Introduction
In 2015, 23,100 new cases of breast cancer were
reported in the US alone, and the disease caused >40,000
mortalities, making it the second leading cause of
cancer-associated mortality (1,2).
Anthracyclines are the anti-tumor drugs most commonly used to treat
patients with breast cancer, yet ≤50% of patients respond
effectively to them (3). Therefore,
paclitaxel is frequently administered as supplementary
chemotherapy, in order to increase the efficacy of anthracyclines.
A meta-analysis study concluded that combining paclitaxel with
anthracyclines leads to better overall survival of patients with
metastatic breast cancer compared with either therapy alone
(4). On the other hand, numerous
studies suggest that paclitaxel may only be given following
anthracyclines, due to the dose-dependent cardiotoxicity of
anthracyclines (5–7), and that paclitaxel may cause febrile
neutropenia in up to 32% of patients (4). Furthermore, studies suggest that
paclitaxel is more effective against breast cancer when used in
combination, compared with on its own. However, one study has
suggested similar efficacy for paclitaxel monotherapy as for
combination therapy involving doxorubicin, cyclophosphamide and
5-fluorouracil (8). Cumulative
paclitaxel doses have been associated with notable toxic effects,
including peripheral neurotoxicity, diarrhea and myalgia (9). Therefore, examining novel combinations
of paclitaxel with other treatments against breast cancer is
essential for enhancing therapeutic efficacy and reducing
paclitaxel-associated toxicity.
A potential treatment that may be effective in
combination with paclitaxel is exposure to mild hyperthermia, in
which artificial heating methods are used in order to increase
local- or whole-body temperatures to 39.5–42°C. The resulting
heating and its secondary effects may kill cancer cells directly,
or induce apoptosis. Induced hyperthermia may selectively kill
tumor cells without damaging normal tissues (10–14), and
it may also sensitize cells to radio- and chemotherapy.
Additionally, mild hyperthermia may reduce the toxic side effects
of radio- and chemotherapy through activation of the immune system,
promoting the release of a large quantity of cytokines, and
preserving hematopoiesis in the bone marrow (15–17).
The optimal temperature for mild hyperthermia is
controversial. Previous studies suggest that the minimum
temperature for hyperthermia alone or combined with radio- or
chemotherapy is 41–43°C (18,19). Consistent with this, other studies
have suggested that inducing hyperthermia at temperatures <41°C
inhibits tumor growth by activating the immune system, however it
may not synergize with chemotherapy to kill tumor cells directly
(20,21). On the other hand, previous studies
have reported that whole-body hyperthermia at temperatures of ~39°C
may synergize with chemotherapy (21).
The present study investigated whether paclitaxel
and mild hyperthermia may synergize in order to inhibit the growth
of the human breast cancer cell line MCF-7. In particular, the
question of whether such synergy may occur at temperatures <41°C
was assessed.
Materials and methods
Cell culture, hyperthermia induction
and treatment with paclitaxel
The human breast cancer cell line MCF-7 (Shanghai
Institute of Biochemistry and Cell Biology, Shanghai, China) was
cultured in standard cell culture plates containing RPMI 1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in an incubator
with 5% CO2 and saturated humidity. Cultures were
maintained at 37°C (control), treated with paclitaxel alone,
exposed to microwave-induced hyperthermia alone (at 40, 40.5 or
41°C), or treated with the combination of paclitaxel and
hyperthermia (at 40, 40.5 or 41°C).
Hyperthermia was induced using a focused-beam
microwave hyperthermia apparatus (UHR-2000; Huayuan Medical
Equipment, Hunan, China) with an effective heating area >16×16×5
cm3. The target temperature (40, 40.5 or 41°C) was
reached within 60 min. Once the target temperature was reached, it
was maintained for 2 h. The overall temperature fluctuated within a
range of ±0.2°C. Following hyperthermia, cultures were returned for
24 or 48 h to an incubator at 37°C with a 5% CO2
atmosphere and saturated humidity.
For treatment with paclitaxel, cells were seeded
into 96-well plates. When cell density reached 5×103/ml,
paclitaxel (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), was
added at the indicated concentrations and cells were cultured for
24 h.
MTT assay of cell proliferation
Cell proliferation following the different
treatments was measured using the MTT Cell Proliferation Assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.). DMSO was used to
dissolve the purple formazan. Absorbance was measured at 570 nm
using Spectramax M2 microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Apoptosis assay and determination of
apoptotic ratio
At 24 and 48 h post-treatment, cultures were assayed
for apoptosis using the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit I (BD
Biosciences, Franklin Lakes, NJ, USA). Cells were washed twice with
PBS, centrifuged (78 × g at 37°C for 5 min) and incubated with
Annexin V-FITC/PI at room temperature in the dark for 30 min.
During flow cytometry with CytExpert software (version 2.0; Beckman
Coulter, Inc., Brea, CA, USA), FITC was detected with excitation at
488 nm and emission at 530 nm. Cells positive for Annexin V-FITC
and negative for PI were defined as early apoptotic cells, while
cells positive for Annexin V-FITC and PI were defined as late
apoptotic cells. The ratios of cells in late to early apoptosis
(apoptotic ratio) were calculated for each treatment group.
