Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common cancer in the world (1). A total of ~600,000 new cases are
reported every year, with a marked proportion of these cases in
China (2). HNSCC frequently occurs as
a heterogeneous tumor with an aggressive phenotype (3). In clinical practice, metastasis,
particularly cervical lymph node metastasis, frequently occurs
during the progression of the disease (4). Regional cervical lymph node metastasis
is closely associated with the poor prognosis of patients with
HNSCC (5). Current treatments for
HNSCC have limited success in improving patient prognosis.
Therefore, identifying potential novel markers may be a promising
strategy to treat HNSCC.
Iron metabolism is associated with tumor growth and
promotes tumor cell proliferation in HNSCC (6,7). The major
storage form of iron in the human body is ferritin (8). Ferritin has been studied for nearly 80
years. However, it remains a key molecule, with novel
characteristics continuing to be discovered. Recent studies
revealed that the expression levels of ferritin were closely
associated with a number of malignant tumors, including lung cancer
(9) and primary hepatocellular
carcinoma (10). However, the role of
ferritin in HNSCC tumorigenesis and development is not fully
understood.
In the present study, serological and histological
experiments, in addition to Gene Expression Omnibus (GEO) datasets,
were used to investigate the expression levels of ferritin in
HNSCC. The primary aim was to clarify whether ferritin may serve as
a biomarker for HNSCC diagnosis and metastasis prediction.
Materials and methods
Patient selection for serum ferritin
(SF) detection
As presented in Table
I, 281 patients, including 44 patients with mucosal
inflammation (nasopharyngeal epithelium or throat mucous membrane),
133 with benign tumors (vocal polyps, n=108; cyst of epiglottis,
n=25), 20 with precancerosis (vocal leukoplakia, n=1; atypical
hyperplasia, n=19) and 84 with cancer (carcinoma in situ,
n=14; HNSCC without metastasis, n=40; HNSCC with metastasis, n=30),
were selected for the study. The inclusion criteria were: i)
patient diagnoses were based on conventional clinical, radiographic
and histopathological or cytological criteria; ii) patients had
previously undergone primary tumor resection. The 70 patients with
HNSCC (not including carcinoma in situ) additionally
underwent appropriate cervical lymph node dissection at Renmin
Hospital of Wuhan University (Wuhan, China) between January 2013
and January 2015. The exclusion criteria were: i) patients with
severe systemic disorders (such as diabetes mellitus, renal or
heart failure); ii) patients with diseases that affect SF levels
(such as liver injury, precancerous anemia or other types of
cancer); iii) patients who underwent previous chemotherapy or
radiotherapy.
 | Table I.Baseline characteristics of the 281
patients. |
Table I.
Baseline characteristics of the 281
patients.
|
| Sex | Age, years | Menopausal
status |
|---|
|
|
|
|
|
|---|
| Group | Male | Female | Range | Mean | Pre-menopausal |
Post-menopausal |
|---|
| Inflammation | 23 | 21 | 13–72 | 45 | 13 | 8 |
| Benign | 48 | 85 | 5–73 | 46 | 53 | 32 |
| Precancerosis | 16 | 4 | 29–73 | 53 | 4 | 0 |
| Cancer | 82 | 2 | 31–84 | 61 | 0 | 2 |
Serum sample collection and
analyses
Venous blood samples (3–5 ml) were collected in a
plain vial at diagnosis, prior to surgery. The samples were stored
at room temperature for 2 h. Serum samples were obtained following
centrifugation at 1,500 × g at 4°C for 5 min and immediately stored
at −70°C until use. The expression levels of SF were detected using
the chemiluminescent immunoassay method with a Siemens Centaur XP
fully-automated chemiluminescence immunoassay analyzer (Siemens
Healthcare GmbH, Erlangen, Germany) in the Department of Clinical
Laboratory, Renmin Hospital of Wuhan University, and the
correlative Quantitative Assay kit for ferritin (cat. no. 012245)
was purchased from Siemens Healthcare GmbH.
Ethics statement
The present study was approved by the ethics
committee of Renmin Hospital of Wuhan University. All specimens
were collected from patients who provided written informed consent,
in accordance with the principles of the Declaration of
Helsinki.
Patient selection for iron staining
and immunohistochemical staining
A total of 70 sets of paraformaldehyde-fixed
paraffin-embedded specimens were obtained from the pathology
department, including 40 patients without and 30 with cervical node
metastasis. Details are presented in Table II. Each set contained one primary
tumor tissue and one corresponding cervical lymph node tissue. The
tissues were fixed in 4% formaldehyde for 24 h at 4°C, and were
routinely processed into paraffin blocks. Each tissue was divided
into at least three paraffin-embedded sections: One for detecting
iron content using iron staining; and the remaining two for
detecting ferritin h (FTH) and ferritin L (FTL), respectively,
using immunohistochemistry (IHC).
 | Table II.Characteristics of patients
selected. |
Table II.
Characteristics of patients
selected.
| Characteristic | Metastasis group
(n=30) | No metastasis group
(n=40) | P-value |
|---|
| Sex |
|
| >0.05 |
|
Male | 30 | 39 |
|
|
Female | 0 | 1 |
|
| Age at
diagnosis |
|
| <0.05 |
| Median
(range) | 59.7 (31–73) | 61.2 (41–81) |
|
| Tumor type |
|
| <0.05 |
|
Glottis | 15 | 37 |
|
|
Hypopharynx | 10 | 2 |
|
|
Supraglottis | 2 | 0 |
|
|
Nasopharynx | 3 | 0 |
|
|
Tonsil | 0 | 1 |
|
| Differentiation
grade |
|
| >0.05 |
|
Well | 25 | 32 |
|
|
Moderate | 5 | 7 |
|
|
Poor | 0 | 0 |
|
| Total | 30 | 40 | >0.05 |
Neck color Doppler
ultrasonography
The neck lymph nodes of the 70 patients with HNSCC
were detected using color Doppler ultrasonography prior to surgery,
and the results were obtained from the radiology department. The
features of the nodes included size (longitudinal diameter, LD) and
shape (long axis/short axis or L/S ratio). For size, the dividing
line was 1 cm LD, and for the shape, 2× L/S ratio.
Tissue microarray for IHC
Two tissue microarrays (TMAs) (no. HN803b) with the
same patients' information were purchased from US Biomax, Inc.
