Introduction
Urothelial carcinoma (UC) is the ninth most common
type of tumor globally (1). In
Western countries, urinary bladder UC (UBUC) is the most common
malignancy of the urinary tract, and upper-tract UC (UTUC) is
comparatively rare, accounting for 5–10% of all urinary tract
malignancies (2). However, in Taiwan,
UTUC accounts for 40% of all types of urothelial tumor due to the
prevalent use of Chinese herbs containing aristolochic acids
including Aristolochia fangchi, named ‘Guang Fang Ji’ in
Mandarin (3). Aristolochia
fangchi is used in slimming pills, in addition to being used as
a diuretic and immune modulator (3).
The Aristolochia herb results in Aristolochic acid-induced
urothelium damage (4). As human
urothelial tissue is rich in peroxidases, the aristolactams
activated by peroxidase may result in the formation of aristolactam
(AL)-DNA adducts in urothelial tissue (4,5) resulting
in mutations in the TP53 tumor suppression gene which may promote
cell-cycle checkpoints, DNA repair and apoptosis (6). The overexpression of TP53 protein in
patients is highly observed in Aristolochic acid nephropathy
resulting in urothelial carcinoma (7).
Nephroureterectomy is the gold standard in the
initial management of invasive localized UTUC (8,9). However,
in patients with high-stage (AJCC 7th edition stage II–IV)
(10) tumors, the survival rate is
poor. The 5-year survival rate is <50% for patients with tumor
(T) stage T2/3 and <10% for patients with T4 UTUC (11). The high recurrence and mortality rates
in patients with high-stage tumors indicates the necessity for
additional effective adjuvant treatments (12).
UC is a chemosensitive tumor (13), and the effectiveness of chemotherapy
in treating UBUC is well established (8,14).
Therefore, considering the high prevalence of distant metastasis of
upper tract urothelial cancer (15),
chemotherapy in a neoadjuvant or adjuvant setting appears to be a
reasonable approach for the treatment of high-stage UTUC. Kwak
et al (16) studied 32
patients receiving cisplatinum-based chemotherapy compared with 11
patients without chemotherapy. Based on multivariate analysis, this
previous study concluded that chemotherapy improved survival.
The objective of the present study was to observe
the outcome of high-stage UTUC and determine whether adjuvant
chemotherapy is beneficial for patients with high-stage UTUC.
Patients and methods
Patients
The present study was approved by the Research
Ethics Committee of China Medical University and Hospital (IRB
approval no. CMUH103-REC1-094; Taichung, Taiwan). Personal records
and data were retrospectively collected and analyzed anonymously. A
tertiary referral center (China Medical University Hospital,
Taichung, Taiwan) database was used, and included 139 patients who
underwent nephroureterectomy between June 2003 and December 2012
for pathological stage II to IV UTUC at the China Medical
University Hospital. Patients with concomitant UBUC (n=5); patients
with incomplete data (n=2); patients with a follow-up duration of
<3 months (n=4); or patients receiving neoadjuvant chemotherapy
(n=2) were excluded. Finally, the study population comprised 126
patients with UTUC of pathological stage II [pT2, node (N)0/X,
metastasis (M)0], stage III (pT3, N0/X, M0) and stage IV (T4, N0/X,
M0; or T1-4, N1-3, M0).
The present retrospective study analyzed age at
surgery, sex, tumor location, adjuvant chemotherapy status and
regimen, estimated glomerular filtration rate (eGFR), tumor
pathology (grade and lymphovascular invasion), tumor recurrence and
cause of mortality. All patients underwent open or laparoscopic
nephroureterectomy with bladder cuff excision and achieved free
pathological margins. Lymph node dissection was performed if
preoperative computerized tomography (CT) of the abdomen and pelvis
revealed lymph node enlargement or if palpable lymph nodes were
identified during surgery. Extended lymphadenectomy was not
routinely performed due to potential complications, including
lymphocele development, vascular injury and bowel injury. Regional
lymph node recurrence was defined according to a previous study by
Kondo et al (17).
Tumor staging and follow-up
Tumor stage was determined according to the American
Joint Committee on Cancer TNM classification (18) and tumor grade was defined according to
the 2004 World Health Organization grading system (19). Lymphovascular invasion was defined as
the presence of tumor cells within an endothelium-lined space
without underlying muscular walls (20). Postoperative renal function was
calculated as per the following equation: eGFR=186 × (serum
creatinine/88.4)−1.154 × (age)-0.203 × (0.742 if female)
(21).
Patients were followed up by physical examination,
blood tests and cystoscopy postoperatively every 3 months for the
first 3 years after surgery, every 6 months for the subsequent 2
years, and then annually for up to 10 years. Abdominal CT or
magnetic resonance imaging were performed every 6 months to
evaluate tumor recurrence, lymph node metastasis and distant
metastasis.
Measurements and definitions
Patients with a stage I tumor were defined as
low-stage while those with stage II–IV were defined as high-stage.
In the high-stage group, patients with pathological stage II tumors
were categorized as the localized UTUC group, and patients with T3,
T4 and/or node-positive non-distant metastatic UTUC were
categorized as the locally advanced UTUC group.
The endpoints assessed were overall survival (OS),
disease-free survival (DFS), distant metastasis-free survival
(DMFS) and locoregional recurrence-free survival (LRFS).
Local recurrence was established by urine cytology,
cystoscopy, retrograde ureteropyeloscopy or biopsy, and/or CT or
magnetic resonance imaging scans of the abdomen and pelvis. Distant
metastasis was diagnosed on the basis of physical examination and
imaging methods. OS was defined as the time from treatment until
mortality; the follow-up of the patients that survived was censored
at the latest date of follow-up (date of mortality). DFS was
defined as the time from treatment until the earliest occurrence of
recurrence or mortality from any cause. For analysis of LRFS and
DMFS, the latencies were recorded (time from date of treatment) to
the first detection of locoregional recurrence or distant
metastasis, respectively.
