Introduction
Leukemia includes a group of heterogeneous
neoplastic malignancies that develop in the bone marrow and affect
normal hematopoiesis. Conventionally, leukemia has been classified
according to its cellular origin, whether of myeloid or lymphoid
lineage, and by the course of the illness, whether acute or
chronic. The four major types are acute myeloid leukemia (AML),
acute lymphocytic leukemia (ALL), chronic myeloid leukemia (CML),
and chronic lymphocytic leukemia (CLL). They differ significantly
in terms of the morphological, cytogenetic, immunophenotypic, and
molecular features of the malignant cells (1). The French-American-British (FAB)
classification divides AML into nine subtypes, identified as M0
through M7. These nine are based on the level of maturity of the
myeloid cells. The category of undifferentiated AML, or M0,
reflects the difficulty in delineating whether a cancer cell is
truly a myeloid-type or a lymphoid-type malignancy when it presents
neither myeloid nor lymphoid markers (2). These specific characteristics of
leukemia suggest the heterogeneity of the underlying biological
alterations involved in cancer cell transformation and the
variations in the levels of hematopoietic progenitor cell
hierarchy.
Acetylcholine (ACh) is a major central and
peripheral neurotransmitter and is also involved in the control of
several non-neuronal functions, including immune function (3). Immune cells, especially lymphocytes,
express essential components of the non-neuronal cholinergic
system. They include the chemical messenger, ACh; the
ACh-synthesizing enzyme, choline acetyltransferase (ChAT); an
ACh-degrading enzyme, acetylcholinesterase (AChE); and both
muscarinic (m) and nicotinic (n) ACh receptors (AChRs) (4). There is accumulating evidence for the
involvement of the non-neuronal cholinergic pathway in regulating
and modulating the immune system by means of its effects on the
differentiation and proliferation of lymphocytes, cytokine
production, antigen presentation, and inflammation (5).
Several studies on the involvement of the
non-neuronal cholinergic pathway in tumorigenesis have been
published, including lung, colon, cervix, prostate, breast, and
bile duct cancers, wherein more information regarding cholinergic
autocrine and paracrine signaling is available (6–9). Molecular
analyses of the expression of essential cholinergic components in
malignant tumors of different histogenesis have indicated that
those tumors largely exhibit different cholinergic component
expression from normal tissue (10).
In addition, enhancement of ACh production has often been observed
in tumors as well as some differences in the expression patterns of
ACh receptors. Among the various types of cholinergic AChRs,
subtype α7 nAChR (α7-nAChR) and subtype M3 mAChR receptor
(M3-mAChR) have been identified as the cholinergic AChRs most
capable of promoting cancer progression, for example, by the
induction of cancer cell growth and metastasis (6,9,11).
Previous studies have demonstrated the
downregulation of certain types of cholinergic ACh receptors during
the thymocyte maturation process, denoting their possible role in
T-cell development (12).
Furthermore, the plasticity of cholinergic AChRs during T-cell
differentiation has also been demonstrated in murine splenic T-cell
models (13). Extensive data obtained
from human mononuclear leukocytes (MNLs), isolated T- and B-cells,
and various leukemia cell lines, have revealed the diversity of the
non-neuronal cholinergic system, particularly of cholinergic AChRs
in human immune cells (14,15). Our previous in vitro study of
NB-4 acute promyeolocytic leukemic cells also demonstrated that the
expression of α7-nAChR and M3-mAChR changes following all-trans
retinoic acid-induced differentiation treatment (16). In addition, induction of AChE activity
was evident in whole blood and lymphocyte samples obtained from
newly diagnosed pediatric patients with T- or B-ALL. However, AChE
activity decreased during the remission period (17). It is important to note the recent
hypothesis that non-neuronal cholinergic machinery may be involved
in leukemogenesis, especially in T-cell leukemia, and that its
components, such as cholinergic AChRs, may represent relevant
therapeutic targets for leukemia (18). To date, there has been no new
information regarding the involvement of the non-neuronal
cholinergic system amongst different types of leukemia. We propose
that the expression patterns of cholinergic systems may differ
among the types and subtypes of leukemia. In the present study, we
compared the expression levels of the major cholinergic AChRs,
including α7-nAChR and M3-mAChR, in healthy subjects and in
patients with the three main types of leukemia, to reveal the
potentiality of the cholinergic pathway as a pharmacological target
in hematopoietic derived neoplasia.