(Rockville, MD, USA) to examine differences in the expression of
FTH and FTL between HNSCC and normal tissues. Each TMA consisted of
11 normal and 69 HNSCC tissue samples, with the mean age of
patients being 53.4 years (range 15–90 years). Details of the TMA
characteristics are presented in Table
III.
 | Table III.Tissue microarray
characteristics. |
Table III.
Tissue microarray
characteristics.
|
| Group |
|---|
|
|
|
|---|
| Total n=80 | HNSCC (n=69) | Normal (n=11) |
|---|
| Sex |
|
Male | 57 | 5 |
|
Female | 12 | 6 |
| Age, years |
|
Mean | 57.3 | 28.8 |
|
Range | 32–90 | 15–48 |
Iron staining
Iron staining (also termed Prussian blue staining)
was performed using a 5% potassium hexacyanoferrate trihydrate and
hydrochloric acid solution. Staining incubation time was ~30 min at
room temperature. Subsequently, the sections were rinsed with
water, counterstained with nuclear fast red, dehydrated, and
covered.
IHC staining
The 3–5 µm sections were deparaffinized with
standard pure xylene for 15 min, three times at room temperature
and hydrated in an alcohol gradient. PBS was used to wash the
sections. Antigen retrieval was performed in boiling citrate buffer
(pH 6.0) for 15 min. The sections were cooled down to room
temperature in the citrate buffer. Following washing of the
sections with PBS three times for 5 min, 0.3% hydrogen peroxide
phosphate-citrate buffer was used to block endogenous peroxidase
activity for 10 min at room temperature. Goat serum (5%; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) was used to block the
samples for 10 min at room temperature. The sections were then
rinsed with PBS for 5 min and incubated with primary antibody
against FTL (cat. no. SAB2108636; 1:500 dilution; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany) and against FTH (cat. no.
SAB2108662; 1:250 dilution; Sigma-Aldrich, Merck KGaA) for 12 h at
4°C. The sections were incubated with the biotinylated goat
anti-rabbit secondary antibody (cat. no. SP KIT-C9; 1:250 dilution;
Fuzhou Maixin Biotech Co., Ltd.) for 30 min at room temperature.
The slides were stained with diaminobenzidine for 5 min at room
temperature. Hematoxylin was used to counterstain the nucleus for 5
min at room temperature, followed by dehydration, and mounting.
Evaluation of iron staining and
IHC
Images of the stained sections were captured using
an Olympus BX40 light microscope and CC-12 Soft-Imaging System
(Olympus Corporation, Tokyo, Japan). The sections were analyzed and
scored for intensity (0–3) and frequency (0–4). The intensity was
scored as follows: Grade (0) negative; grade (1) weak intensity; grade (2) moderate intensity; and grade (3) strong intensity. The frequency scores
were assigned when 0–25, 26–50, 51–75 and 76–100% of the tumor
cells were positive, respectively. For statistical analysis, the
intensity and frequency were transformed into a Composite
Expression Score (CES) using the formula: CES=intensity ×
frequency. The range of CES was 0–12. The CES was scored as
negative (0) weak positive (1–4) positive
(5–8)
or strong positive (9–12).
GEO dataset
The GSE33205 (Cancer Outlier Gene Profile Sets
Elucidate Pathways and patient-Specific Targets in Head and Neck
Squamous Cell Carcinoma), GSE6631 (Expression Data from Head and