Statistical analysis
Data were analyzed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Continuous outcomes were expressed as the mean ±
standard deviation. Data were presented as bar charts, and
inter-group differences were assessed for significance by one-way
analysis of ANOVA with a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
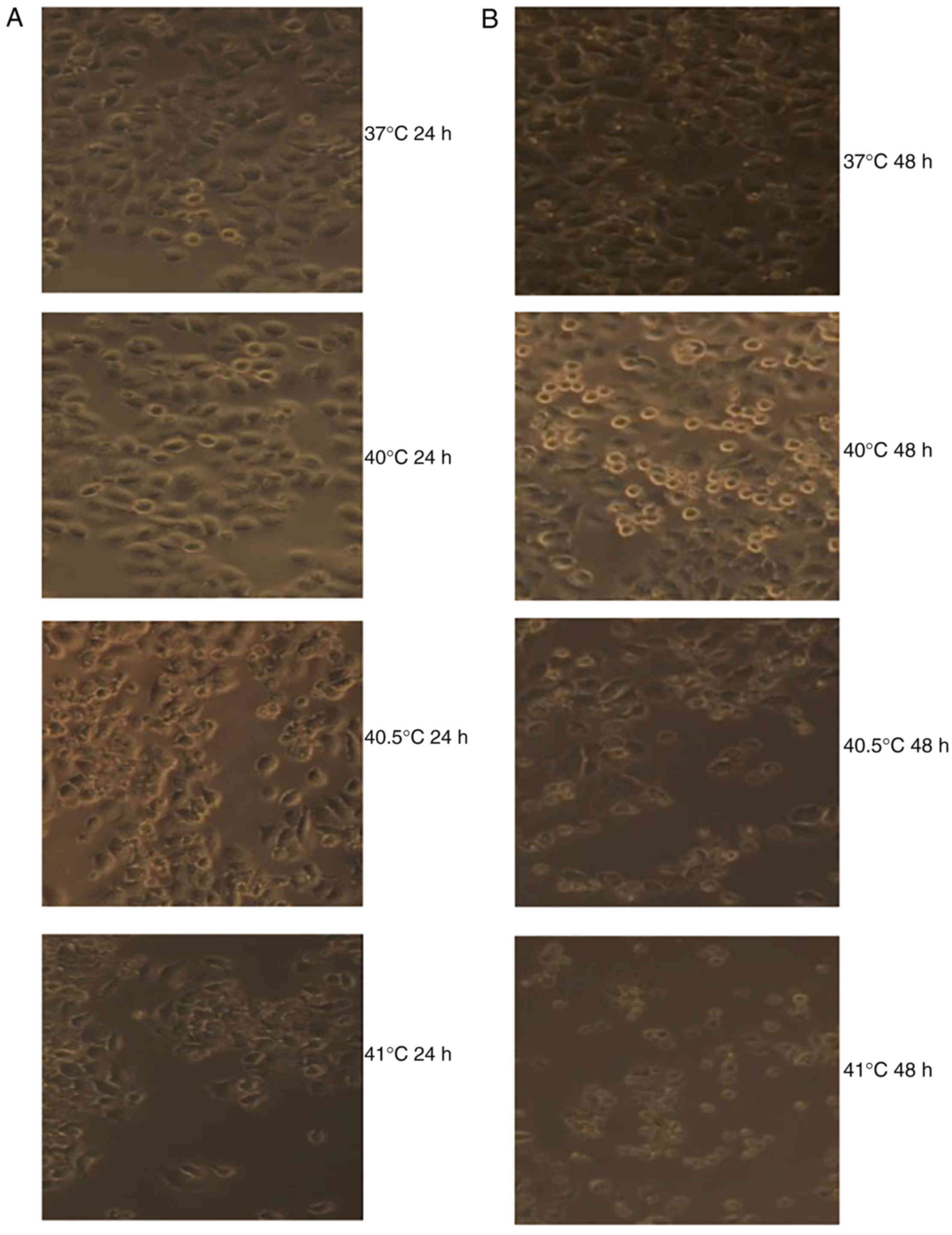
Effects of mild hyperthermia on MCF-7
proliferation
At 24 and 48 h following hyperthermia at 40 and
40.5°C, MCF-7 cells displayed epithelial-cell-like adherent growth
and obvious, gradual cell contours; cells were connected to one
another in a uniform arrangement. They displayed no obvious
morphological differences compared with the control group. By
contrast, at 24 and 48 h after hyperthermia at 41°C, a proportion
of MCF-7 cells shrank and became rounded, with decreased
transparency and loose intercellular connections. However, the
majority of cells still displayed epithelial-cell-like adherent
growth, vague contours, intercellular connections and an overall
healthy appearance. A small number of cells appeared to undergo
morphological alterations consistent with apoptosis (Fig. 1).
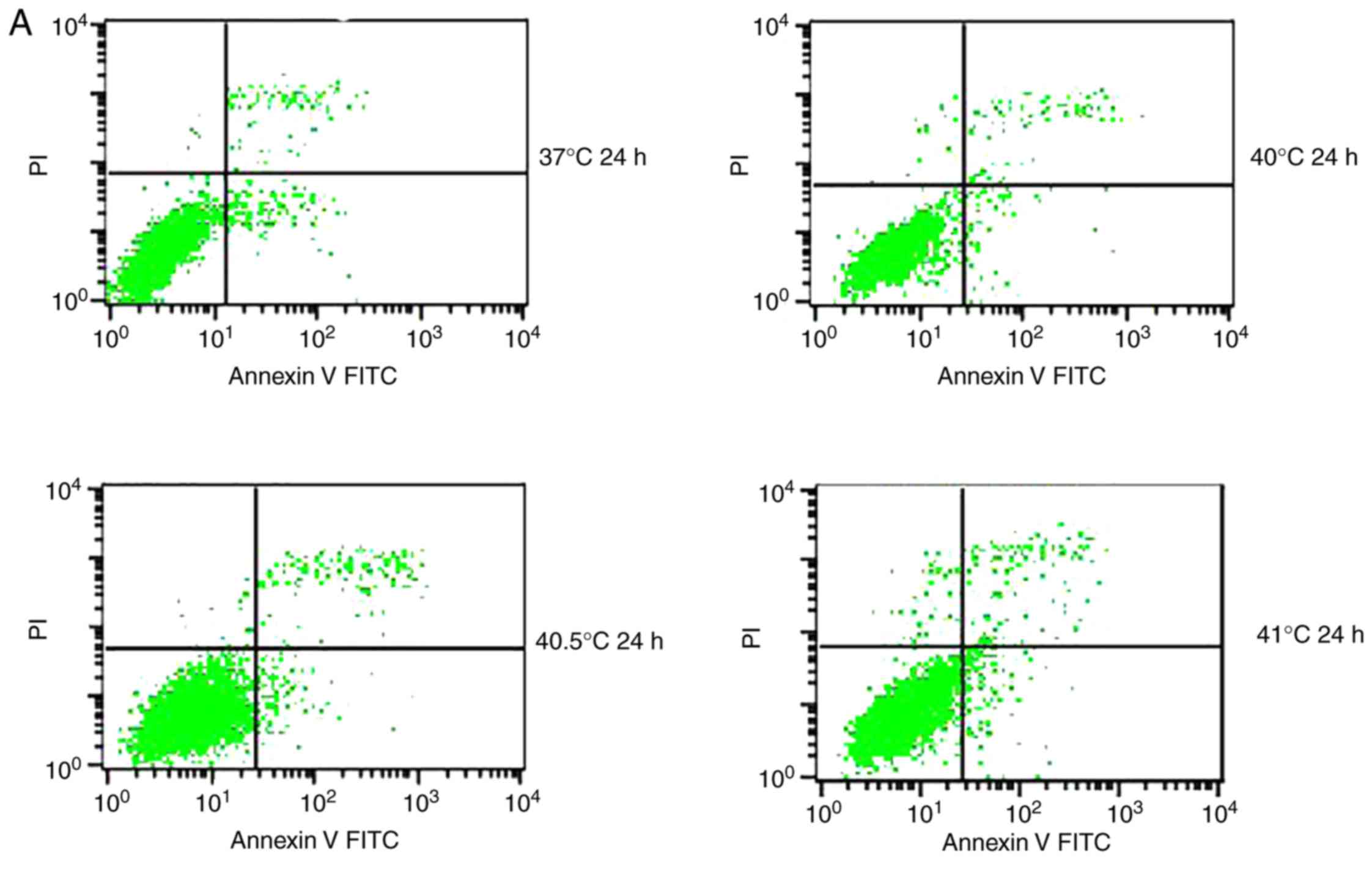