Materials and methods
Patients
Inclusion criteria for the subjects were as follows:
i) Diagnosis of leukemia at Siriraj Hospital (Bangkok, Thailand);
ii) patients with leukemia were in the novel diagnostic-phase and
not undergoing treatment that might influence the expression of
cholinergic AChRs in lymphocytes; iii) written informed consent was
obtained. The exclusion criteria were as follows: i) Presence of
multiple tumors; ii) being pregnant or too young (age <15
years); iii) presence of acute or chronic diseases such as
diabetes, parasitosis or any immune dysfunction. The exclusions
were intended to minimize potential complications due to the impact
of these complex diseases on immune cells. This study was conducted
in accordance with the provisions of the International Conference
on Harmonization of Good Clinical Practice guidelines and the
Helsinki Declaration. The study was approved by the Committee on
Human Rights Related to Research Involving Human Subjects of the
Chulabhorn Research Institute (CRI project number: 33/2554,
approval date: 29/02/2012) and the Ethics Committee of Siriraj
Hospital (Project number: 255/2555, approval date: 01/08/2012).
Written informed consent was obtained from each patient prior to
sample collection. A total of 51 peripheral blood (PB) or bone
marrow (BM) samples were obtained from patients at the time of
diagnosis between 01/2013 and 12/2016. PB samples were also taken
from healthy subjects (n=5) to serve as a control group. The
peripheral blood mononuclear cells (PBMCs) of patients with
leukemia and healthy subjects were isolated from 6 ml of
heparinized venous blood by density gradient centrifugation using
Ficoll-Paque (Isoprep; Robbins Scientific, Sunnyvale, CA, USA) at
500 × g for 30 min. The mononuclear cells were washed three times
in Hank's balanced salt solution (Gibco-Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Western immunoblotting assay
PBMCs and BM samples were lysed in lysis buffer
containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM
Na3VO4, 20 mM NaF, 1 mM PMSF, 1% Triton
X-100, and 1X protease inhibitor cocktail set I (Calbiochem; Merck
KGaA, Darmstadt, Germany). Sample lysates were sonicated and then
incubated at 4°C for 30 min. Samples were centrifuged at 16,000 × g
for 15 min at 4°C. Sample supernatants were collected and stored at
−80°C for further analysis. Concentrations of proteins in the
supernatants were determined by using a Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The sample (50 µg total
protein) was mixed with Laemmli loading buffer (62.5 mM Tris-HCl pH
6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, and 5%
2-mercaptoethanol) and then boiled at 95°C for 5 min. Proteins were
separated via 7.5% SDS-PAGE in a Mini-PROTEAN II system (Bio-Rad
Laboratories, Inc.). The separated proteins were then transferred
onto a nitrocellulose membrane (GE Healthcare Life Sciences, Little
Chalfont, UK), and the membrane was incubated in blocking buffer
containing 5% non-fat dry milk in TBS-T buffer (10 mM Tris-HCl, pH
8.0, 0.05% Tween-20, and 150 mM NaCl) for 1 h at room temperature,
followed by overnight incubation at 4°C with the primary
antibodies. Antibodies against α7-nAChR (sc-5544; 1:1,000) and
M3-mAChR (sc-9108; 1:500) were obtained from Santa Cruz
Biotechnology (Dallas, TX, USA). The antibody against GAPDH (2118;
1:2,000) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). After washing with TBS-T buffer 3 times (10 min
each), the membrane was incubated with a horseradish-peroxidase
conjugated secondary antibody (GE Healthcare Life Sciences) for 2 h
at room temperature. The protein bands stained by the targeted
antibodies were visualized using an enhanced chemiluminescence
assay kit (GE Healthcare Life Sciences) followed by exposure to
x-ray film (Pierce, Perbio, Brazil). To avoid variation between
gels, the exposure time of α7-nAChR, M3-mAChR, and GAPDH, was fixed
at 2, 20, and 2 min, respectively. Relative protein expression
levels of α7-nAChR and M3-mAChR were calculated from the band
intensities using computerized densitometry with ImageQuantTL
software (GE Healthcare Life Sciences). Notably, the M3-mAChR
immunoblot had two bands, which were considered and quantified as
M3-mAChR in accordance with a previous study (19).
