05). Following
cell transfection and oxaliplatin treatment, the cell proliferation
in the experimental group at 5 time points was determined. The
absorbance values of the experimental group were significantly
lower, compared with the other two groups, as measured by the CCK-8
assay (Fig. 3B; P<0.01). While no
significant difference was observed between the 2 control groups
(P>0.05), indicating that inhibiting AEBP1 expression in HT-29
cells under the effect of chemotherapy drugs may inhibit cell
proliferation ability.
The miR-214 mimic and pMIR-REPORT luciferase vectors
containing the AEBP1 3′-UTR binding site fragment were
co-transfected. Compared with the negative or blank co-transfection
group, the enzyme activity decreased by 30% in the mimic
co-transfection group, demonstrating that AEBP1 is the target of
miR-214 (Fig. 3C). AEBP1 was
identified as the main downstream target of miR-214. Following
transfection of the miR-214 mimic into the HT-29 cells, AEBP1
expression was significantly lower than that of the negative
control group (P<0.01, blank control group gray value is set to
1). No significant differences were detected between the negative
control and the BC group (Fig. 3D;
P>0.05).
Discussion
At present, CRC is one of the most common types of
malignant tumor in humans but its pathogenic mechanisms remain
unclear. Additionally, chronic inflammation often destroys the
normal connection between epithelial and stromal cells, thereby
inducing tumor development (15).
Previous studies have demonstrated that AEBP1 is an important
regulatory factor in inflammation, serving a key function in the
pathogenesis of atherosclerosis, and have proven its use as a
target of the prevention and treatment of atherosclerosis (16,17). In
rat prostate cancer cell lines, AEBP1 has been demonstrated to
function as a transcription-inhibiting factor with carboxypeptidase
activity, and has been reported to be methylated and to participate
in the regulation of mitogen-activated protein kinase activity
(18). In PLX4032-resistant melanoma
cells, Hu et al (11) reported
that AEBP1 was demonstrated to be highly upregulated because of the
hyperactivation of the PI3K/Akt-cAMP response element-binding
protein signaling pathway, which resulted in NF-κB pathway
activation. These results demonstrated that AEBP1 may be a novel
genetic therapy target for BRAF inhibitor-resistant melanoma [AEBP1
upregulation confers acquired resistance to BRAF (V600E) inhibition
in melanoma]. Holloway et al (17) demonstrated that AEBP1, implicated as a
novel pro-inflammatory mediator, has an effect on tumor cell growth
and survival through aberrant sonic hedgehog expression and that it
regulates the cross-talk between the mammary epithelium and stroma
that may predispose the mammary tissue to tumorigenesis (17).
To date, there has been no systematic study on AEBP1
expression in CRC and the results of the present study are the
first to demonstrate that the AEBP1 protein is involved in the
development of CRC. AEBP1 expression is strongly associated with
clinical stage, lymph node metastasis and recurrence. High
expression of AEBP1 leads to a poor prognosis and is an independent
risk factor for CRC prognosis. Therefore, AEBP1 expression may be
used to monitor disease progression in patients with CRC tumor
markers.
The primary auxiliary treatment of advanced-stage
CRC is chemotherapy. Adjuvant chemotherapy following surgery may
prolong the DFS and OS time and improve patient quality of life
(19). However, clinical
chemoresistance in tumor cells is the leading cause of treatment
failure (20). Therefore,
investigation into the cellular mechanisms underlying tumor
chemoresistance has clinical significance. The present study
revealed that, in HT-29 cells, the inhibition of AEBP1 expression
combined with oxaliplatin treatment resulted in significantly lower
cell proliferation and markedly increased apoptosis, indicating
that the expression of AEBP1 has an effect on the sensitivity of
tumor cells to chemotherapy. Previously, it was demonstrated that
Bim, a B-cell lymphoma 2 family member, is an important apoptosis
regulating protein; that Myc and E2F transcription factors regulate
cell proliferation and apoptosis, and that their expression at the
cellular level determines whether they function as oncogenes or
tumor suppressor genes involved in tumorigenesis (21). Downregulated phosphatase and tensin
homolog (PTEN) protein expression and the Akt pathway
hyperactivation resulting from the PTEN downregulation may lead to
cisplatin resistance in gastric cancer (22). Valeri et al (23) observed that miR-21 downregulated hMSH2
protein expression in CRC, leading to fluorouracil resistance.