Neck Squamous Cell Carcinoma) and GSE27020 (Identification and
Validation of a Multi-gene Predictor of Recurrence in Primary
Laryngeal Cancer) datasets were obtained from the GEO (https://www.ncbi.nlm.nih.gov/). The expression values
of FTH and FTL were transformed into relative expression values,
and expression levels were compared between HNSCC and normal
tissues, using GEO2R software (https://www.ncbi.nlm.nih.gov/geo/geo2r/).
Statistical analysis
All statistical analyses were performed using SPSS
version 20.0 (IBM Corporation, Armonk, NY, USA). Quantitative data
are expressed as the mean ± standard deviation. Each test was
performed at least three times. A Student's t-test was used to
compare the differences between two groups. One-way analysis of
variance (ANOVA) was performed for the comparison of multiple
groups. If the results of the ANOVA indicated significant
differences, post-hoc analysis was performed with the Tukey test.
The diagnostic accuracy was evaluated using receiver operating
characteristic (ROC) curve analysis. A 4-fold Table χ2
test was used to determine the sensitivity and specificity of size
and shape. Correlation analysis was performed using Pearson's
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
SF levels are higher in patients with
HNSCC and metastasis compared with patients with HNSCC without
metastasis
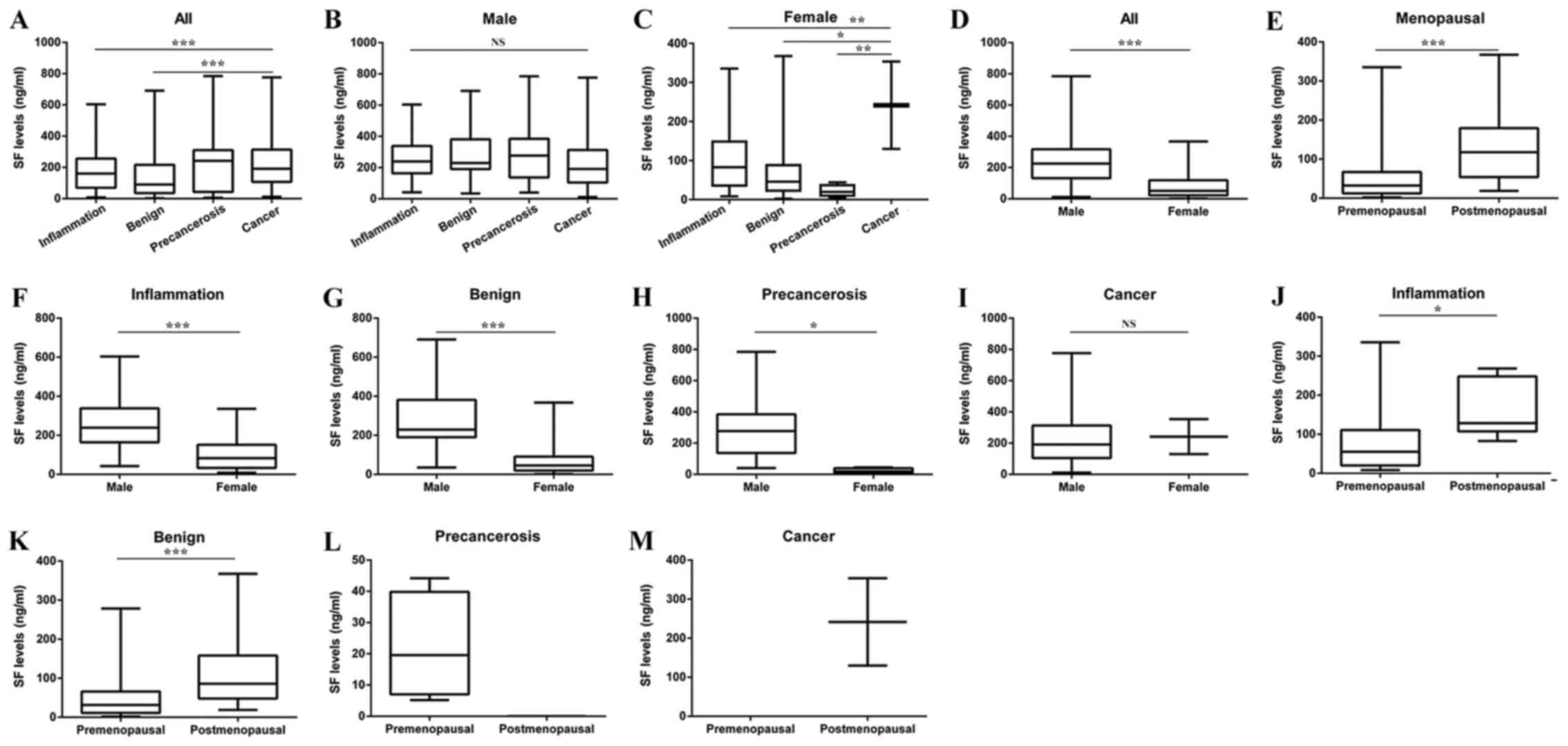
All patients were divided into four groups: i)
Inflammation; ii) benign tumor; iii) precancerosis; and iv) cancer.
The SF level in the cancer group was higher compared with the
benign group (Fig. 1A). However, in
male patients, no significant difference was reported among the
four groups (Fig. 1B). In female
patients, the SF level in the cancer group was significantly higher
compared with the benign and precancerosis groups (Fig. 1C). The differences in the SF levels
between male and female patients were compared, and the SF level of
male patients was significantly higher compared with that of female
patients (Fig. 1D). Postmenopausal
women had a higher SF level compared with premenopausal women
(Fig. 1E).
The differences in the SF level between male and
female patients were compared in each group. In general, male
patients had a higher SF level compared with female patients
(Fig. 1F-I). Taking menopausal status
into consideration, the SF level was reported to be higher in
postmenopausal women compared with premenopausal women in the
benign and inflammation groups (Fig.
1J-M).
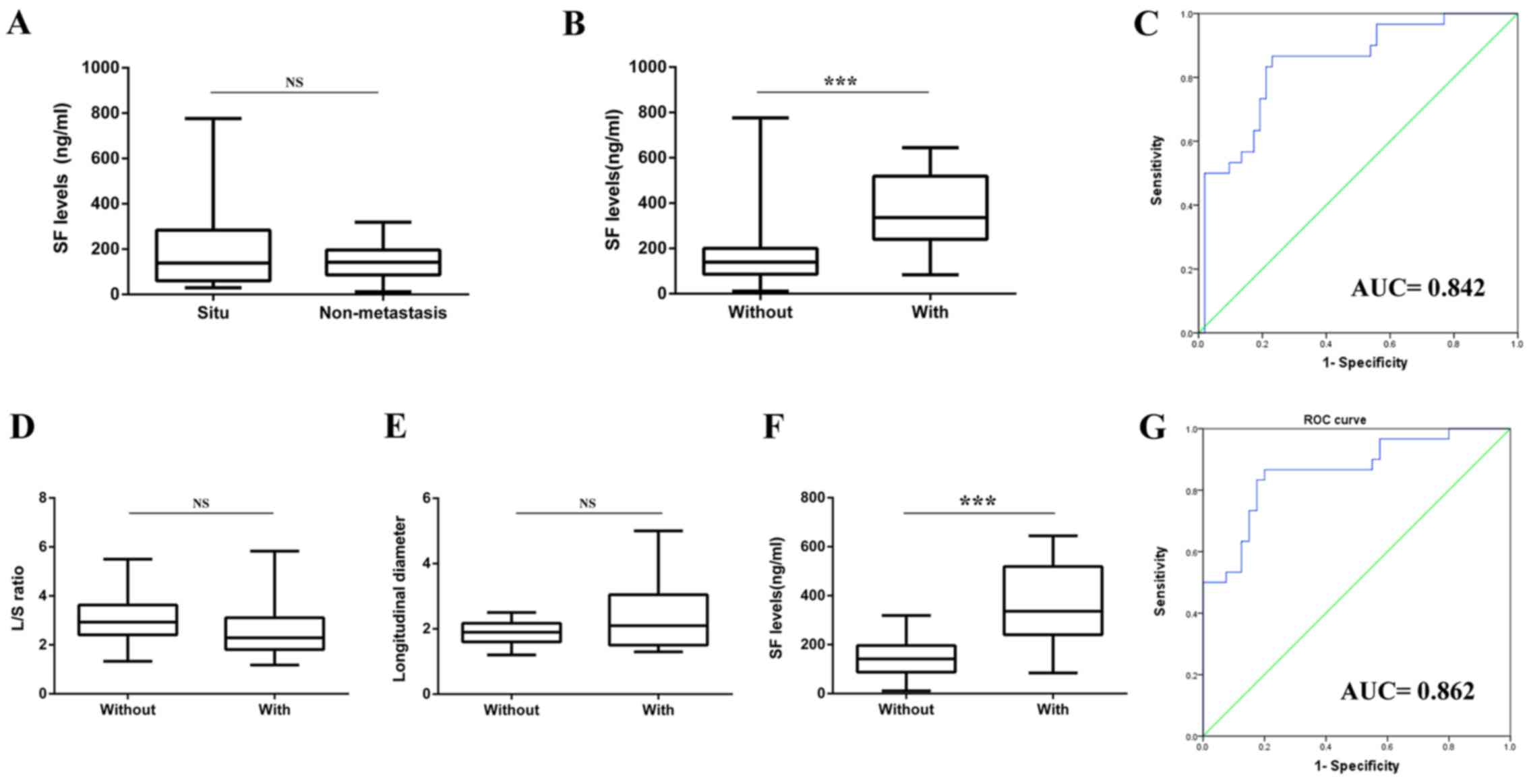
The SF level was compared between carcinoma in
situ (n=14) and HNSCC without metastasis groups (n=40). No
significant difference was observed between these two groups
(Fig. 2A). Therefore, carcinoma in
situ was considered as a part of the HNSCC without metastasis
group. Subsequently, the cancer group was divided into two
subgroups according to the cervical metastasis status: HNSCC with
metastasis, n=30; HNSCC without metastasis, n=54. It was revealed
that the SF level in the HNSCC with metastasis group was
significantly higher compared with the HNSCC without metastasis
group (Fig. 2B). The ROC analysis
revealed that the area under the curve (AUC) for SF to predict
cervical metastasis was 0.842, and the cutoff value of the SF level
was 205.55 ng/ml (Fig. 2C).