Statistical analysis
All data are expressed as the means ± standard
deviation (SD). To determine whether the data set was normally
distributed, a Shapiro-Wilk normality test was performed. As some
groups contained only small sample numbers, statistically
significant differences were assessed using the non-parametric
Kruskal-Wallis one-way analysis of variance for rank with the post
hoc Dunn's test. A P-value <0.05 is considered to indicate a
statistically significant difference.
Results
Patient characteristics
The study included 5 healthy subjects and 51
patients with leukemia [AML (n=33), CML (n=5), and ALL (n=13)].
According to the WHO classification (2), AML cases were further classified into
subtypes: AML with minimal differentiation (AML-M0; n=6), AML
without maturation (AML-M1; n=5), AML with maturation (AML-M2;
n=8), acute promyelocytic leukemia (AML-M3; n=6), or acute
myelomonocytic leukemia (AML-M4; n=8). ALL was further classified
as T lymphoblastic leukemia/lymphoma (T-ALL; n=9), and B
lymphoblastic leukemia/lymphoma (B-ALL; n=4). Clinical
characteristics are listed in Table
I. AML and ALL occur in children as well as in adults (subject
age range 15–85) but CML has only been found in adults (subject age
range 26–57). Hemoglobin levels and percentages of hematocrit in
all groups of leukemia seem to be relatively lower than in healthy
subjects. As predicted, the white blood cell count (WBC) was
higher; meanwhile the platelet count was much lower in the patients
with leukemia than in the healthy subject group.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Disease
diagnosis | No. of case | No. of
males/females | Age (years) | Hemoglobin
(g/dl) | Hematocrit (%) | WBC count
(×109/l) | Platelet count
(×109/l) | Blast cells (%) |
|---|
| AML-M0 | 6 | 3/3 | 16–76 (42) | 6.2–8.5 (8) | 18.2–25.5 (24.6) | 2.4–273.4 (62.1) | 8–133 (28) | 80.5–88 (85.8) |
| AML-M1 | 5 | 4/1 | 15–81 (54) | 6.9–12.2 (8.8) | 21.3–37.0 (26.3) | 4.8–190.2 (16) | 24–507 (152) | 24–92.2 (39.8) |
| AML-M2 | 8 | 4/4 | 16–66 (47.5) | 4.2–11.7 (8.1) | 13.5–33.8 (23.4) | 0.5–131 (20.6) | 8–85 (33) | 65.7–80.4 (76.6) |
| AML-M3 | 6 | 4/2 | 33–85 (56.5) | 7.3–10 (7.95) | 21–29.8 (23) | 0.6–133.8 (13.9) | 9–113 (41) | 64.5–92.5 (82.3) |
| AML-M4 | 8 | 5/3 | 21–71 (58.5) | 4.9–9.9 (8.4) | 15.7–30.5 (25.7) | 9.2–205.1 (31.4) | 20–189 (38.5) | 66.3–85 (78.8) |
| CML | 5 | 3/2 | 26–57 (49) | 8.8–12.3 (9.8) | 26.4–37.3 (32.1) | 2.4–312.4 (5.7) | 100–601 (209) | ND |
| T-ALL | 9 | 8/1 | 19–50 (26) | 5–16.6 (8.9) | 16–50.8 (26.9) | 3.1–280 (9.4) | 17–406 (131) | 44.3–90.4 (74.4) |
| B-ALL | 4 | 2/2 | 15–56 (29) | 5.6–12.5 (8.3) | 18.8–27.9 (25.8) | 6.65–153.1
(56.2) | 21–63 (31) | 65.1–77.7 (65.2) |
| Normala | 5 | 2/3 | 22–34 (28) | 12.1–14.5 (13.3) | 38.1–44.1 (39.7) | 4.55–7.84 (5.7) | 170–341 (231) | ND |
Expression levels of α7-nAChR and
M3-mAChR in patients with leukemia
Expression levels of α7-nAChR in PBMCs or BM in
different types of patients with leukemia were compared with
healthy subjects. It might be expected that all types of patients
with leukemia would have lower expression levels of α7-nAChR than
healthy subjects, but significant expression levels were observed
in T-ALL, CML, AML-M40 and AML-M4 (Fig.