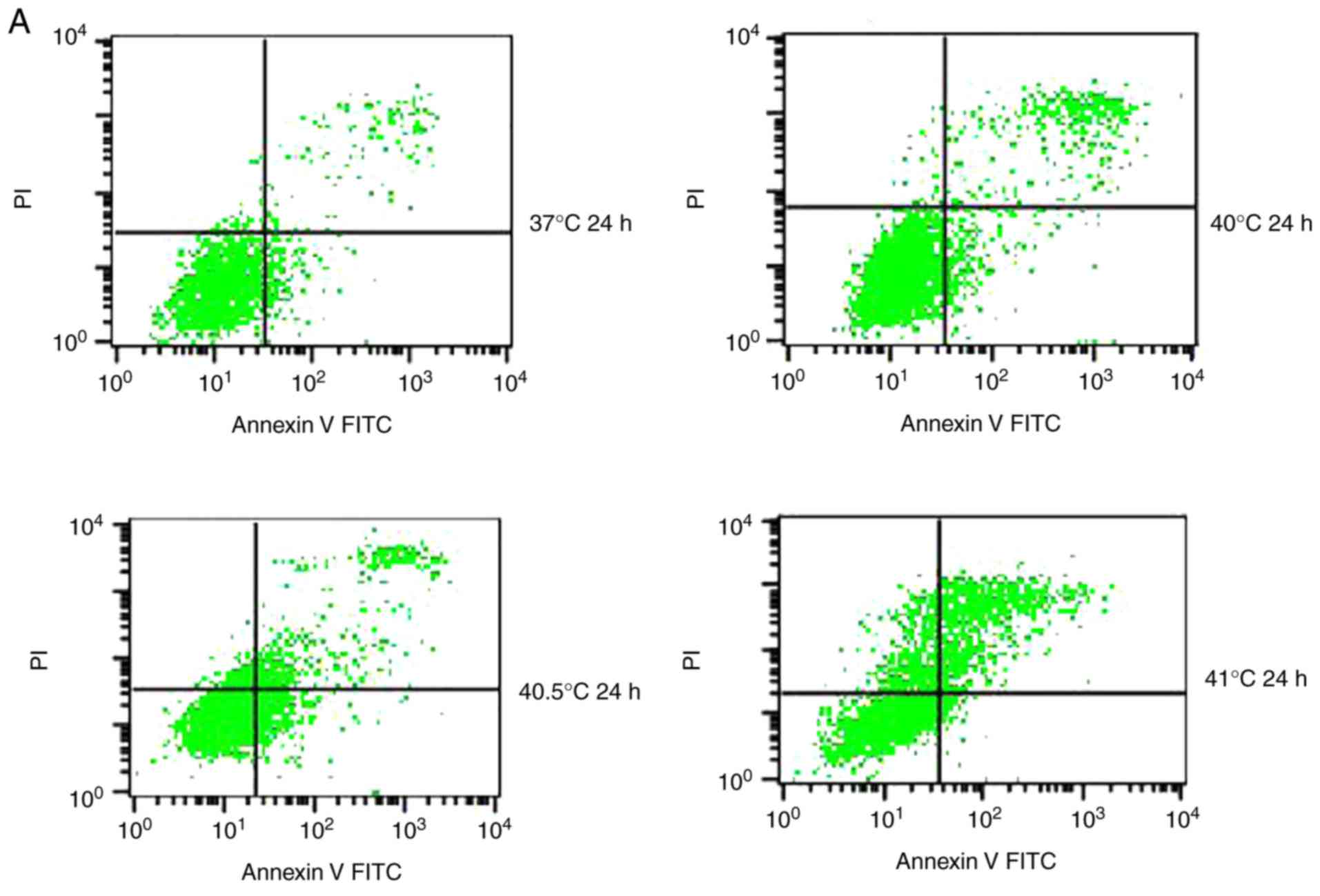
Effects of mild hyperthermia on MCF-7
apoptotic ratio
Previous studies have reported that mild
hyperthermia at 41°C does not synergize with chemotherapy to kill
tumor cells directly; instead, it exerts its anti-tumor effects by
regulating the immune system. In the present study, the hypothesis
that mild hyperthermia at temperatures <41°C may induce
anti-tumor effects by inducing apoptosis was tested. Therefore,
cultures were exposed to hyperthermia at varying temperatures and
the apoptotic ratios measured using flow cytometry. The apoptotic
ratio was significantly higher following hyperthermia at 41°C
compared with the control incubation at 37°C. The apoptotic ratio
significantly increased from 6.11±0.45% at 24 h following
hyperthermia, to 11.38±1.75% at 48 h following hyperthermia
(P<0.01). The apoptosis ratio was also significantly higher at
48 h at 41°C compared with 40 or 40.5°C (P<0.05; Fig. 2). These results demonstrate that
microwave-induced hyperthermia for 2 h at 41°C alone may induce
apoptosis in MCF-7 cells at 24 h later. Apoptosis was increased
when hyperthermia involved higher temperatures, or when cultures
were examined at later time points following hyperthermia.