Mishra et al (24) revealed
that miR-24 binding sites, including single-nucleotide mutations,
cause cells to develop resistance to methotrexate, and that miR-221
inhibited the expression of p27kip1, which mediates tumor cells to
develop drug resistance. In CRC, AEBP1 affects the specific
function of HT-29 chemoresistance, but the associated mechanisms
require further investigation.
The present study demonstrates that, through AEBP1
upstream target gene prediction, miR-214 may negatively regulate
the expression of AEBP1 in CRC. miR-214 is an important miRNA
molecule, and a number of previous studies have demonstrated that
miR-214 is expressed at varying levels in human malignant tumors
and that it affects the progression of the tumor and
chemoresistance through different target genes and molecular
mechanisms. Qiang et al (25)
demonstrated that miR-214 expression in cervical cancer tissues is
significantly lower than that in normal cervical tissues. The
expression of miR-214 is negatively associated with the invasive
ability of the tumor and patient clinical outcomes, and miR-214 may
inhibit the expression of the target gene plexin-B1 to inhibit the
proliferation of HeLa cells (25).
Yang et al (26) also
demonstrated that miR-214 was expressed at a lower level in
cervical cancer tissues than in normal cervical tissues and may
negatively regulate the target genes MEK3 and JNK1 to inhibit cell
proliferation in cervical cancer HeLa cells.
In conclusion, the present study revealed that
miR-214 may serve as the upstream gene involved in AEBP1
regulation, most likely by negatively regulating AEBP1 to enhance
the sensitivity of HT-29 cells to oxaliplatin chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL conceived the study and wrote the manuscript. CL
performed the experiments. ZF assisted in collecting and analyzing
the patient data regarding the clinicopathological parameters of
colorectal cancer. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Weifang People's Hospital and informed consent to participate was
signed by the patients.
Patient consent for publication
Written informed consent was obtained from all
participants for the publication of any data/associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fukushima K, Tsuchiya K, Kano Y, Horita N,
Hibiya S, Hayashi R, Kitagaki K, Negi M, Itoh E, Akashi T, et al:
Atonal homolog 1 protein stabilized by tumor necrosis factor α
induces high malignant potential in colon cancer cell line. Cancer
Sci. 106:1000–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Research on
Cancer: Estimated incidence, mortality and 5-year prevalence. Both
sexes [EB/OL]. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx2017
|
|
3
|
National cancer institute: SEER stat fact
sheets. Colon and rectum cancer [EB/OL]. http://seer.cancer.gov/statfacts/html/colorect.html2017.
|
|
4
|
Andersen HS, Bertelsen CA, Henriksen R,
Campos AH, Kristensen B, Ingeholm P and Gögenur I: The pathological
phenotype of colon cancer with microsatellite instability. Dan Med
J. 63(pii): A51982016.PubMed/NCBI
|
|
5
|
van de Velde CJ, Aristei C, Boelens PG,
Beets-Tan RG, Blomqvist L, Borras JM, van den Broek CB, Brown G,
Coebergh JW, Cutsem EV, et al; EURECCA colorectal, .
Multidisciplinary mission statement on better care for patients
with colon and rectal cancer in Europe. Eur J Cancer. 49:2784–2790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ku G, Tan IB, Yau T, Boku N, Laohavinij S,
Cheng AL, Kang YK and de Lima Lopes G Jr: Management of colon
cancer: Resource-stratified guidelines from the Asian oncology
summit 2012. Lancet Oncol. 13:e470–e481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan X, Chen W, Liu Z, Jiang Z, Hu H, Zhao
Z, Wang S, Chen Y, Wang G and Wang X: Whether regional lymph nodes
evaluation should be equally required for both right and left colon
cancer. Oncotarget. 7:59945–59956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang B, Feng Y, Zhu L, Xu T, Huang L and
Cai G: Smaller tumor size is associated with poor survival in stage
II colon cancer: An analysis of 7,719 patients in the SEER
database. Int J Surg. 33:Pt A. 157–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bogachev O, Majdalawieh A, Pan X, Zhang L
and Ro HS: Adipocyte enhancer-binding protein 1 (AEBP1) (a novel
macrophage proinflammatory mediator) overexpression promotes and
ablation attenuates atherosclerosis in ApoE (−/-) and LDLR (−/-)
mice. Mol Med. 17:1056–1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Majdalawieh A and Ro HS: Regulation of
IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel
proinflammatory mediator in macrophages. Mediators Inflamm.