Statistical analysis
χ2 or Fisher's exact tests were used to
assess differences between groups. All survival data were analyzed
using the Kaplan-Meier method with a log-rank test to compare the
disease free survival and overall survival rate. Multivariate
analysis was carried out using a Cox proportional hazards
regression model. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS statistical software (version 22.0; IBM Corp.,
Armonk, NY, USA).
Results
Patient characteristics
The median follow-up period was 23.6 months (range,
3.1–120.9 months). Clinicopathological characteristics are listed
in Table I. A total of 38 patients
with pathological stage II (T2, N0, M0) UTUC were categorized as
the localized group, and 88 patients with stage III/IV and/or
node-positive non-distant metastatic UTUC were categorized as the
locally advanced group.
 | Table I.Clinicopathological characteristics of
patients with high stage (II–IV) upper-tract urothelial
carcinoma. |
Table I.
Clinicopathological characteristics of
patients with high stage (II–IV) upper-tract urothelial
carcinoma.
| Clinicopathological
characteristic | Value |
|---|
| Age (years); median
(range) | 70.0 (26–86) |
| Sexa |
|
| Male | 43 (34.1) |
|
Female | 83 (65.9) |
| eGFR
(ml/min)a |
|
|
>60 | 30 (23.8) |
|
<60 | 96 (76.2) |
| Pathological
stagea |
|
| II | 38 (30.2) |
|
III | 73 (57.9) |
| IV | 15 (11.9) |
| Gradea |
|
|
Low | 35 (27.8) |
|
High | 91 (72.2) |
| Lymphovascular
invasiona |
|
| No | 80 (63.5) |
|
Yes | 46 (36.5) |
| Perineural
permeationa |
|
| No | 104 (82.5) |
|
Yes | 22 (17.5) |
|
Locationa |
|
|
Ureter | 52 (41.3) |
| Renal
pelvis | 74 (58.7) |
| Lymph node
metastasisa |
|
|
Negative | 121 (96.0) |
|
Positive | 5 (4.0) |
In the localized group, the 5-year OS, DFS, LRFS and
DMFS were 70.7, 82.2, 82.0 and 97.3%, respectively; whereas, in the
locally advanced group with and without chemotherapy, these rates
were 49.5, 56.8, 71.1 and 75.2%, respectively. Overall, in the
locally advanced group, 29 patients (33.3%) succumbed to UTUC, and
5 patients (5.7%) succumbed to other causes. Furthermore, 19
patients with locally advanced UTUC presented with distant
metastasis at diagnosis of disease recurrence and 17 presented with
locoregional recurrence. By contrast, in the localized UTUC group,
4 patients (10.5%) succumbed to UTUC and 4 (10.5%) succumbed to
other causes; 1 patient presented with distant metastasis at
diagnosis of disease recurrence; and 5 patients presented with
locoregional recurrence.
Lymph node metastasis was detected in 5 patients,
and all of these patients received adjuvant chemotherapy. The mean
age was 60.6 years. Of the 5 patients, 3 (60%) survived for >5
years and 1 patient (20%) developed distant metastasis. No patients
suffered from local recurrence. These patients were included in the
locally advanced with chemotherapy group to expand the case
number.
Univariate log-rank analysis was performed for OS.
Age, pathological stage and lymphovascular invasion were identified
as significant prognostic factors for OS, while there were no
significant differences in terms of sex, tumor location, tumor
grade or renal function (eGFR) (Table
II).
 | Table II.Univariate analysis of overall
survival in patients with high stage (II–IV) upper-tract urothelial
carcinoma. |
Table II.
Univariate analysis of overall
survival in patients with high stage (II–IV) upper-tract urothelial
carcinoma.
| Variable | 5-year overall
survival rate (%) | P-value |
|---|
| Age (years) |
| 0.018 |
|
>70 | 39.9 |
|
|
<70 | 70.8 |
|
| Sex |
| 0.900 |
|
Male | 38.9 |
|
|
Female | 60.5 |
|
| Location |
| 0.681 |
| Renal
pelvis | 56.9 |
|
|
Ureter | 53.3 |
|
| Grade |
| 0.231 |
|
Low | 59.0 |
|
|
High | 52.7 |
|
| Lymphovascular
invasion |
| 0.002 |
|
With | 42.4 |
|
|
Without | 61.3 |
|
| Stage |
| <0.001 |
| II | 70.7 |
|
|
III | 56.7 |
|
| IV | 16.0 |
|
| eGFR (ml/min) |
| 0.303 |
|
<60 | 51.0 |
|
|
>60 | 63.1 |
|
Chemotherapy regimens
Adjuvant chemotherapy was not administered to the
localized group. Of the locally advanced group, 38 (43.2%) received
adjuvant chemotherapy. These patients were scheduled to receive
>4 courses of carboplatin-based chemotherapy, including the
carboplatin, methotrexate and vinblastine (CarMV) regimen (13
patients), and the gemcitabine and carboplatin (GCar) regimen (25
patients). The median interval between surgery and the beginning of
systemic therapy was 1.2 months (range, 0.7–2.3 months).
Patients treated with the CarMV regimen received
methotrexate (30 mg/m2), vinblastine (3
mg/m2), and carboplatin [area under the plasma drug
concentration-time curve (AUC)=5] intravenously on day 1, and
methotrexate (30 mg/m2) and vinblastine (3
mg/m2) intravenously on days 15 and 22. Cycles were
repeated every 4 weeks.
The GCar regimen was administered in a 4-week cycle,
with gemcitabine (1,000 mg/m2) administered
intravenously on days 1, 8, and 15, and carboplatin (AUC=5)
administered intravenously on day 1. The majority of patients
received >4 cycles of chemotherapy (median, 5 cycles; range, 2–6
cycles), with the exception of 1 patient who received 2 cycles, and
3 patients who received 3 cycles of chemotherapy.