1 and Table II). The expression
level of α7-nAChR was lowest in T-ALL, significantly different from
in AML-M1, AML-M2 and AML-M4.
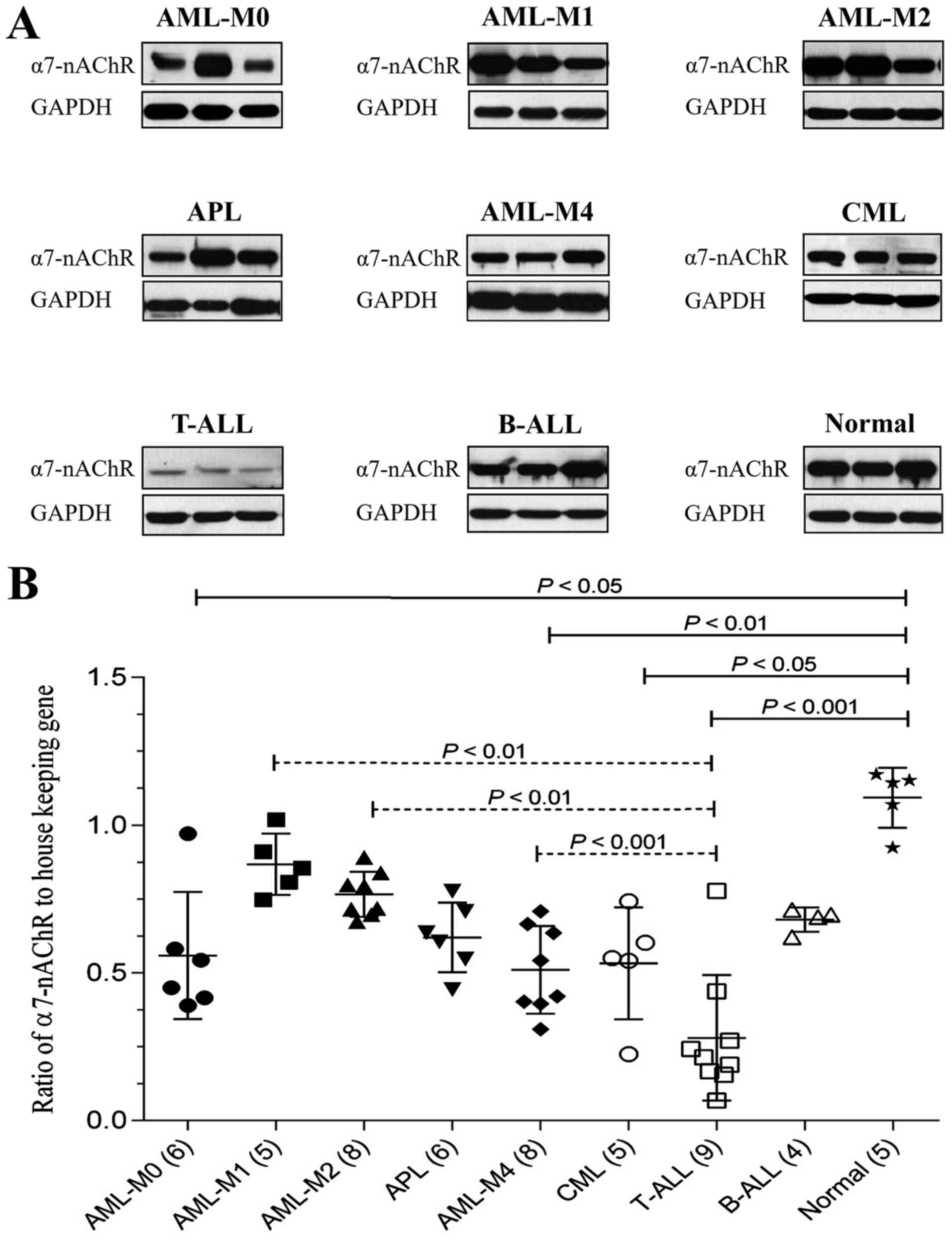 | Figure 1.Expression levels of α7-nAChR in
patients with leukemia. (A) Representative immunoblots of α7-nAChR
in the three main types of leukemia, including five subtypes of
AML, two subtypes of ALL, and CML. GAPDH was used as the loading
control. (B) The ratio of α7-nAChR to GAPDH was determined by
densitometric analysis. Data are presented as the means ± standard
deviation. The number of cases in each group is presented in
parentheses on the × axis. P<0.05 was considered to indicate a
statistically significant difference. α7-nAChR, nicotinic subtype
α7 acetylcholine receptors; AML-M0, AML with minimal
differentiation; AML-M1, AML without maturation; AML-M2, AML with
maturation; APL, acute promyelocytic leukemia; AML-M4, acute
myelomonocytic leukemia; CML, chronic myeloid leukemia; T-ALL, T
lymphoblastic leukemia/lymphoma; B-ALL, B lymphoblastic
leukemia/lymphoma. |
 | Table II.Relative expression levels of α7-nAChR
and M3-mAChR in patients with leukemia compare to healthy
subjects. |
Table II.