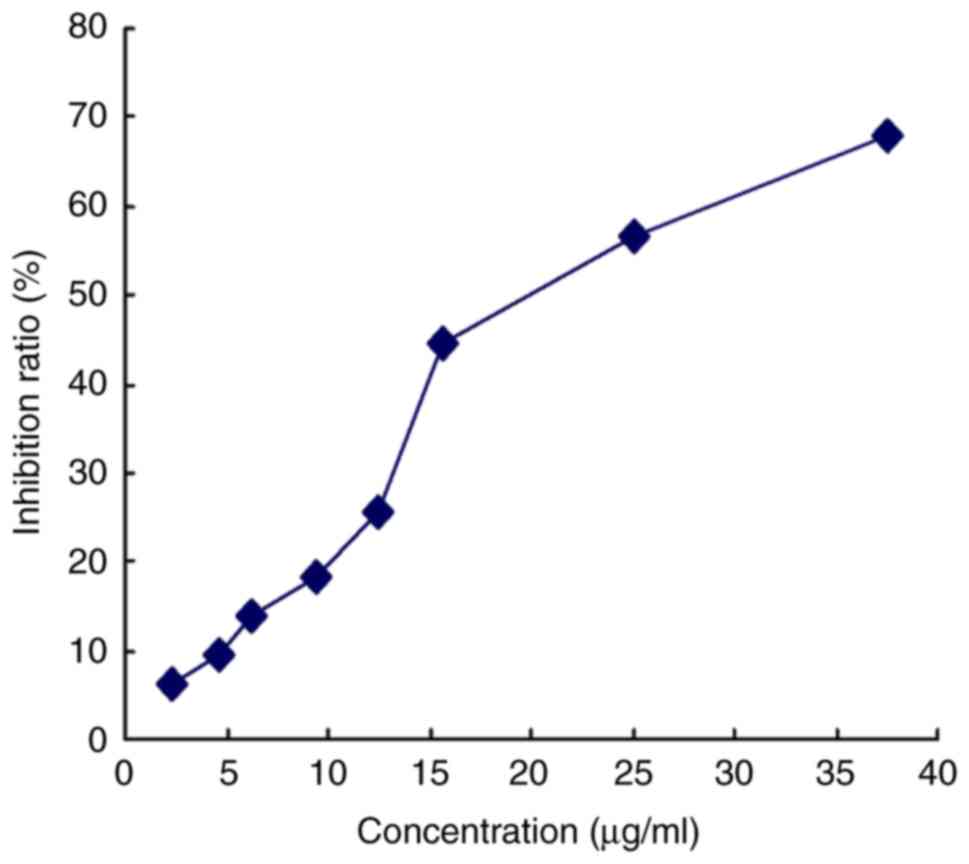
Effects of mild hyperthermia combined
with paclitaxel on MCF-7 growth
The effective killing concentration with paclitaxel
was determined under the MCF-7 culture conditions using the MTT
assay: The 10% inhibitory concentration (IC10) and 20%
inhibitory concentration (IC20) were found to be 5 and
10 µg/ml, respectively (Fig. 3).
Cultures were treated with one of these doses of paclitaxel and
incubated at 37°C for 24 h. A proportion of cells appeared to
shrink and become rounded, with decreased transparency, obvious
contours and a dark brown color. A number of cells, likely
apoptotic, floated in the culture medium. Over time, the number of
apoptotic cells increased and the number of adherent,
healthy-looking cells decreased.
Treatment with paclitaxel (5 or 10 µg/ml) was
combined with hyperthermia for 2 h at 40, 40.5 or 41°C. Cultures
were observed 24 or 48 h later. In cultures treated with 5 µg/ml
paclitaxel and hyperthermia at 40°C, cell apoptosis was relatively
rare; in fact, it was similar to the level of apoptosis observed in
cultures treated with 10 µg/ml paclitaxel alone. By contrast, a
notably increased number of dead cells, as a result of either
apoptosis or necrosis, were observed in cultures treated with 5 or
10 µg/ml of paclitaxel in combination with hyperthermia at 40.5 or
41°C for 2 h. The number of dead cells increased over time
following hyperthermia (Fig. 4).