Doppler ultrasonography may be less
optimal for metastasis prediction compared with SF
The Doppler results of the 70 patients in the cancer
group were collected to further examine the importance of SF in
metastasis prediction. No statistically significant difference in
SF was reported between male and female patients in the cancer
group (Fig. 1I). The results of the
Doppler and SF levels are presented in Table IV. No statistically significant
difference in L/S ratio (Fig. 2D) and
LD (Fig. 2E) was noted between these
two groups, yet the SF level (Fig.
2F) exhibited a statistically significant difference. The
sensitivity of LD and the L/S ratio for detecting metastasis was 60
and 20%, and the specificity was 37.5 and 10%, respectively. The
ROC analysis of the SF level revealed that the AUC for metastasis
was 0.862, the cutoff value of the SF level was 205.60 ng/ml
(Fig. 2G), and the sensitivity and
specificity of SF for predicting neck metastasis were 86.7 and
80.0%, respectively.
 | Table IV.Doppler and SF results in 70 HNSCC
patients. |
Table IV.
Doppler and SF results in 70 HNSCC
patients.
|
| LD (cm) | L/S ratio | SF (ng/ml) |
|---|
|
|
|
|
|
|---|
| Variable | <1 | ≥1 | <2 | ≥2 | <205.60 | ≥205.60 |
|---|
| Metastasis | 12 | 18 | 6 | 24 | 4 | 26 |
| No metastasis | 15 | 25 | 4 | 36 | 31 | 9 |
| P-value | >0.05 |
| >0.05 |
| <0.001 |
|
Ferritin expression levels are higher
in the tumor tissues of HNSCC with metastasis
Tissue samples of the cancer group were obtained
from the pathology department to clarify whether the expression of
ferritin was also upregulated in the tumor tissues of HNSCC with
metastasis. IHC detection demonstrated that whether in primary
tumor or lymph node tissues, levels of FTH (Fig. 3A) and FTL (Fig. 3B) were higher in the HNSCC with
metastasis group compared with the HNSCC without metastasis group.
Iron staining (Fig. 3C) demonstrated
that the iron content was higher in the HNSCC with metastasis group
compared with the HNSCC without metastasis group, in primary tumor