Relative expression levels of α7-nAChR
and M3-mAChR in patients with leukemia compare to healthy
subjects.
|
| Relative expression
levels (% of healthy subjects) |
|---|
|
|
|
|---|
|
| α7-nAChR | M3-mAChR |
|---|
|
|
|
|
|---|
| Leukemia types | Mean | Median | Mean | Median |
|---|
| Normala (5) | 100 | 100 | 100 | 100 |
| AML-M0 (6) | 51 | 43 | 70 | 80 |
| AML-M1 (5) | 79 | 75 | 129 | 147 |
| AML-M2 (8) | 70 | 66 | 127 | 145 |
| APL (6) | 57 | 54 | 47 | 57 |
| AML-M4 (8) | 47 | 42 | 57 | 58 |
| CML (5) | 49 | 48 | 164 | 187 |
| T-ALL (9) | 26 | 19 | 40 | 49 |
| B-ALL (4) | 62 | 60 | 135 | 147 |
Regarding M3-mAChR expression, the results showed a
large variation in M3-mAChR expression among leukemia types
(Fig. 2 and Table II). The expression level of M3-mAChR
was lowest in T-ALL, significantly different from in B-ALL, CML,
AML-M1 and AML-M2. In addition, CML showed the highest level of
M3-mAChR, significantly different from in APL and AML-M4. Notably,
there was no significant difference between expression in the
control group any in type of leukemia types.
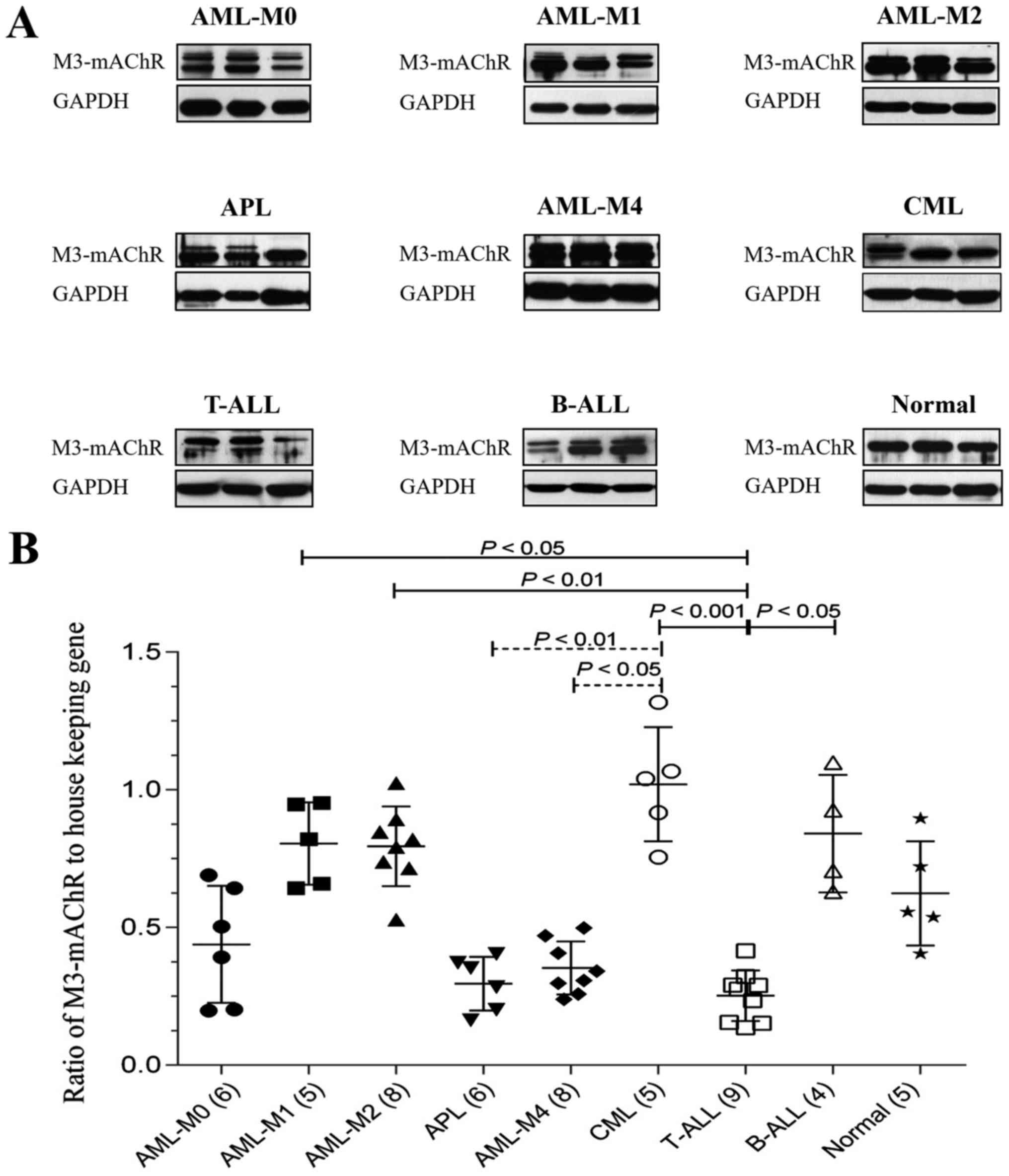 | Figure 2.Expression levels of M3-mAChR in
patients with leukemia. (A) Representative immunoblots of M3-mAChR
in the three main types of leukemia, including five subtypes of
AML, two subtypes of ALL, and CML. (B) Ratio of M3-mAChR to GAPDH
as determined by densitometric analysis. Data are presented as the
means ± standard deviation. The number of cases in each group is
presented in parentheses at the × axis. P<0.05 was considered to
indicate a statistically significant difference. M3-mAChR,
muscarinic subtype M3 acetylcholine receptors; AML-M0, AML with