Survival analysis
In the locally advanced UTUC group, there were no
significant differences in clinicopathological characteristics
between patients who received and those who did not receive
adjuvant chemotherapy, except that an eGFR of <60 ml/min was
more prevalent among patients who did not receive adjuvant
chemotherapy (Table III).
 | Table III.Characteristics of patients in the
locally advanced group stratified by adjuvant chemotherapy. |
Table III.
Characteristics of patients in the
locally advanced group stratified by adjuvant chemotherapy.
| Clinicopathological
characteristics | Adjuvant
chemotherapy | No adjuvant
chemotherapy | P-value |
|---|
| Age (years),
mean | 63.57 | 68.86 | 0.735 |
| Sexa |
|
Male | 17 | 13 | 0.107 |
|
Female | 21 | 37 |
|
| eGFR
(ml/min)a |
|
>60 | 15 | 8 | 0.025 |
|
<60 | 23 | 42 |
|
| Pathological
stagea |
|
III | 30 | 43 | 0.558 |
| IV | 8 | 7 |
|
| Gradea |
|
Low | 8 | 13 | 0.774 |
|
High | 30 | 37 |
|
| Lymphovascular
invasiona |
| No | 21 | 27 | 1.000 |
|
Yes | 17 | 23 |
|
| Perineural
permeationa |
| No | 30 | 38 | 0.944 |
|
Yes | 8 | 12 |
|
|
Locationa |
|
Ureter | 13 | 15 | 0.850 |
| Renal
pelvis | 25 | 35 |
|
Of the 50 patients who did not receive adjuvant
chemotherapy, 14 (28%) developed distant metastasis, and 10 (20%)
developed locoregional recurrence. On the other hand, of the 38
patients administered with adjuvant chemotherapy, 5 (13.2%)
developed distant metastasis and 7 (18.4%) developed locoregional
recurrence.
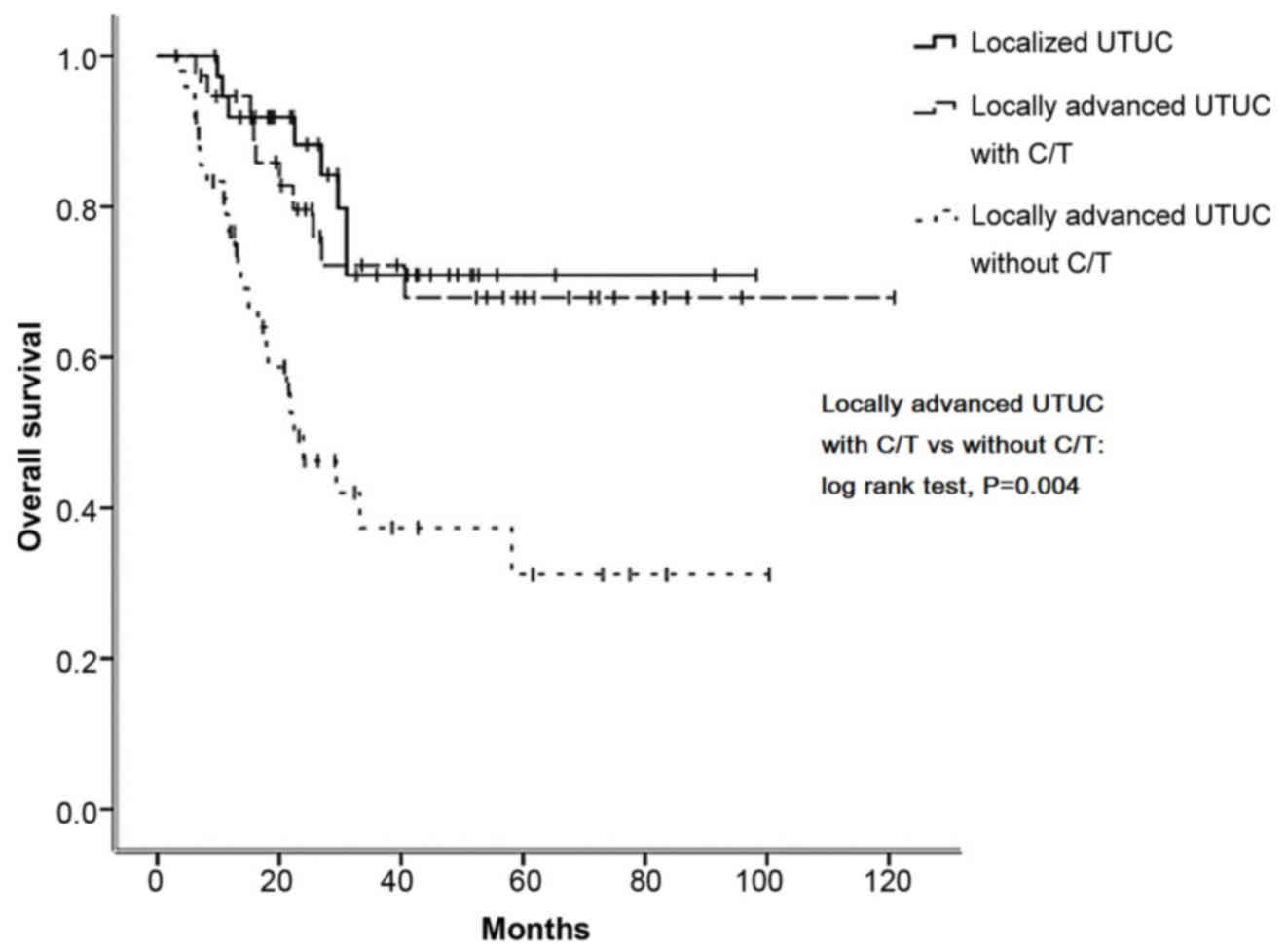