and lymph node tissues. Furthermore, the correlation analysis
illustrated that the iron content was significantly correlated with
SF, FTH and FTL (Fig. 3D).
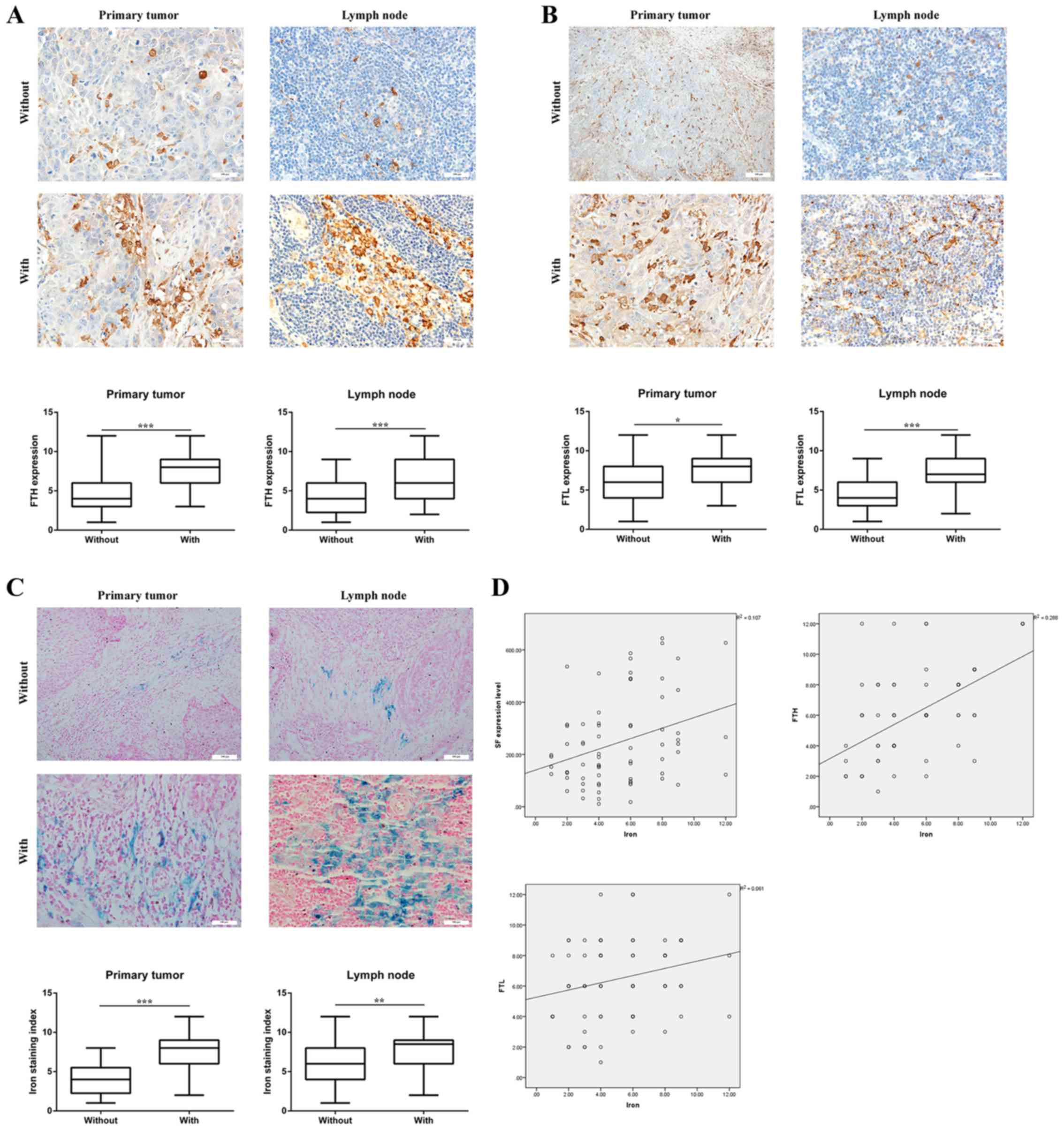 | Figure 3.Ferritin expression levels are higher
in HNSCC tumor tissues with metastasis. (A) Expression levels of
FTH in 70 patients with HNSCC. Whether in primary tumor (t=3.948)
or lymph node tissue (t=3.511), the expression of FTH was higher in
the HNSCC with metastasis group compared with the HNSCC without
metastasis group. Magnification, ×200. (B) Expression levels of FTL
in 70 patients with HNSCC. Whether in primary tumor (t=2.609) or
lymph node tissue (t=3.710), the expression levels of FTL were
higher in the HNSCC with metastasis group compared with the HNSCC
without metastasis group. Magnification, ×200. (C) Iron content in
70 patients with HNSCC. Whether in primary tumor (t=6.262) or lymph
node tissue (t=3.192), the iron content was higher in the HNSCC
with metastasis group compared with the HNSCC without metastasis
group. Magnification, ×200. (D) Correlation between iron content
and FTH levels, and the protein expression level of FTL in 70
patients with HNSCC. Iron content was significantly correlated with
SF levels (r=0.327; P<0.01), FTH levels (r=0.537; P<0.001)
and FTL levels (r=0.247; P<0.05). *P<0.05, **P<0.01,
***P<0.001. NS, not significant; FTH, ferritin H subunit; FTL,
ferritin L subunit; SF, serum ferritin; HNSCC, head and neck
squamous cell carcinoma. |
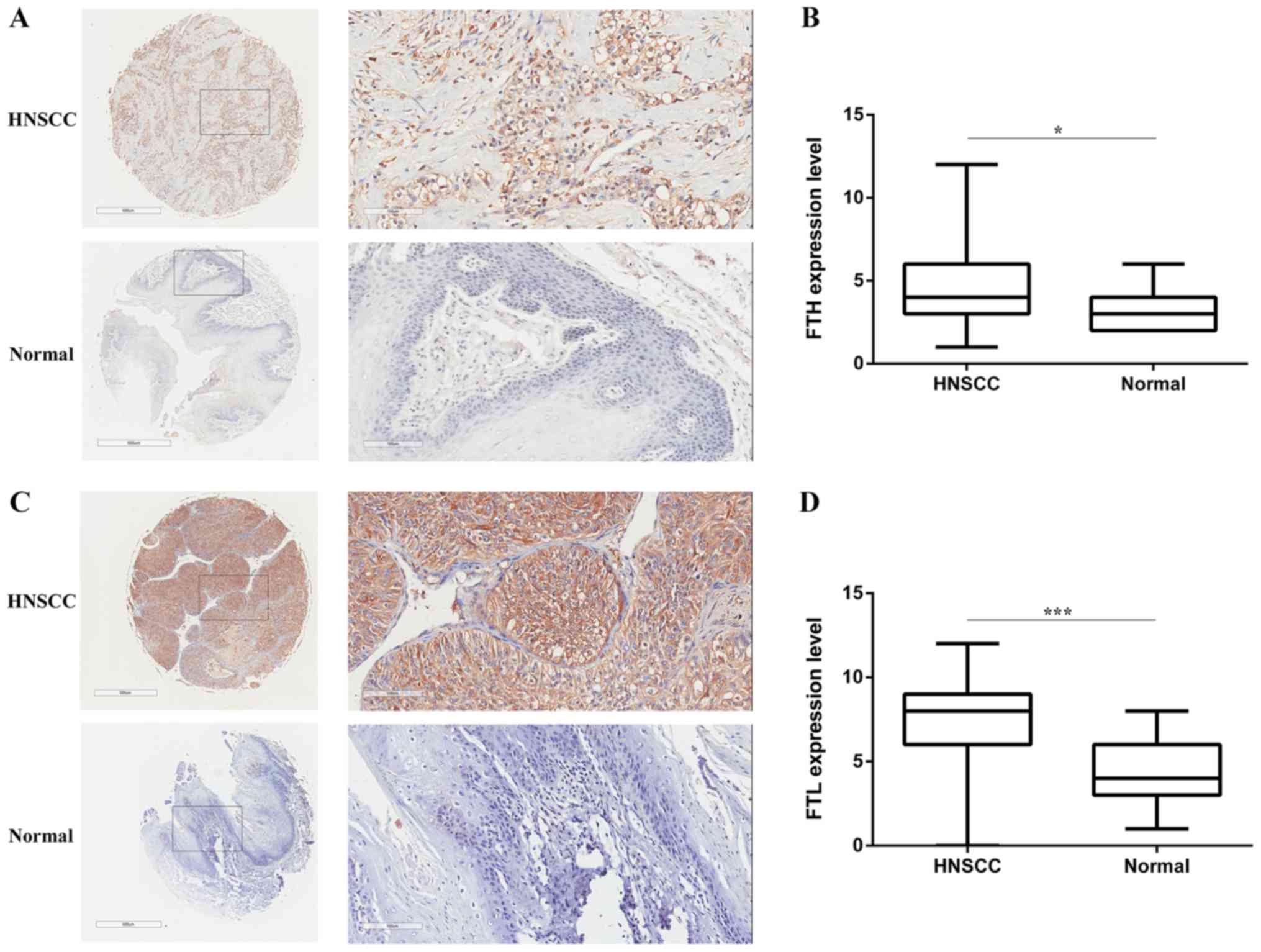
Expression levels of FTH and FTL are
upregulated in HNSCC tissues
Based on the above findings, two TMAs were purchased
to investigate the differences in the protein expression levels of
FTH and FTL between HNSCC and normal tissues. However, unlike the
expression of ferritin in serum, the protein expression levels of
FTH (Fig. 4A and B) and FTL (Fig. 4C and D) were significantly higher in
HNSCC tissues compared with normal tissues.