minimal differentiation; AML-M1, AML without maturation; AML-M2,
AML with maturation; APL, acute promyelocytic leukemia; AML-M4,
acute myelomonocytic leukemia; CML, chronic myeloid leukemia;
T-ALL, T lymphoblastic leukemia/lymphoma; B-ALL, B lymphoblastic
leukemia/lymphoma. |
Discussion
The putative involvement of the non-neuronal
cholinergic system in the etiology of hematopoietic derived
neoplasia is of particular importance in view of various recent
reports (16–18). Several studies have provided evidence
demonstrating that blood cells, especially lymphocytes, possess
non-neuronal cholinergic components including cholinergic AChRs
(14,20). Our results showed a variation of
cholinergic AChRs, including α7-nAChR and M3-AChR, in the BM or
PBMC samples from patients with the three main types of leukemia:
AML, ALL, and CML. In detail, α7-nAChR in T-ALL, CML, but not
B-ALL, were lower in patients with leukemia than in healthy
subjects. It has been reported that cholinergic signals regulate
thymic differentiation and selection (21). Furthermore, previous studies have
shown that α7-nAChR is involved in both T and B lymphocyte
development in the bone marrow and spleen (22). In AML subtypes based on levels of
myeloid maturation, variation in α7-nAChR expression was observed.
The α7-nAChR expression was lowest in AML-M0 (AML with minimal
differentiation); its level of expression increased to a maximum in
AML-M1, then continuously declined in AML-M2, APL, and AML-M4. This
pattern of variation was also observed in the expression of
α4-nAChR subunits during B lymphocyte development in wild-type mice
(22).