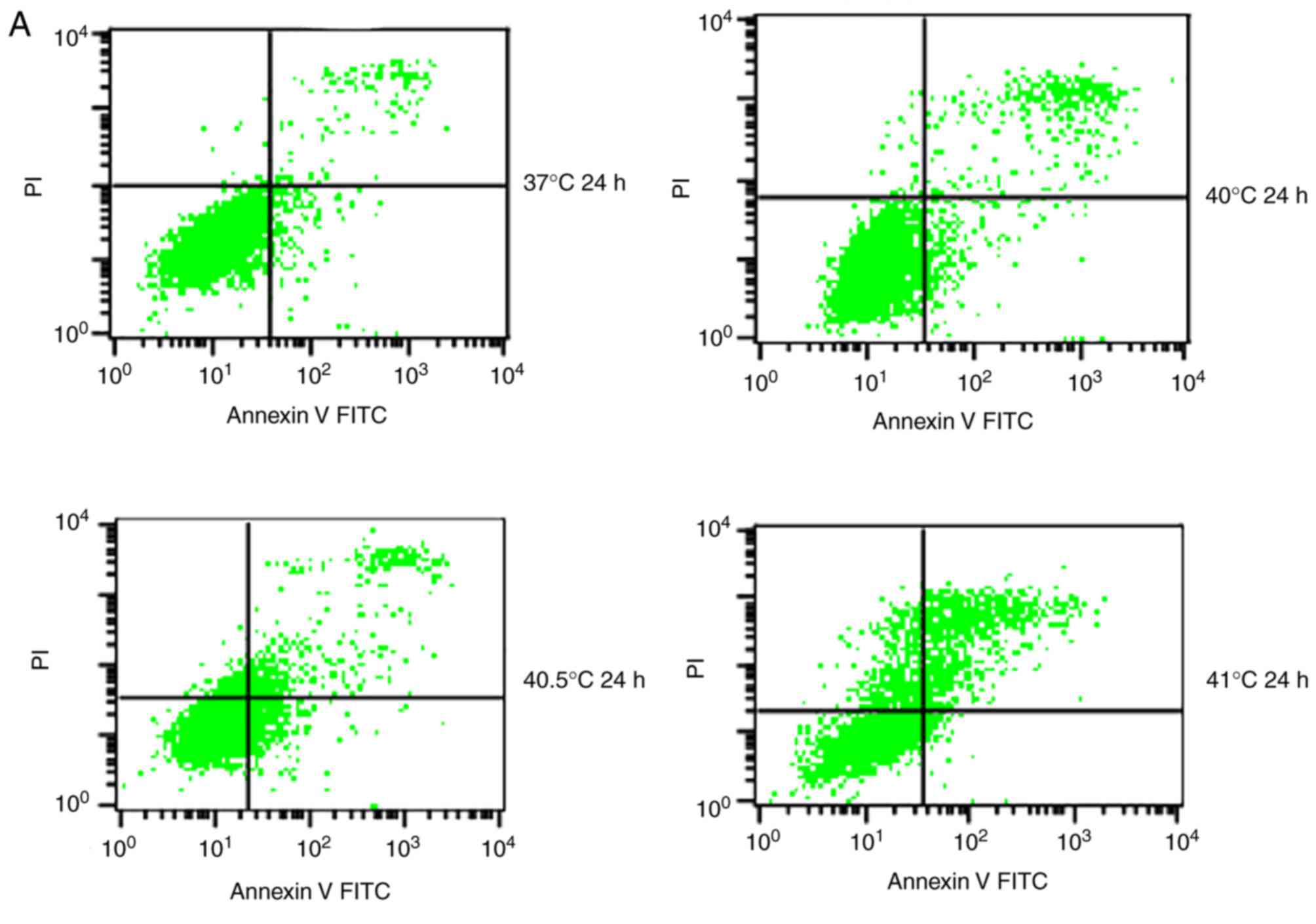
Effects of mild hyperthermia combined
with 5 µg/ml paclitaxel on the MCF-7 apoptotic ratio
To quantify the aforementioned visible differences
in apoptosis observed, cultures were exposed to the IC10
paclitaxel dose of 5 µg/ml, followed by exposure to 2 h of
hyperthermia at different temperatures. The apoptotic ratio was
measured at 24 or 48 h post-hyperthermia. At 24 h later, the
apoptotic ratio was significantly higher in cultures treated with
paclitaxel and hyperthermia at 40.5°C (12.21±1.02%) compared with
cultures treated with paclitaxel alone (8.54±1.24%), or
hyperthermia alone (4.95±0.20%) (P<0.05). This indicated that
paclitaxel and mild hyperthermia may act synergistically to induce
apoptosis in MCF-7 cells.
The apoptotic ratio further increased, and the
synergistic action became more notable with increasing hyperthermia
temperatures (from 40.5°C to 41°C) (P<0.05 or 0.01), and with
increasing lengths of time following hyperthermia (from 24 to 48 h)
(P<0.05). Notably, a lower apoptotic ratio in cultures treated
with paclitaxel and hyperthermia at 40°C was observed compared with
cultures treated with paclitaxel alone. This suggests that
low-temperature thermo-chemotherapy may enhance the activity of
MCF-7 cells (Fig. 5).
Effects of mild hyperthermia combined
with 10 µg/ml paclitaxel on the MCF-7 apoptotic ratio
Similar to the aforementioned experiments conducted
with 5 µg/ml paclitaxel, cultures treated with 10 µg/ml of the drug
and exposed to hyperthermia for 2 h at 40.5°C displayed a
significantly higher apoptotic ratio at 24 h post-treatment
(25.88±1.21%), compared with cultures treated with paclitaxel alone
(16.87±2.59%), or hyperthermia alone (4.95±0.20%) (P<0.05). This
provides further evidence to suggest that paclitaxel and
hyperthermia may act synergistically.
This synergy became increasingly apparent at the
higher hyperthermia temperature of 41°C, since the apoptotic ratio
was significantly higher (P<0.01). While the apoptotic ratio
decreased between 24 and 48 h following hyperthermia, the number of
necrotic cells increased (P<0.01), consistent with synergistic
action.
The results from the current study with
thermo-chemotherapy at two different paclitaxel doses demonstrate
that mild hyperthermia may induce anti-tumor effects by inducing
cell apoptosis. The results further suggest that at higher
paclitaxel doses and with increasing lengths of time following
hyperthermia, necrosis may become a secondary mechanism helping to
drive the anti-tumor effects of thermo-chemotherapy (Fig. 6).
Discussion
Over the last two decades, there have been
continuous improvements in clinical hyperthermia technology; hence,
exposure to hyperthermia has become a routine method for treating
malignant tumors (22–24). Numerous basic and clinical studies
have demonstrated that the combination of hyperthermia with radio-
or chemotherapy may significantly improve tumor control rates and
prolong survival (25–28). The majority of these studies have
suggested that 42–45°C is the minimal effective temperature for
hyperthermia induced by microwave or infrared radiation, or by
incubation in a water bath (29–33). This
poses a challenge for clinical implementation, as 41.8°C is
considered the upper limit of whole-body temperature in humans,
thus achieving temperatures of 42–45°C places a substantial burden
on patients and equipment.