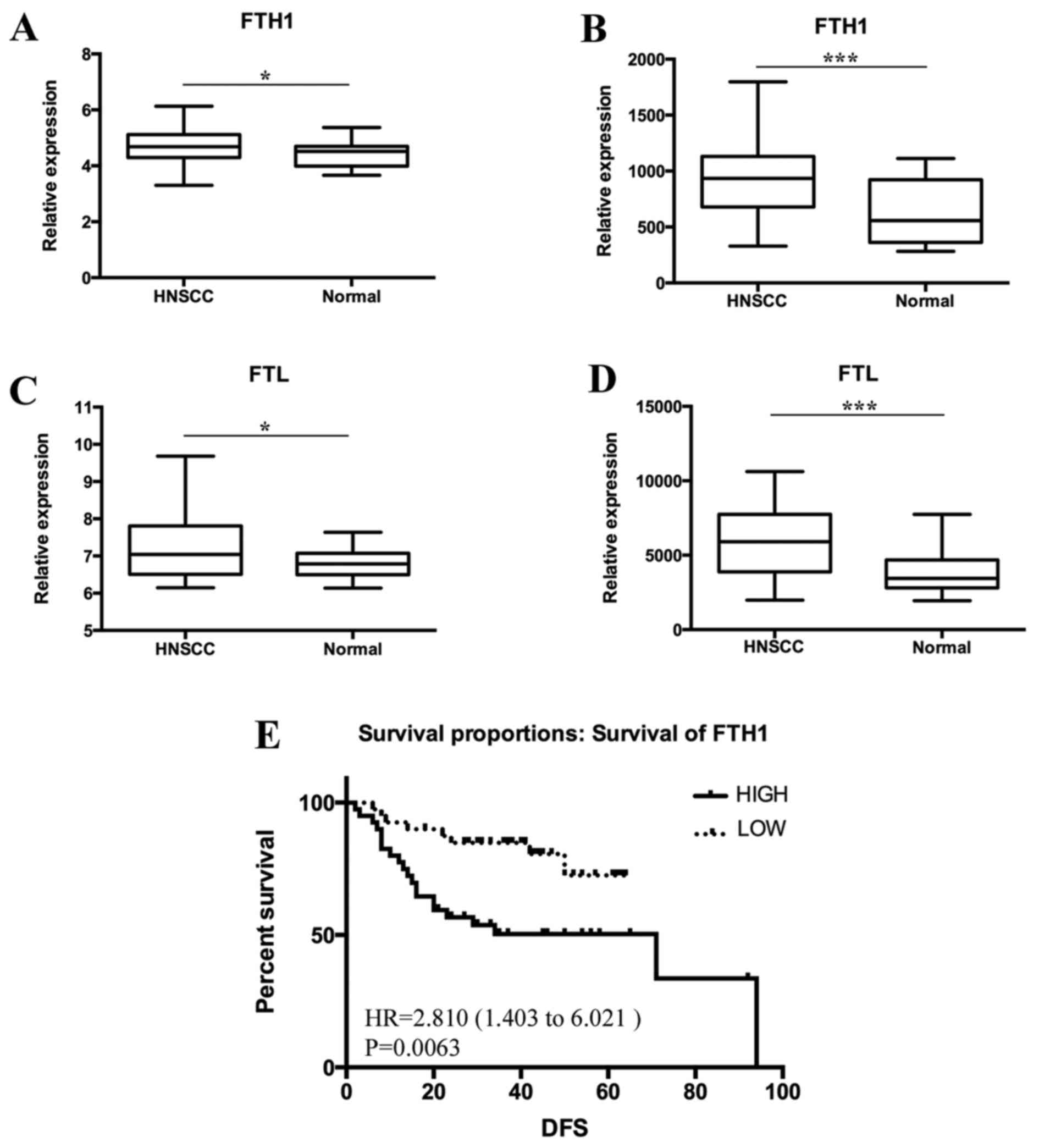
The associated data from the GEO dataset were
downloaded and analyzed. Using GSE33205 and GSE6631, the relative
expression levels of FTH (Fig. 5A and
B) and FTL (Fig. 5C and D) were
compared between HNSCC and normal tissues. The gene expression
levels of FTH and FTL were higher in HNSCC compared with normal
tissues, which was consistent with the histochemical findings.
Furthermore, the results of GSE27020 revealed that a high
expression level of FTH was associated with poor survival in
patients with HNSCC (Fig. 5E).
However, no data relating to the association between FTL and
survival were reported.
Discussion
Ferritin has a typical structure of a 24-subunit
spherical protein encapsulating an iron oxide core (11). The subunit comprises two different
types: FTH (21 kDa) and FTL (19 kDa) (12). Only FTH has the enzymatic activity to
convert Fe2+ into Fe3+ (13,14). The
SF level was higher in male patients compared with female patients.
Additionally, the SF level was higher in postmenopausal women
compared with premenopausal women (15). These results are in accordance with
previous findings. This may be due to menstruation, which causes a
loss of ~250 mg iron per year (16).
Undiminished iron may lead to a higher SF level in postmenopausal
women.
Currently, the correlation between iron metabolism
and tumor development has become a major concern (17,18).
Numerous studies have revealed that the expression levels of
ferritin are upregulated in various types of tumors, including lung
cancer and prostate cancer (19). The
present study demonstrated that the iron content and expression
levels of ferritin were higher in HNSCC compared with normal
tissues. As reported by other studies, iron metabolism is vital for
normal cell function (20). However,
in tumor growth, endocytosis leads to the storage of a large amount
of iron in cancer cells (21).