2010:8238212010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu W, Jin L, Jiang CC, Long GV, Scolyer
RA, Wu Q, Zhang XD, Mei Y and Wu M: AEBP1 upregulation confers
acquired resistance to BRAF (V600E) inhibition in melanoma. Cell
Death Dis. 4:e9142013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ladha J, Sinha S, Bhat V, Donakonda S and
Rao SM: Identification of genomic targets of transcription factor
AEBP1 and its role in survival of glioma cells. Mol Cancer Res.
10:1039–1051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu H, Krasinskas A and Willis J:
Perspectives on current tumor-node-metastasis (TNM) staging of
cancers of the colon and rectum. Semin Oncol. 38:500–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Chen P, Chang Y, Qi J, Fu H and Guo
H: Let-7a inhibits tumor cell growth and metastasis by directly
targeting RTKN in human colon cancer. Biochem Biophys Res Commun.
478:739–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Majdalawieh A and Ro HS: PPARgamma1 and
LXRalpha face a new regulator of macrophage cholesterol homeostasis
and inflammatory responsiveness, AEBP1. Nucl Recept Signal.
8:e0042010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holloway RW, Bogachev O, Bharadwaj AG,
McCluskey GD, Majdalawieh AF, Zhang L and Ro HS: Stromal adipocyte
enhancer-binding protein (AEBP1) promotes mammary epithelial cell
hyperplasia via proinflammatory and hedgehog signaling. J Biol
Chem. 287:39171–39181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita S, Takahashi S, McDonell N,
Watanabe N, Niwa T, Hosoya K, Tsujino Y, Shirai T and Ushijima T:
Methylation silencing of transforming growth factor-beta receptor
type II in rat prostate cancers. Cancer Res. 68:2112–2121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sumpio C, Knobf MT and Jeon S: Treatment
complexity: A description of chemotherapy and supportive care
treatment visits in patients with advanced-stage cancer diagnoses.
Support Care Cancer. 24:285–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young JI, Mongoue-Tchokote S, Wieghard N,
Mori M, Vaccaro GM, Sheppard BC and Tsikitis VL: Treatment and
survival of small-bowel adenocarcinoma in the United States: A
comparison with colon cancer. Dis Colon Rectum. 59:306–315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muthalagu N, Junttila MR, Wiese KE, Wolf
E, Morton J, Bauer B, Evan GI, Eilers M and Murphy DJ: BIM is the
primary mediator of MYC-induced apoptosis in multiple solid
tissues. Cell Rep. 8:1347–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Shen H, Li H, Cao Y, Qin R, Long
L, Zhu X, Xie C and Xu W: miR-106a confers cisplatin resistance by
regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim
Biophys Sin (Shanghai). 45:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valeri N, Gasparini P, Braconi C, Paone A,
Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, et al:
MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating
human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA.
107:21098–21103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra PJ, Humeniuk R, Mishra PJ,
Longo-Sorbello GS, Banerjee D and Bertino JR: A miR-24 microRNA
binding-site polymorphism in dihydrofolate reductase gene leads to
methotrexate resistance. Proc Natl Acad Sci USA. 104:13513–13518.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiang R, Wang F, Shi LY, Liu M, Chen S,
Wan HY, Li YX, Li X, Gao SY, Sun BC and Tang H: Plexin-B1 is a
target of miR-214 in cervical cancer and promotes the growth and
invasion of HeLa cells. Int J Biochem Cell Biol. 43:632–641. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X,
Liu T and Tang H: MicroRNA-214 is aberrantly expressed in cervical
cancers and inhibits the growth of HeLa cells. IUBMB Life.
61:1075–1082. 2009. View
Article : Google Scholar : PubMed/NCBI
|