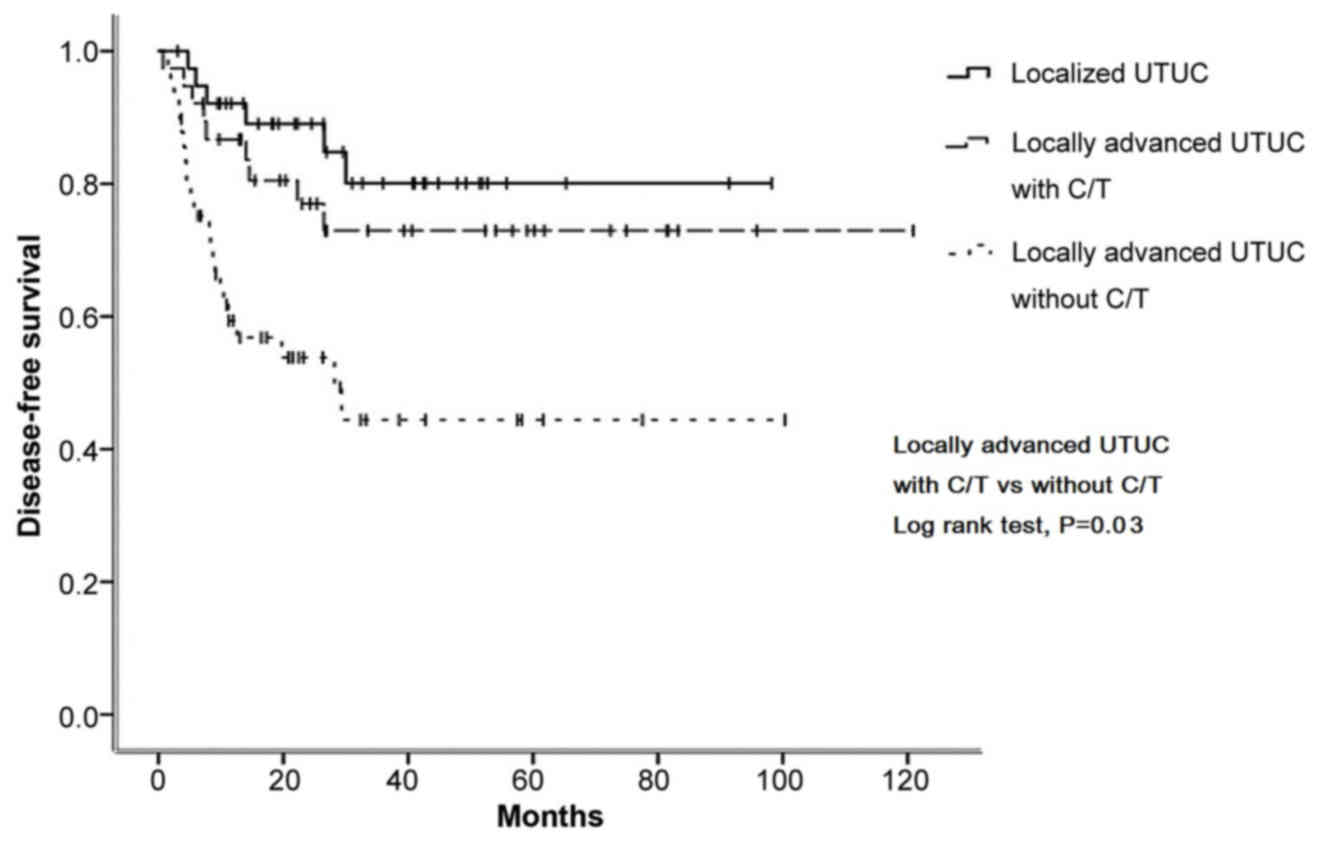
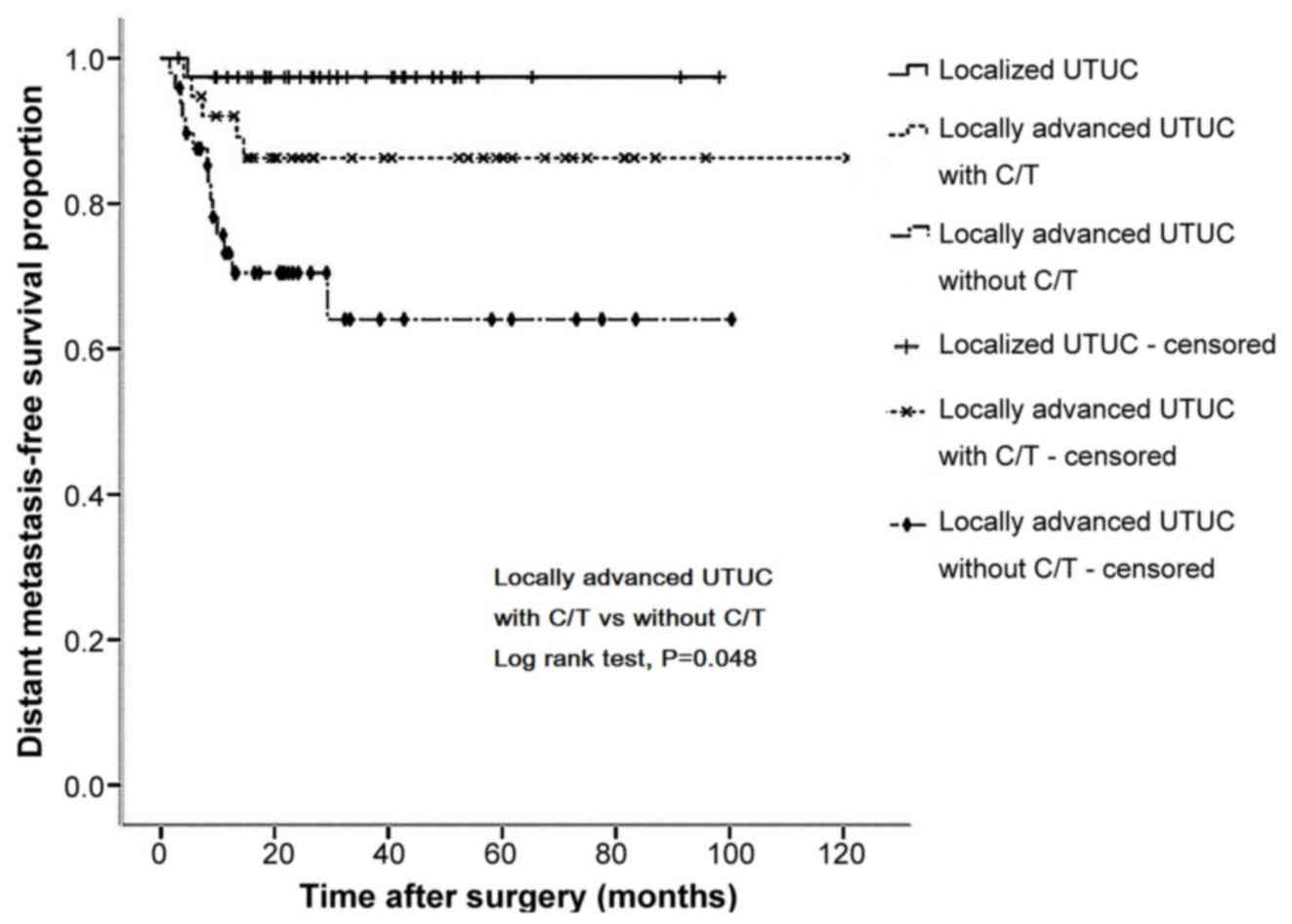
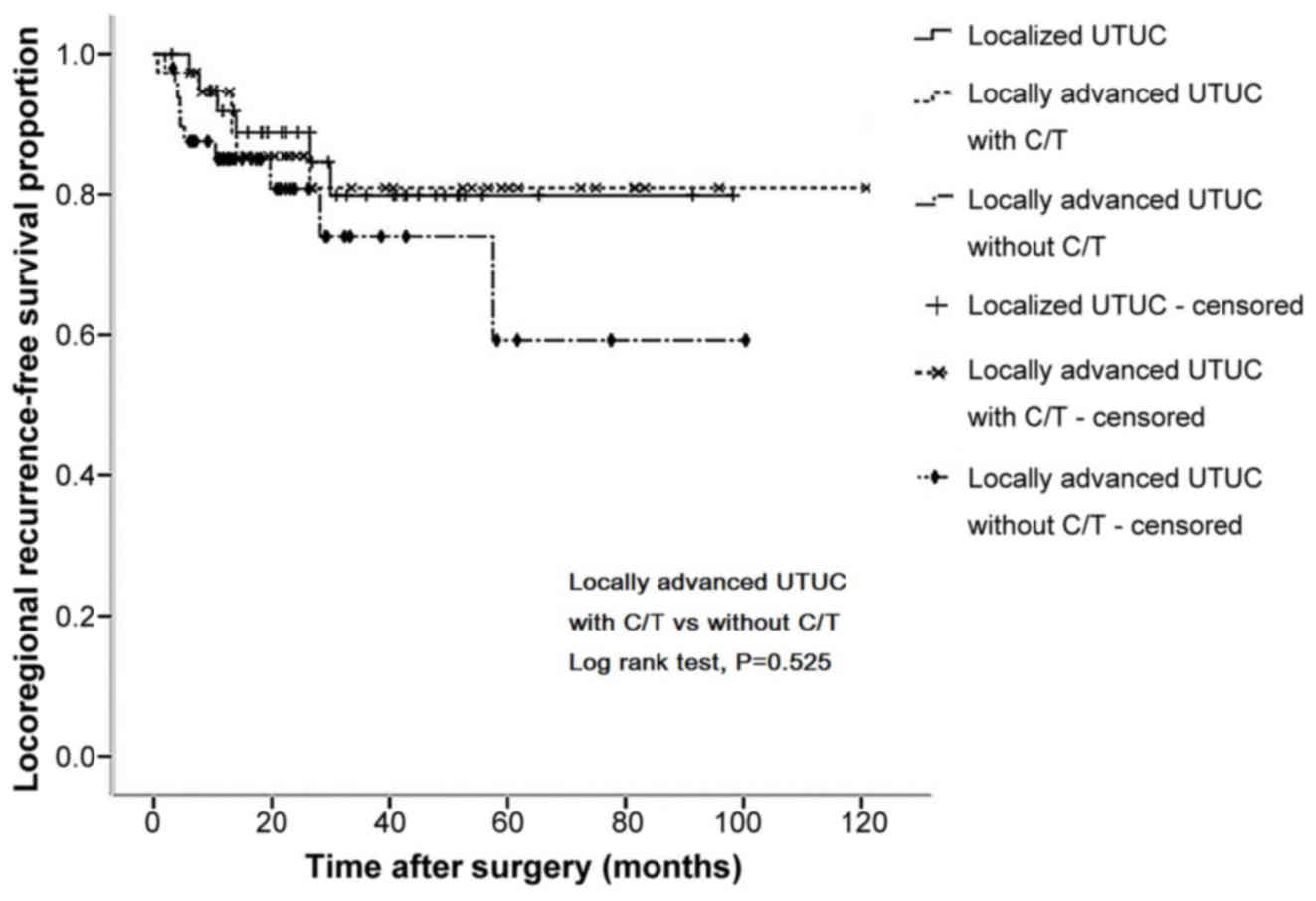
Kaplan-Meier curve analysis revealed that, in
locally advanced patients, those who received adjuvant chemotherapy
had significantly better 5-year OS (67.1 vs. 33.7%, P=0.004;
Fig. 1), DFS (70.2 vs. 46.0%,
P=0.030; Fig. 2) and DMFS (86.3 vs.
65.2%, P=0.048; Fig. 3) rates
compared with those who did not receive adjuvant chemotherapy.
However, there was no significant difference in 5-year LRFS rates
(78.2 vs. 62.5%, P=0.525; Fig. 4).
Notably, the survival curves of the locally advanced group who
received adjuvant chemotherapy were similar to those of the
localized group who underwent surgery only (Figs. 1–4).
Log-rank analysis was performed for OS and DFS.