On the one hand, the expression of transferrin
receptor 1 (TFR1) is upregulated in the tumor membrane and
transports increased levels of iron into cancer cells (22,23). On
the other hand, the major protein that transports iron out of cells
is ferroportin, and its expression is always downregulated in
cancer cells (24,25). Therefore, a large amount of iron is
stored in cancer cells and subsequently promotes the expression of
ferritin. With the development of the tumor, high levels of
ferritin are secreted or leaked from damaged tumor cells, leading
to a higher SF level (26). Notably,
no statistically significant difference in the SF level was
reported between HNSCC and benign tumors or inflammation in the
present study. This may be due to that fact that in a few
pathological cases, disturbance in iron metabolism may lead to a
higher SF level (27). Therefore, SF
may be not a potential serum marker for the early diagnosis of
HNSCC.
The present study reported that the expression
levels of ferritin were significantly higher in the HNSCC with
metastasis group compared with the HNSCC without metastasis group.
This is the first time, to the best of our knowledge, that this
phenomenon has been described. A study indicated that ferritin may
regulate vascular remodeling and angiogenesis. It was also
demonstrated that ferritin may block the antiangiogenic effects of
cleaved high-molecular-weight kininogen (HKa) through specific
binding to the antiangiogenic domain of HKa (28). This may be a possible mechanism;
however, whether it promotes the metastasis of HNSCC to cervical
lymph nodes requires investigation.
Data from the GEO dataset demonstrated that high
expression levels of FTH were associated with poor survival. A
similar phenomenon has recently been described in breast cancer and
astrocytic brain tumors (29,30). However, no relevant data about the
prognostic value of FTL in HNSCC are available. Growing evidence
suggests that artificial iron compounds may inhibit tumor growth
and cell proliferation (31–33). For example, the iron oxide
nanoparticle has become a research focus; this may inhibit neoplasm
growth by inducing pro-inflammatory macrophage polarization
(34).
A recent study has indicated that the surgical
staging of cervical lymph nodes will not be replaced even by
advanced imaging modalities in the next few years (35). The performance of Doppler, computed
tomography, magnetic resonance imaging or positron emission
tomography in the nodal staging of HNSCC is not perfect in the
clinical setting. The present study revealed that SF had marked
clinical importance in predicting metastasis. It is an inexpensive,
routinely available marker that may serve as a valuable tool and
may improve predictive accuracy for lymph node metastasis of
HNSCC.
In conclusion, ferritin may be used a biomarker for
the early diagnosis of other malignancies, yet it may not be
applicable for HNSCC. However, the association of ferritin with
HNSCC may help identify patients at high risk of metastasis.
Ferritin may be a novel potential biomarker for detecting cervical
node metastasis in HNSCC.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant nos. 81372880 and 81172569), the
Natural Science Foundation of Zhejiang Province (grant no.
LY17H160065) and the Research Foundation of Zhejiang Provincial
Administration of Traditional Chinese Medicine (grant no.
2016ZB018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH, LW, CC and ZT designed the study and prepared
the manuscript. LW, YH, FL, AZ and YX collected clinical samples
and analyzed data. ZH, LW, FL, FW and BX conducted the experiments.
AZ, YX, FW and BX critically reviewed the manuscript and supervised
the study. ZH and LW wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Renmin Hospital of Wuhan University. All specimens
were collected from patients who provided written informed consent,
in accordance with the principles of the Declaration of Helsinki
and Good Clinical Practice Guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Busch C, Becker B, Kriegs M, Gatzemeier F,
Krüger K, Möckelmann N, Fritz G, Petersen C, Knecht R, Rothkamm K,
et al: Similar cisplatin sensitivity of HPV-positive and -negative
HNSCC cell lines. Oncotarget. 7:35832–35842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suarez C, Rodrigo JP, Robbins KT, Paleri
V, Silver CE, Rinaldo A, Medina JE, Hamoir M, Sanabria A, Mondin V,
et al: Superselective neck dissection: Rationale, indications, and
results. Eur Arch Oto-Rhino-L. 270:2815–2821. 2013. View Article : Google Scholar
|
|
3
|
Vigneswaran N and Williams MD:
Epidemiologic trends in head and neck cancer and aids in diagnosis.
Oral Maxillofac Surg Clin North Am. 26:123–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trivedi S, Mattos J, Gooding W, Godfrey TE
and Ferris RL: Correlation of tumor marker expression with nodal
disease burden in metastatic head and neck cancer. Otolaryngol Head
Neck Surg. 149:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
SV T and FM T: Iron and cancer: More ore
to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lenarduzzi M, Hui AB, Yue S, Ito E, Shi W,
Williams J, Bruce J, Sakemura-Nakatsugawa N, Xu W, Schimmer A, et
al: Hemochromatosis enhances tumor progression via upregulation of
intracellular iron in head and neck cancer. PLoS One. 8:e740752013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn SH, Lee S, Kim H, Lee SH, Kim BJ and
Koh JM: Higher serum ferritin level and lower femur neck strength
in women at the stage of bone loss (≥45 years of age): The fourth
Korea national health and nutrition examination survey (Knhanes
IV). Endocr Res. 41:334–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi HB, Li XD, Jiang JT, Zhao WQ, Ji M and
Wu CP: Serum ferritin is elevated in advanced non-small cell lung
cancer patients and is associated with efficacy of platinum-based
chemotherapy. J Cancer Res Ther. 10:681–685. 2014.PubMed/NCBI
|
|
10
|
Zhao Y, Wang M, Cui C, Zhang L, Liao F, Li
H and Wu X: Significance of combined tests of serum golgi
glycoprotein 73 and other biomarkers in diagnosis of small primary
hepatocellular carcinoma. Cancer Biomark. 15:677–683. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arenas-Salinas M, Townsend PD, Brito C,
Marquez V, Marabolli V, Gonzalez-Nilo F, Matias C, Watt RK,
López-Castro JD, Domínguez-Vera J, et al: The crystal structure of
ferritin from chlorobium tepidum reveals a new conformation of the
4-fold channel for this protein family. Biochimie. 106:39–47. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Memon S, Gao RC, Jiang RZ, Si WN and Zhang
XH: Deciphering the evolution of ferritin gene family in various
living organisms. Pak J Agr Sci. 52:1055–1063. 2015.
|
|
13
|
Timoshnikov VA, Kobzeva TV, Polyakov NE
and Kontoghiorghes GJ: Inhibition of Fe(2+)- and Fe(3+)-induced
hydroxyl radical production by the iron-chelating drug deferiprone.