Among AML subtypes, we found that M3-mAChR
expression was lowest in APL and AML-M4. Our previous in
vitro study on the NB-4 acute promyeolocytic leukemic cell line
demonstrated that M3-mAChR markedly increases after
all-trans-retinoic acid-induced differentiation treatment
(16). Hence, administration of a
specific M3-mAChR agonist along with differentiation-inducing drugs
may be a potential treatment for the APL subtype. Amongst the
leukemia types, the highest expression of M3-mAChR was detected in
CML, where most myeloid cells are mature. Collectively, these
observations support the association of M3-mAChR in myeloid
maturation. It is notable that in previous studies of the CML K562
cell line, muscarinic receptor activation stimulated intracellular
cAMP, decreased c-Fos and cyclin D1 expression, and inhibited cell
proliferation (19,23). Altogether, mAChR activation by its
agonist may also be a potential approach for treatment of CML.
However, this hypothesis needs further study.
Previous studies have demonstrated that ACh
production in various leukemic T-cell lines, including CEM, Jurkat,
HSB-2, MOLT-3, and MOLT-4, was considerably higher compared to
fresh PBMCs obtained from healthy subjects (24). Moreover, it has been suggested that
ACh may not be rapidly hydrolyzed in T-ALL as AChE is decreased in
these types of leukemic cells, compared with in mature normal
T-cell lymphocytes (25). Previous
studies have shown that Jurkat, T-cell-derived leukemic cells,
increased M3-mAChR expression (26).
In addition, it has been proposed that high levels of ACh in T-ALL
may act as an autocrine growth factor and play a significant role
in leukemia T-cell clonal expansion via shaping of intracellular
calcium signaling pathways (18).
However, our study showed the lowest expression of both α7-nAChR
and M3-mAChR was in T-ALL patients. It could be hypothesized that
the reduction of cholinergic receptors may represent an adaptation
mechanism of cholinergic over-activation.
In conclusion, our observations highlight the
differential expression profiles of α7-nAChR and M3-mAChR among the
three main types of leukemia, and that these expression patterns
may mediate leukemogenesis. Moreover, these findings may support
the utilization of cholinergic AChRs as potential prognostic
markers and alternative therapeutic treatments. However, further
studies with larger cohorts, and functional studies, are necessary
to elucidate this proposal.
Acknowledgements
The authors would like to thank Assistant Professor
Janice M. Wongsurawat of the Chulabhorn Research Institute
(Bangkok, Thailand) for proofreading the manuscript.
Funding
This study was supported by a research grant from
Chulabhorn Research Institute (grant no. PH2011-02, 2011).
Availability of data and materials
All data generated and analyzed during this study
are included in this article.
Authors' contributions
TS performed the experiment, analyzed the data, and
drafted the manuscript. SC and KC performed the experiments. CUA
participated in the design of the study and supervised subject
selection and sample collection. OP performed the sample
collection. JS was responsible for initiation, conception,
experimental design, execution of the entire project and for
critical revision of the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Committee on
Human Rights Related to Research Involving Human Subjects of the
Chulabhorn Research Institute (CRI project number: 33/2554,
approval date: 29/02/2012) and the Ethics Committee of Siriraj
Hospital (Project number: 255/2555, approval date: 01/08/2012). All
patients provided written informed consent for the publication of
data in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin MW, Xu SM, An Q and Wang P: A review
of risk factors for childhood leukemia. Eur Rev Med Pharmacol Sci.
20:3760–3764. 2016.PubMed/NCBI
|
|
2
|
Schlenk RF, Döhner K, Krauter J, Fröhling
S, Corbacioglu A, Bullinger L, Habdank M, Späth D, Morgan M, Benner
A, et al: Mutations and treatment outcome in cytogenetically normal
acute myeloid leukemia. N Engl J Med. 358:1909–1918. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawashima K and Fujii T: The lymphocytic
cholinergic system and its biological function. Life Sci.
72:2101–2109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawashima K and Fujii T: Expression of
non-neuronal acetylcholine in lymphocytes and its contribution to
the regulation of immune function. Front Biosci. 9:2063–2085. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ofek K and Soreq H: Cholinergic
involvement and manipulation approaches in multiple system
disorders. Chem Biol Interact. 203:113–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russo P, Del Bufalo A, Milic M, Salinaro
G, Fini M and Cesario A: Cholinergic receptors as target for cancer
therapy in a systems medicine perspective. Curr Mol Med.
14:1126–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paleari L, Grozio A, Cesario A and Russo
P: The cholinergic system and cancer. Semin Cancer Biol.