Administration of adjuvant chemotherapy (without vs. with) was a
significant independent risk factor for OS [hazard ratio (HR),
0.291; 95% CI, 0.129–0.654; P=0.003] and DFS (HR, 0.381; 95% CI,
0.168–0.865; P=0.021). Additionally, tumor stage (III vs. IV) was
an independent predictor of OS and DFS, while lymphovascular
invasion (absent vs. present) predicted OS only (HR, 2.248; 95% CI,
1.124–4.497; P=0.022) (Table
IV).
 | Table IV.Multivariate analysis for overall
survival and disease-free survival in patients in the locally
advanced group. |
Table IV.
Multivariate analysis for overall
survival and disease-free survival in patients in the locally
advanced group.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Adjuvant
chemotherapy: Without vs. with | 0.291
(0.129–0.654) | 0.003 | 0.381
(0.168–0.865) | 0.021 |
| Age: <70 vs.
>70 years | 2.042
(0.987–4.224) | 0.054 | 1.750
(0.841–3.645) | 0.135 |
| Stage: III vs.
IV | 4.286
(2.006–9.159) | <0.001 | 3.928
(1.755–8.794) | 0.001 |
| Lymphovascular
invasion: Without vs. with | 2.248
(1.124–4.497) | 0.022 | 1.235
(0.603–2.530) | 0.564 |
| eGFR: <60 vs.
>60 ml/min | 1.072
(0.464–2.473) | 0.871 | 0.816
(0.346–1.928) | 0.644 |
Discussion
In the present study, 65.9% of the patients with
UTUC were female. Although the majority of UTUC cases occur in men,
the high prevalence of UTUC in Taiwan is notably impacted by the
high use of Aristolochia fangchi by women for weight-loss
and immune modulation to become healthy, which results in an
increased female incidence in Taiwan compared with men (22). The present study aimed to determine
whether adjuvant chemotherapy is beneficial for patients with
high-stage UTUC. It was revealed that patients with localized UTUC
had good survival outcomes without adjuvant chemotherapy compared
with patients with locally advanced UTUC. The results of the
present study demonstrated that adjuvant chemotherapy following
nephroureterectomy may improve the survival of patients with
locally advanced high-stage UTUC. In particular, the significant
improvement in DMFS implies that the eradication of micrometastasis
by systemic chemotherapy is feasible, but warrants further
research.
A carboplatin-based regimen was selected for
adjuvant chemotherapy, as carboplatin had been demonstrated to
provide an equivalent effect and improved tolerability compared
with cisplatin in numerous other types of cancer (23). However, it has not been well studied
whether carboplatin is an adequate alternative for patients who are
considered fit for the administration of cisplatin. Only one phase
III study compared a cisplatin-based regimen (methotrexate,
vinblastine, doxorubicin and cisplatin) to carboplatin/paclitaxel
in patients with metastatic urothelial cancer, which revealed no
significant differences in the response and OS rates of patients
(24); however, the study was
terminated early, meaning no definitive conclusions could be drawn
due to the slow accrual rate (the accrual rate was 31 cases per
year). A previous meta-analysis (25)
demonstrated an apparent lack of efficacy of non-cisplatin regimens
with regard to OS, DFS and disease-specific survival in patients
with UTUC, whereas it demonstrated improved OS and DFS rates for
patients treated with cisplatin-based regimens. The single
prospective cohort (n=36) of patients treated with a
non-cisplatin-based regimen in the meta-analysis indicated that
carboplatin-based chemotherapy is feasible and may reduce the risk
of distant metastasis. Another retrospective analysis enrolled
patient groups with unbalanced characteristics between the adjuvant
chemotherapy and patients who had not received adjuvant
chemotherapy, and used non-uniform treatment regimens
(cisplatin-based and non-cisplatin-based regimens were all
included) (16). Therefore, the
efficacy of carboplatin in the treatment of upper tract urothelial
cancer remains unclear (16). In the
present study, a carboplatin-based regimen was selected due to the
fact that a majority of patients with upper UC will have declining
renal function and decreased tolerability for chemotherapy
following surgery, which may impair the achievement of an adequate
dose-intensity of cisplatin-based chemotherapy to successfully
eradicate micrometastasis. The majority of the patients enrolled in
the present study were able to complete >4 cycles of full-dose
chemotherapy, which may explain the markedly decreased rate of
distant metastasis compared with those who cannot tolerate
chemotherapy (26–28).
Owing to the relatively low frequency of UTUC, thus
far, no randomized trial has been conducted to evaluate the impact
of chemotherapy on patients with UTUC. However, a number of
retrospective studies have been published (Table V). In 2011, Vassilakopoulou et
al (26) conducted the largest
retrospective study, which included 140 patients with UTUC who had
received diverse adjuvant chemotherapy regimens based on what they
were predicted to be able to tolerate according to their renal
function. Univariate and multivariate analyses revealed that
adjuvant chemotherapy significantly improved metastasis-free
survival. In a previous retrospective study (26), overall survival was not improved
following adjuvant chemotherapy as nearly 25% of all patients had
metastatic disease, and 15% of the patients had positive surgical
margins. These patients, who had potential metastasis and received
chemotherapy following surgery, may consider a palliative
chemotherapy, inhibiting the cancer progression and easing symptoms
without, however, the possibility of a cure. As the previous
investigation was not conducted in a completely ‘adjuvant’ setting,
the ability of chemotherapy to eradicate micrometastasis was
underestimated. This may have additionally resulted in an
underestimation of the OS benefit. In addition, considering the
diversity of chemotherapy regimens, including single-agent
chemotherapy and administration of fluorouracil, which has weak
cytotoxicity for transitional cell carcinoma, a number of patients
in the adjuvant group may have been in a relatively poor condition
and therefore not apt candidates for adjuvant chemotherapy.
 | Table V.Outcomes of adjuvant chemotherapy for
patients with locally advanced UTUC. |
Table V.