Achieving whole-body hyperthermia of 38–41°C may be
technically and clinically more feasible (11–14),
however various studies have reported conflicting results regarding
whether hyperthermia at 40–41°C exerts anti-tumor effects. One
study reported that 90 min of hyperthermia at 39–41°C activated
cellular and humoral anti-tumor responses (34). In the present study, it was
demonstrated that microwave-induced hyperthermia at 41°C lasting
for 2 h induced apoptosis in the human breast cancer cell line
MCF-7. Apoptosis was increased following hyperthermia at 41°C
compared with 40°C, and it was increased at 48 h following
hyperthermia compared with at 24 h following hyperthermia. The
results from the current study are consistent with previous work
demonstrating that 2 h of hyperthermia at 41–44°C induced apoptosis
in the leukemia cell lines HL-60, K562 and TF-1star at 96 h
post-treatment, with temperatures of 43–44°C inducing the most
obvious apoptotic morphology (35).
These results suggest that 2 h of hyperthermia at 41°C may induce
anti-tumor effects by inducing apoptosis. This justifies further
preclinical studies into the potential of mild hyperthermia for
treating cancer.
Although mild hyperthermia may be feasible in the
clinic, available basic and clinical evidence suggests that
hyperthermia on its own lacks the efficacy required for
comprehensive treatment of malignant tumors (36,37).
Therefore, hyperthermia has generally been studied as an adjuvant
to chemotherapy for improving the curative effect, while allowing
lower chemotherapy doses to be used (16,17,38–41).
The minimum effective temperature for thermo-chemotherapy appears
to be 40–43°C. One study found that thermo-chemotherapy involving 2
h of hyperthermia at 41°C induced apoptosis in an increased number
of tumor cells, and had more potent anti-tumor effects compared
with hyperthermia or chemotherapy alone (42). Similarly, another study reported that
combining 20 µmol/l docetaxel with 2 h of hyperthermia at 40–41°C
induced apoptosis in an increased number tumor cells compared with
chemotherapy alone (43).
Additionally, it was reported that the apoptotic ratio increased
with increasing hyperthermia temperature. However, the study did
not compare thermo-chemotherapy with hyperthermia alone. In
addition, few reports have examined the combination of low-dose
chemotherapy and microwave-induced hyperthermia at temperatures
<41°C.
In the present study, it was demonstrated that the
combination of 5 µg/ml paclitaxel and 2 h of hyperthermia at 40.5°C
induced apoptosis in MCF-7 cells at 24 h post-treatment, and that
this pro-apoptotic induction was greater compared with either
therapy alone, indicating synergistic action. This synergistic
effect became even more apparent when the time following
hyperthermia was increased to 48 h compared with 24 h, and when the
hyperthermia temperature was 41°C compared with 40 or 40.5°C.
Therefore, the results from the current study suggest that
paclitaxel and mild hyperthermia at temperatures as low as 40.5°C
may induce apoptosis in tumor cells and interact synergistically in
order to kill tumor cells.
Notably, it was reported that the combination of 5
or 10 µg/ml paclitaxel and 2 h of hyperthermia at 40°C was
associated with a significantly smaller apoptotic ratio at 24 or 48
h following treatment, compared with paclitaxel treatment alone.
Cells treated with the combination therapy demonstrated a
proliferative trend. These results are consistent with a previous
study that reported that thermo-chemotherapy involving hyperthermia
at 39–40°C increased tumor cell activity (42). The results from the current study
suggest that paclitaxel dosage and hyperthermia temperature require
careful optimization in order to enhance the cytotoxicity of
paclitaxel, and avoid the promotion of tumor cell growth.
The cytotoxic mechanism of the combination of
paclitaxel and mild hyperthermia may involve apoptosis and
necrosis. Treating MCF-7 cultures with 10 µg/ml paclitaxel and 2 h
of hyperthermia at 40.5 or 41°C induced tumor cell apoptosis 24 h
post-treatment, in a synergistic fashion. At 48 h following
treatment, the extent of necrosis was greater compared with that of
apoptosis. The extent of necrosis relative to apoptosis was even
greater when the hyperthermia temperature was 41°C. These results
are concordant with previous work (44), and may reflect an enhancement of
paclitaxel cytotoxicity at higher temperatures. With increased
durations of hyperthermia exposure, the cytotoxic effect of
paclitaxel was stronger, and it was demonstrated that cell necrosis
prevailed over apoptosis. However, results from Michalakis et
al (44) suggested that
hyperthermia (at 41.5 or 43°C) exerted a cytostatic effect to all
cell lines, including the human breast cell MCF-7, the ovarian
SKOV-3 cell line and the hepatocarcinoma HepG2 cell line. The
results of the present study suggested that mild hyperthermia
<41.5°C may induce apoptosis in the human breast cell line
MCF-7, and the combination of mild hyperthermia at 40.5–41°C with
low-dose paclitaxel at 5 or 10 µg/ml (IC10/IC20) may exert
synergistic anti-tumor effects. This has previously been reported
for various other chemotherapy drugs (45,46). In
conclusion, the present study reports that mild hyperthermia (41°C)
alone may induce apoptosis in the human breast cancer cell line
MCF-7, and that this effect is enhanced with increasing lengths of
time following hyperthermia. The results further suggest that the
combination of mild hyperthermia at 40.5–41°C with low-dose
paclitaxel may exert synergistic anti-tumor effects, which are
enhanced at higher hyperthermia temperatures and with longer
periods following hyperthermia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medication
and Health Care Research Program of Guangxi (grant no. S201418-03
and S201634), the Guangxi Natural Science Foundation (grant no.
2017GXNSFAA198103), the Key Planning Development Research Program
of Guangxi (grant no. guikeAB16380215) and the Health and Family
Planning Commission Project of Guangxi (grant no. Z20170452).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SNL conceptualised the study, analysed and
interpreted the data and was involved in drafting the manuscript.