18:211–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah N, Khurana S, Cheng K and Raufman JP:
Muscarinic receptors and ligands in cancer. Am J Physiol Cell
Physiol. 296:C221–C232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campoy FJ, Vidal CJ, Munoz-Delgado E,
Montenegro MF, Cabezas-Herrera J and Nieto-Cerón S: Cholinergic
system and cell proliferation. Chem Biol Interact. 259:257–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spindel ER: Muscarinic receptor agonists
and antagonists: Effects on cancer. Handb Exp Pharmacol. 451–468.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dang N, Meng X and Song H: Nicotinic
acetylcholine receptors and cancer. Biomed Rep. 4:515–518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawashima K, Fujii T, Moriwaki Y and
Misawa H: Critical roles of acetylcholine and the muscarinic and
nicotinic acetylcholine receptors in the regulation of immune
function. Life Sci. 91:1027–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian J, Galitovskiy V, Chernyavsky AI,
Marchenko S and Grando SA: Plasticity of the murine spleen T-cell
cholinergic receptors and their role in in vitro differentiation of
naïve CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes
Immun. 12:222–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato KZ, Fujii T, Watanabe Y, Yamada S,
Ando T, Kazuko F and Kawashima K: Diversity of mRNA expression for
muscarinic acetylcholine receptor subtypes and neuronal nicotinic
acetylcholine receptor subunits in human mononuclear leukocytes and
leukemic cell lines. Neurosci Lett. 266:17–20. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tayebati SK, El-Assouad D, Ricci A and
Amenta F: Immunochemical and immunocytochemical characterization of
cholinergic markers in human peripheral blood lymphocytes. J
Neuroimmunol. 132:147–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chotirat S, Suriyo T, Hokland M, Hokland
P, Satayavivad J and Auewarakul CU: Cholinergic activation enhances
retinoic acid-induced differentiation in the human NB-4 acute
promyelocytic leukemia cell line. Blood Cells Mol Dis. 59:77–84.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Battisti V, Schetinger MR, Maders LD,
Santos KF, Bagatini MD, Correa MC, Spanevello RM, do Carmo Araújo M
and Morsch VM: Changes in acetylcholinesterase (AchE) activity in
lymphocytes and whole blood in acute lymphoblastic leukemia
patients. Clin Chim Acta. 402:114–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dobrovinskaya O, Valencia-Cruz G,
Castro-Sánchez L, Bonales-Alatorre EO, Liñan-Rico L and Pottosin I:
Cholinergic machinery as relevant target in acute lymphoblastic T
leukemia. Front Pharmacol. 7:2902016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cabadak H, Aydin B and Kan B: Regulation
of M2, M3, and M4 muscarinic receptor expression in K562 chronic
myelogenous leukemic cells by carbachol. J Recept Signal Transduct
Res. 31:26–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawashima K and Fujii T: Extraneuronal
cholinergic system in lymphocytes. Pharmacol Ther. 86:29–48. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rinner I, Globerson A, Kawashima K,
Korsatko W and Schauenstein K: A possible role for acetylcholine in
the dialogue between thymocytes and thymic stroma.
Neuroimmunomodulation. 6:51–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skok M, Grailhe R, Agenes F and Changeux
JP: The role of nicotinic acetylcholine receptors in lymphocyte
development. J Neuroimmunol. 171:86–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aydin B, Kan B and Cabadak H: The role of
intracellular pathways in the proliferation of human K562 cells
mediated by muscarinic receptors. Leuk Res. 37:1144–1149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujii T, Tsuchiya T, Yamada S, Fujimoto K,
Suzuki T, Kasahara T and Kawashima K: Localization and synthesis of
acetylcholine in human leukemic T cell lines. J Neurosci Res.
44:66–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubinstein H, Lubrano T, Dainko J, Mathews
H, Lange C, Silberman S and Minowada J: Acetylcholinesterase in
cultured human leukemia/lymphoma cell lines. Leuk Res. 8:741–744.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alea MP, Borroto-Escuela DO,
Romero-Fernandez W, Fuxe K and Garriga P: Differential expression
of muscarinic acetylcholine receptor subtypes in Jurkat cells and
their signaling. J Neuroimmunol. 237:13–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
