Outcomes of adjuvant chemotherapy for
patients with locally advanced UTUC.
|
| Number of
patients |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | With adjuvant
chemotherapy | Without adjuvant
chemotherapy | UTUC stage | Chemotherapy
regimen (%) | Survival outcomes
(with vs. without chemotherapy) | (Refs.) |
|---|
| Vassilakopoulou
et al, 2011 | 140 | 487 | T3N0, T4N0 and/or
N+ and/or M+ | Cisplatin-based
(52.8%); carboplatin-based (39.4%) | HR (95% CI) for
cancer-specific survival: 4.63 (0.16–128.69) | (26) |
| Hellenthal et
al, 2009 | 121 | 421 | T3N0M0, T4N0M0
and/or N+ | Cisplatin-based
(89%); methotrexate + vinblastine + doxorubicin + cisplatin
(59%) | HR (95% CI) for OS:
1.06 (0.80–1.40) | (27) |
| Kwak et al,
2006 | 32 | 11 | T2N0M0 and
T3N0M0 |
Cisplatin-based | 5-year OS rate:
78.1 vs. 36.4% | (16) |
| Soga et al,
2008 | 24 | 22 | T2N0M0 and
T3N0M0 | Methotrexate +
vinblastine + doxorubicin + cisplatin | 5-year OS rate:
95.8 vs. 86.5% | (28) |
| Chang et al
(the present study) | 38 | 50 | T3N0, T4N0 and/or
N+ | Gemcitabine +
carboplatin; or carboplatin + methotrexate + vinblastine | 5-year OS rate:
67.1 vs. 33.7% | – |
In 2009, Hellenthal et al (27) compared 121 patients with UTUC who
received adjuvant chemotherapy with 421 patients who did not
receive adjuvant chemotherapy, and revealed that chemotherapy did
not result in any significant differences in OS. Platinum-based
chemotherapy was used in 97% of patients and primarily comprised
MVAC. Furthermore, there were a number of notable limitations: Data
on the surgical margin and cycles of adjuvant chemotherapy were
unavailable and data were incomplete in ~10% of the cases (27). In 2009, Soga et al (28
conducted a case-control study including patients with good renal
function and of a young age (<80 years old) in order to avoid
severe adverse side effects induced by MVAC; a majority of patients
had received >2 cycles of chemotherapy. The authors reported
decreased bladder cancer recurrence and improved survival outcomes
in patients treated with adjuvant chemotherapy (28). In 2006, Kwak et al (16) demonstrated the therapeutic benefit of
adjuvant chemotherapy in a cohort of 43 patients with invasive but
non-metastatic UTUC. In this previous study, all patients received
>4 cycles of cisplatin-based chemotherapy.
In contrast to the studies of adjuvant chemotherapy
by Vassilakopoulou et al, Kwak et al, and Soga et
al (16,26,28), in
the present study, locally advanced, lymph node-positive and
non-metastatic patients with UTUC were enrolled. In our locally
advanced group, the 5-year OS rate of patients who did not receive
adjuvant chemotherapy was 33.7%, which was similar to that reported
by Hellenthal et al (27).
Taking into consideration the renal toxicity of cisplatin, the
advanced age of the patients, and the comorbidities following
nephroureterectomy, a carboplatin-based combination chemotherapy
was used, and the majority of the patients in the cohort of the
present study completed >4 cycles of chemotherapy. Although
there is a lack of studies comparing cisplatin and carboplatin in
patients with UTUC, a number of studies on transitional cell
carcinoma of the bladder have revealed that carboplatin-based
chemotherapy yields pathological and survival outcomes similar to
those of cisplatin-based combination chemotherapy (29,30).
Therefore, consistent with the findings of Soga et al and
Kwak et al (16,28), either by administering less toxic
regimens (as was performed in the present study) or by selecting
patients with a greater capacity to tolerate chemotherapy [as was
performed by Soga et al (28)], it is possible to achieve optimal
dosing of adjuvant chemotherapy in patients with locally advanced
UTUC and improve survival outcomes.
In the present study, distant metastasis in patients
with locally advanced UTUC was the most probable cause of
mortality. The implementation of adjuvant chemotherapy
significantly improved DMFS but not LRFS rates, resulting in
improved OS rates in patients with locally advanced UTUC. Similar
results were observed in the study by Vassilakopoulou et al
(26). Locoregional recurrence may
usually be treated by surgical intervention; however, if distant
metastasis occurs, curative treatment is not possible.
There were a number of limitations to the present
study. First, this was a retrospective study, and therefore it was
not possible to determine the rationale behind administering or not
administering chemotherapy to these patients. Second, the sample
size was relatively small, and the treatments were not uniform.
Third, a number of patient demographics, including performance
status and comorbidities, were not recorded in the present study,
and thus there may have been a selection bias in the study cohort.
Therefore, the results of the present study should be considered as
hypothesis-forming.
In summary, survival rates were higher in patients
with localized UTUC compared with locally advanced patients who did
not receive adjuvant chemotherapy. The present study supports the
hypothesis that adjuvant chemotherapy may improve outcomes in
patients with locally advanced UTUC. Further large-scale,
prospective, randomized studies are required in order to verify the
results of the present study and determine the precise
effectiveness of adjuvant chemotherapy in patients with UTUC.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
David KA, Mallin K, Milowsky MI, Ritchey
J, Carroll PR and Nanus DM: Surveillance of urothelial carcinoma:
Stage and grade migration, 1993–2005 and survival trends,
1993–2000. Cancer. 115:1435–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang MH, Chen KK, Yen CC, Wang WS, Chang
YH, Huang WJ, Fan FS, Chiou TJ, Liu JH and Chen PM: Unusually high
incidence of upper urinary tract urothelial carcinoma in Taiwan.
Urology. 59:681–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stiborová M, Frei E, Breuer A, Bieler CA
and Schmeiser HH: Aristolactam I a metabolite of aristolochic acid
I upon activation forms an adduct found in DNA of patients with
chinese herbs nephropathy. Exp Toxicol Pathol. 51:421–427. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arlt VM, Stiborova M and Schmeiser HH:
Aristolochic acid as a probable human cancer hazard in herbal
remedies: A review. Mutagenesis. 17:265–277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng CX: BRCA1: Cell cycle checkpoint,
genetic instability, DNA damage response and cancer evolution.