SNL also gave final approval of the version to be published. XLL
and YQL designed the study and revised the manuscript. XHH, ZHL,
YL, RL, YMZ, QL contributed the conception design and editing of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zeichner SB, Ambros T, Zaravinos J,
Montero AJ, Mahtani RL, Ahn ER, Mani A, Markward NJ and Vogel CL:
Defining the survival benchmark for breast cancer patients with
systemic relapse. Breast Cancer (Auckl). 9:9–17. 2015.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taatjes DJ, Fenick DJ and Koch TH: Nuclear
targeting and nuclear retention of anthracycline-formaldehyde
conjugates implicates DNA covalent bonding in the cytotoxic
mechanism of anthracyclines. Chem Res Toxicol. 12:588–596. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carrick S, Parker S, Thornton CE, Ghersi
D, Simes J and Wilcken N: Single agent versus combination
chemotherapy for metastatic breast cancer. Cochrane Database Syst
Rev. CD003372:2009.
|
|
5
|
O'Brien ME, Wigler N, Inbar M, Rosso R,
Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP,
et al: Reduced cardiotoxicity and comparable efficacy in a phase
III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil)
versus conventional doxorubicin for first-line treatment of
metastatic breast cancer. Ann Oncol. 15:440–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keller AM, Mennel RG, Georgoulias VA,
Nabholtz JM, Erazo A, Lluch A, Vogel CL, Kaufmann M, von Minckwitz
G, Henderson IC, et al: Randomized phase III trial of pegylated
liposomal doxorubicin versus vinorelbine or mitomycin C plus
vinblastine in women with taxane-refractory advanced breast cancer.
J Clin Oncol. 22:3893–3901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piccart-Gebhart MJ, Burzykowski T, Buyse
M, Sledge G, Carmichael J, Luck HJ, Mackey JR, Nabholtz JM,
Paridaens R, Biganzoli L, et al: Taxanes alone or in combination
with anthracyclines as first-line therapy of patients with
metastatic breast cancer. J Clin Oncol. 26:1980–1986. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buzdar AU, Singletary SE, Theriault RL,
Booser DJ, Valero V, Ibrahim N, Smith TL, Asmar L, Frye D, Manuel
N, et al: Prospective evaluation of paclitaxel versus combination
chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide
as neoadjuvant therapy in patients with operable breast cancer. J
Clin Oncol. 17:3412–3417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapiro CL and Recht A: Side effects of
adjuvant treatment of breast cancer. N Engl J Med. 344:1997–2008.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klostergaard J, Leroux ME, Auzenne E,
Khodadadian M, Spohn W, Wu JY and Donato NJ: Hyperthermia engages
the intrinsic apoptotic pathway by enhancing upstream caspase
activation to overcome apoptotic resistance in MCF-7 breast
adenocarcinoma cells. J Cell Biochem. 98:356–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohamed F, Marchettini P, Stuart OA, Urano
M and Sugarbaker PH: Thermal enhancement of new chemotherapeutic
agents at moderate hyperthermia. Ann Surg Oncol. 10:463–468. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westermann AM, Grosen EA, Katschinski DM,
Jager D, Rietbroek R, Schink JC, Tiggelaar CL, Jager E, Zum Vorde
sive Vörding P, Neuman A, et al: A pilot study of whole body
hyperthermia and carboplatin in platinum-resistant ovarian cancer.
Eur J Cancer. 37:1111–1117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ressel A, Weiss C and Feyerabend T: Tumor
oxygenation after radiotherapy, chemotherapy, and/or hyperthermia
predicts tumor free survival. Int J Radiat Oncol Biol Phys.
49:1119–1125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burd R, Dziedzic TS, Xu Y, Caligiuri MA,
Subjeck JR and Repasky EA: Tumor cell apoptosis, lymphocyte
recruitment and tumor vascular changes are induced by low
temperature, long duration (fever-like) whole body hyperthermia. J
Cell Physiol. 177:137–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peer AJ, Grimm MJ, Zynda ER and Repasky
EA: Diverse immune mechanisms may contribute to the survival
benefit seen in cancer patients receiving hyperthermia. Immunol
Res. 46:137–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Issels RD, Schlemmer M and Lindner LH: The
role of hyperthermia in combined treatment in the management of
soft tissue sarcoma. Curr Oncol Rep. 8:305–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones EL, Oleson JR, Prosnitz LR, Samulski
TV, Vujaskovic Z, Yu D, Sanders LL and Dewhirst MW: Randomized
trial of hyperthermia and radiation for superficial tumors. J Clin
Oncol. 23:3079–3085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Westermann AM, Jones EL, Schem BC, van der
Steen-Banasik EM, Koper P, Mella O, Uitterhoeve AL, de Wit R, van
der Velden J, Burger C, et al: First results of triple-modality
treatment combining radiotherapy, chemotherapy, and hyperthermia
for the treatment of patients with stage IIB III, and IVA cervical
carcinoma. Cancer. 104:763–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Kareh AW and Secomb TW: A theoretical
model for intraperitoneal delivery of cisplatin and the effect of
hyperthermia on drug penetration distance. Neoplasia. 6:117–127.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frey B, Weiss EM, Rubner Y, Wunderlich R,
Ott OJ, Sauer R, Fietkau R and Gaipl US: Old and new facts about
hyperthermia-induced modulations of the immune system. Int J
Hyperthermia. 28:528–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada Y, Itoh Y, Aoki S, Nakamura K, Taki
T, Naruse K, Tobiume M, Zennami K, Katsuda R, Kato Y, et al:
Preliminary results of M-VAC chemotherapy combined with mild
hyperthermia, a new therapeutic strategy for advanced or metastatic
transitional cell carcinoma of the urothelium. Cancer Chemother
Pharmacol. 64:1079–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yellin A, Simansky DA, Paley M and Refaely
Y: Hyperthermic pleural perfusion with cisplatin: Early clinical
experience. Cancer. 92:2197–2203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mujoomdar AA and Sugarbaker DJ:
Hyperthermic chemoperfusion for the treatment of malignant pleural
mesothelioma. Semin Thorac Cardiovasc Surg. Winter. 20:298–304.
2008.
|
|
24
|
Murthy R, Honavar SG, Naik M and Reddy VA:
Thermochemotherapy in hereditary retinoblastoma. Br J Ophthalmol.