Free Radic Biol Med. 78:118–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Wang x, Gao X, Wang L, Lu Y, Gao P,
Deng W, Yu P, Ma J, Guo J, et al: Identification of five human
novel genes associated with cell proliferation by cell-based
screening from an expressed cDNA ORF library. Life Sci.
81:1141–1151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han LL, Wang YX, Li J, Zhang XL, Bian C,
Wang H, Du S and Suo LN: Gender differences in associations of
serum ferritin and diabetes, metabolic syndrome, and obesity in the
China health and nutrition survey. Mol Nutr Food Res. 58:2189–2195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato I, Dnistrian AM, Schwartz M, Toniolo
P, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A and
Riboli E: Risk of iron overload among middle-aged women. Int J
Vitam Nutr Res. 70:119–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rychtarcikova Z, Lettlova S, Tomkova V,
Korenkova V, Langerova L, Simonova E, Zjablovskaja P,
Alberich-Jorda M, Neuzil J and Truksa J: Tumor-initiating cells of
breast and prostate origin show alterations in the expression of
genes related to iron metabolism. Oncotarget. 8:6376–6398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pizzamiglio S, Bortoli MD, Taverna E,
Signore M, Veneroni S, Cho WC, Orlandi R, Verderio P and Bongarzone
I: Expression of iron-related proteins differentiate non-cancerous
and dancerous breast tumors. Int J Mol Sci. 18:E4102017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Peng A, Zeng J, Liu X, Wang B,
Fang X, Wang F, Ren G and Min J: Serum ferritin in combination with
prostate-specific antigen improves predictive accuracy for prostate
cancer. Oncotarget. 8:17862–17872. 2017.PubMed/NCBI
|
|
20
|
Daher R, Manceau H and Karim Z: Iron
metabolism and the role of the iron-regulating hormone hepcidin in
health and disease. Presse Med. 46:E272–E278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jablonska E, Socha K, Reszka E, Wieczorek
E, Skokowski J, Kalinowski L, Fendler W, Seroczynska B, Wozniak M,
Borawska MH, et al: Cadmium, arsenic, selenium and
iron-Implications for tumor progression in breast cancer. Environ
Toxicol Pharmacol. 53:151–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong SM, Hwang S and Seong RH:
Transferrin receptor regulates pancreatic cancer growth by
modulating mitochondrial respiration and ROS generation. Biochem
Biophys Res Commun. 471:373–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong SM, Lee J, Finley LW, Schmidt PJ,
Fleming MD and Haigis MC: SIRT3 regulates cellular iron metabolism
and cancer growth by repressing iron regulatory protein 1.
Oncogene. 34:2115–2124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo W, Zhang S, Chen Y, Zhang D, Yuan L,
Cong H and Liu S: An important role of the hepcidin-ferroportin
signaling in affecting tumor growth and metastasis. Acta Bioch
Bioph Sin (Shanghai). 47:703–715. 2015. View Article : Google Scholar
|
|
25
|
Chen Y, Zhang Z, Yang K, Du J, Xu Y and
Liu S: Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor
growth through enforcing ferroportin-conducted iron egress.
Oncogene. 34:3839–3847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kell DB and Pretorius E: Serum ferritin is
an important inflammatory disease marker, as it is mainly a leakage
product from damaged cells. Metallomics. 6:748–773. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gartner A, Berger J, Bour A, El Ati J,
Traissac P, Landais E, El Kabbaj S and Delpeuch F: Assessment of
iron deficiency in the context of the obesity epidemic: Importance
of correcting serum ferritin concentrations for inflammation. Am J
Clin Nutr. 98:821–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coffman LG, Parsonage D, D'Agostino R,
Torti FM and Torti SV: Regulatory effects of ferritin on
angiogenesis. Proc Natl Acad Sci USA. 106:570–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu NQ, De Marchi T, Timmermans AM,
Beekhof R, Trapman-Jansen AM, Foekens R, Look MP, van Deurzen CH,
Span PN, Sweep FC, et al: Ferritin heavy chain in triple negative
breast cancer: A favorable prognostic marker that relates to a
cluster of differentiation 8 positive (CD8+) effector
T-cell response. Mol Cell Proteomics. 13:1814–1827. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosager AM, Sorensen MD, Dahlrot RH,
Hansen S, Schonberg DL, Rich JN, Lathia JD and Kristensen BW:
Transferrin receptor-1 and ferritin heavy and light chains in
astrocytic brain tumors: Expression and prognostic value. PloS One.
12:e01829542017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhukova OS, Smirnova ZS, Chikileva IO and
Kiselevskii MV: Antiproliferative activity of a new nitrosyl iron
complex with cysteamine in human tumor cells in vitro. Bull Exp
Biol Med. 162:583–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahajan UM, Teller S, Sendler M, Palankar
R, van den Brandt C, Schwaiger T, Kühn JP, Ribback S, Glöckl G,
Evert M, et al: Tumour-specific delivery of siRNA-coupled
superparamagnetic iron oxide nanoparticles, targeted against PLK1,
stops progression of pancreatic cancer. Gut. 65:1838–1849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kir D, Saluja M, Modi S, Venkatachalam A,
Schnettler E, Roy S and Ramakrishnan S: Cell-permeable iron
inhibits vascular endothelial growth factor receptor-2 signaling
and tumor angiogenesis. Oncotarget. 7:65348–65363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zanganeh S, Hutter G, Spitler R, Lenkov O,
Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M,
et al: Iron oxide nanoparticles inhibit tumour growth by inducing
pro-inflammatory macrophage polarization in tumour tissues. Nat
Nanotechnol. 11:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heusch P, Sproll C, Buchbender C, Rieser
E, Terjung J, Antke C, Boeck I, Macht S, Scherer A, Antoch G, et
al: Diagnostic accuracy of ultrasound, 18F-FDG-PET/CT,
and fused 18F-FDG-PET-MR images with DWI for the
detection of cervical lymph node metastases of HNSCC. Clin Oral
Investig. 18:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|