Nucleic Acids Res. 34:1416–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cosyns JP, Jadoul M, Squifflet JP, Wese FX
and van Ypersele de Strihou C: Urothelial lesions in Chinese-herb
nephropathy. Am J Kidney Dis. 33:1011–1017. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG:
Upper Tract Urothelial Carcinoma Collaboration The Upper Tract
Urothelial Carcinoma Collaboration: Outcomes of radical
nephroureterectomy: A series from the Upper Tract Urothelial
Carcinoma Collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abouassaly R, Alibhai SM, Shah N,
Timilshina N, Fleshner N and Finelli A: Troubling outcomes from
population-level analysis of surgery for upper tract urothelial
carcinoma. Urology. 76:895–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Audenet F, Yates DR, Cussenot O and
Rouprêt M: The role of chemotherapy in the treatment of urothelial
cell carcinoma of the upper urinary tract (UUT-UCC). Urol Oncol.
31:407–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Geller N, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N,
et al: Methotrexate, vinblastine, doxorubicin, and cisplatin for
advanced transitional cell carcinoma of the urothelium. Efficacy
and patterns of response and relapse. Cancer. 64:2448–2458. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freiha F, Reese J and Torti FM: A
randomized trial of radical cystectomy versus radical cystectomy
plus cisplatin, vinblastine and methotrexate chemotherapy for
muscle invasive bladder cancer. J Urol. 155:495–500. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koppie TM, Shariat SF, Nelson EC, Margulis
V, Remzi M, Montorsi F, Raman JD, Ziguener R, Suardi N, Weizer AZ,
et al: Adjuvant chemotherapy for upper tract transitional cell
carcinoma: Results from the Upper Tract UCC Consortium. J Urol.
179:1192008. View Article : Google Scholar
|
|
16
|
Kwak C, Lee SE, Jeong IG and Ku JH:
Adjuvant systemic chemotherapy in the treatment of patients with
invasive transitional cell carcinoma of the upper urinary tract.
Urology. 68:53–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kondo T, Nakazawa H, Ito F, Hashimoto Y,
Toma H and Tanabe K: Primary site and incidence of lymph node
metastases in urothelial carcinoma of upper urinary tract. Urology.
69:265–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobin LH, Gospodarowicz MK and Wittekind
C: Renal pelvis and ureter. TNM Online. 258–261. 2010.https://doi.org/10.1002/9780471420194.tnmc43.pub2
View Article : Google Scholar
|
|
19
|
Lopez-Beltran A, Bassi P, Pavone-Macaluso
M and Montironi R: Handling and pathology reporting of specimens
with carcinoma of the urinary bladder, ureter, and renal pelvis.
Eur Urol. 45:257–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito K, Kawakami S, Fujii Y, Sakura M,
Masuda H and Kihara K: Lymphovascular invasion is independently
associated with poor prognosis in patients with localized upper
urinary tract urothelial carcinoma treated surgically. J Urol.
178:2291–2296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Kidney Foundation: K/DOQI
clinical practice guidelines for chronic kidney disease:
Evaluation, classification, and stratification. Am J Kidney Dis. 39
2 Suppl 1:S1–S266. 2002.PubMed/NCBI
|
|
22
|
Hsiao PJ, Hsieh PF, Chang CH, Wu HC, Yang
CR and Huang CP: Higher risk of urothelial carcinoma in the upper
urinary tract than in the urinary bladder in hemodialysis patients.
Ren Fail. 38:663–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: Comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dreicer R, Manola J, Roth BJ, See WA,
Kuross S, Edelman MJ, Hudes GR and Wilding G: Phase III trial of
methotrexate, vinblastine, doxorubicin, and cisplatin versus
carboplatin and paclitaxel in patients with advanced carcinoma of
the urothelium. Cancer. 100:1639–1645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leow JJ, Martin-Doyle W, Fay AP, Choueiri
TK, Chang SL and Bellmunt J: A systematic review and meta-analysis
of adjuvant and neoadjuvant chemotherapy for upper tract urothelial
carcinoma. Eur Urol. 66:529–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vassilakopoulou M, de la Motte Rouge T,
Colin P, Ouzzane A, Khayat D, Dimopoulos MA, Papadimitriou CA,
Bamias A, Pignot G, Nouhaud FX, et al: Outcomes after adjuvant
chemotherapy in the treatment of high-risk urothelial carcinoma of
the upper urinary tract (UUT-UC): Results from a large multicenter
collaborative study. Cancer. 117:5500–5508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hellenthal NJ, Shariat SF, Margulis V,
Karakiewicz PI, Roscigno M, Bolenz C, Remzi M, Weizer A, Zigeuner
R, Bensalah K, et al: Adjuvant chemotherapy for high risk upper
tract urothelial carcinoma: Results from the Upper Tract Urothelial
Carcinoma Collaboration. J Urol. 182:900–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soga N, Arima K and Sugimura Y: Adjuvant
methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy
has potential to prevent recurrence of bladder tumors after
surgical removal of upper urinary tract transitional cell
carcinoma. Int J Urol. 15:800–803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koie T, Ohyama C, Hashimoto Y, Hatakeyama
S, Yamamoto H, Yoneyama T and Kamimura N: Efficacies and safety of
neoadjuvant gemcitabine plus carboplatin followed by immediate
cystectomy in patients with muscle-invasive bladder cancer,
including those unfit for cisplatin: A prospective single-arm
study. Int J Clin Oncol. 18:724–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mertens LS, Meijer RP, Kerst JM, Bergman
AM, van Tinteren H, van Rhijn BW and Horenblas S: Carboplatin based
induction chemotherapy for nonorgan confined bladder cancer-a
reasonable alternative for cisplatin unfit patients? J Urol.
188:1108–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|