87:14322003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin Y, Ma X, Zhang S, Meng H, Xu M, Yang
X, Xu W and Tian J: A tantalum oxide-based core/shell nanoparticle
for triple-modality image-guided chemo-thermal synergetic therapy
of esophageal carcinoma. Cancer Lett. 397:61–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huilgol NG: A retrospective analysis of
patients with head and neck cancer treated with radiation,
hyperthermia, and cetuximab: A brief report of outcome. J Cancer
Res Ther. 12:1164–1166. 2016.PubMed/NCBI
|
|
27
|
Tao Y, Guo Y, Liu W, Zhang J, Li X, Shen
L, Ru Y, Xue Y, Zheng J, Liu X, et al: AKT inhibitor suppresses
hyperthermia-induced Ndrg2 phosphorylation in gastric cancer cells.
Braz J Med Biol Res. 46:394–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong G, Braun RD and Dewhirst MW:
Characterization of the effect of hyperthermia on nanoparticle
extravasation from tumor vasculature. Cancer Res. 61:3027–3032.
2001.PubMed/NCBI
|
|
29
|
Xie X, Shao X, Gao F, Jin H, Zhou J, Du L,
Zhang Y, Ouyang W, Wang X, Zhao L, et al: Effect of hyperthermia on
invasion ability and TGF-beta1 expression of breast carcinoma MCF-7
cells. Oncol Rep. 25:1573–1579. 2011.PubMed/NCBI
|
|
30
|
Kanaya Y, Doihara H, Shiroma K, Ogasawara
Y and Date H: Effect of combined therapy with the antiestrogen
agent toremifene and local hyperthermia on breast cancer cells
implanted in nude mice. Surg Today. 38:911–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sawaji Y, Sato T, Takeuchi A, Hirata M and
Ito A: Anti-angiogenic action of hyperthermia by suppressing gene
expression and production of tumour-derived vascular endothelial
growth factor in vivo and in vitro. Br J Cancer. 86:1597–1603.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saga T, Sakahara H, Nakamoto Y, Sato N,
Ishimori T, Mamede M, Kobayashi H, Masunaga S, Sasai K, Kuroki M
and Konishi J: Enhancement of the therapeutic outcome of
radio-immunotherapy by combination with whole-body mild
hyperthermia. Eur J Cancer. 37:1429–1434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rong Y and Mack P: Apoptosis induced by
hyperthermia in Dunn osteosarcoma cell line in vitro. Int J
Hyperthermia. 16:19–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang YH, Haegerstrand A and Frostegard J:
Effects of in vitro hyperthermia on proliferative responses and
lymphocyte activity. Clin Exp Immunol. 103:61–66. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deezagi A, Manteghi S, Khosravani P,
Vaseli-Hagh N and Soheili ZS: Induced apoptosis by mild
hyperthermia occurs via telomerase inhibition on the three human
myeloid leukemia cell lines: TF-1, K562, and HL-60. Leuk Lymphoma.
50:1519–1527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canbay E, Ishibashi H, Sako S, Mizumoto A,
Hirano M, Ichinose M, Takao N and Yonemura Y: Preoperative
carcinoembryonic antigen level predicts prognosis in patients with
pseudomyxoma peritonei treated with cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy. World J Surg.
37:1271–1276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robins HI and Longo W: Whole body
hyperthermia: Simple complexities. Intensive Care Med. 25:898–900.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang Z, Yan W, Ming J and Yu Y: Docetaxel
weekly regimen in conjunction with RF hyperthermia for pretreated
locally advanced non-small cell lung cancer: A preliminary study.
BMC Cancer. 7:1892007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hildebrandt B, Drager J, Kerner T, Deja M,
Loffel J, Stroszczynski C, Ahlers O, Felix R, Riess H and Wust P:
Whole-body hyperthermia in the scope of von Ardenne's systemic
cancer multistep therapy (sCMT) combined with chemotherapy in
patients with metastatic colorectal cancer: A phase I/II study. Int
J Hyperthermia. 20:317–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takahashi I, Emi Y, Hasuda S, Kakeji Y,
Maehara Y and Sugimachi K: Clinical application of hyperthermia
combined with anticancer drugs for the treatment of solid tumors.
Surgery. 131:S78–S84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wendtner C, Abdel-Rahman S, Baumert J,
Falk MH, Krych M, Santl M, Hiddemann W and Issels RD: Treatment of
primary, recurrent or inadequately resected high-risk soft-tissue
sarcomas (STS) of adults: Results of a phase II pilot study
(RHT-95) of neoadjuvant chemotherapy combined with regional
hyperthermia. Eur J Cancer. 37:1609–1616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kappel M, Stadeager C, Tvede N, Galbo H
and Pedersen BK: Effects of in vivo hyperthermia on natural killer
cell activity, in vitro proliferative responses and blood
mononuclear cell subpopulations. Clin Exp Immunol. 84:175–180.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lv F, Yu Y, Zhang B, Liang D, Li ZM and
You W: Inhibitory effects of mild hyperthermia plus docetaxel
therapy on ER(+/-) breast cancer cells and action mechanisms. J
Huazhong Univ Sci Technolog Med Sci. 33:870–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michalakis J, Georgatos SD, de Bree E,
Polioudaki H, Romanos J, Georgoulias V, Tsiftsis DD and
Theodoropoulos PA: Short-term exposure of cancer cells to
micromolar doses of paclitaxel, with or without hyperthermia,
induces long-term inhibition of cell proliferation and cell death
in vitro. Ann Surg Oncol. 14:1220–1228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Michalakis J, Georgatos SD, Romanos J,
Koutala H, Georgoulias V, Tsiftsis D and Theodoropoulos PA:
Micromolar taxol, with or without hyperthermia, induces mitotic
catastrophe and cell necrosis in HeLa cells. Cancer Chemother
Pharmacol. 56:615–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Urano M, Kuroda M and Nishimura Y: For the
clinical application of thermochemotherapy given at mild
temperatures. Int J Hyperthermia. 15:79–107. 1999. View Article : Google Scholar : PubMed/NCBI
|