Introduction
Despite improvements in diagnostic technologies and
breakthroughs in effective treatment in recent years, breast cancer
remains the leading cause of cancer-related deaths in females
worldwide, and the incidence and mortality rate generally increase
with age (1). Triple-negative breast
cancer (TNBC) is a sub-group of breast cancer that is characterized
by the lack of estrogen receptor (ER), progesterone receptor (PR)
and human epidermal growth factor receptor 2 (HER2) expressions.
TNBC accounts for approximately 15–20% of breast cancer cases and
is associated with a poorer overall survival compared with other
types of breast cancer (2). TNBC is
the most aggressive breast cancer sub-type with a high possibility
of metastasis (3). Moreover, due to
the lack of ER, PR and HER2, TNBC is unresponsive to any hormonal
treatment and there is currently no available targeted therapy,
thereby increasing the chance of relapse, worsening prognosis and
making TNBC difficult to treat (4,5).
Therefore, there is an urgent need to identify novel therapeutic
targets for TNBC, and our study focused only on TNBC.
TNBC patients are commonly treated with chemotherapy
drugs, such as Taxol. Taxol is able to polymerize tubulin, promote
the assembly/stabilization of microtubules, disrupt normal
microtubule dynamics and arrest cells in mitosis. Cancer cells in
TNBC should be killed by Taxol, but they maintain their viability
via cellular responses, thereby promoting malignance (6). TNBC frequently recurs and metastasizes
due to the acquirement of resistance to Taxol (7), which is one of the major obstacles to
effective treatment.
MiRNAs are small, non-coding, endogenous RNAs, 22–25
nucleotides in length, which regulate gene expression (8). Mounting evidence has indicated that
miRNAs act as oncogenes or tumor suppressor genes in different
types of cancer, including TNBC (9),
indicating their potential as therapeutic targets (10). MiR-1207 was identified to be
upregulated in younger breast cancer patients, and related to cell
motility, invasion and proliferation (11). MiR-1207 overexpression was also found
to promote cancer stem cell-like traits in ovarian cancer (12). In colorectal cancer, significant
overexpression of miR-1207 was detected (13). These findings demonstrate the
participation of miR-1207 in cancer progression. Consequently, the
function of miR-1207 in the sensitivity of TNBC to Taxol was
investigated in the present study.
In summary, we observed a high level of miR-1207-5p
in MDA-MB-231 cells. Treatment with antagomiR-1207-5p enhanced the
cell growth arrest and cell apoptosis induced by Taxol in
MDA-MB-231 cells, by regulating and increasing the protein level of
LZTS1. Combined treatment with Taxol and antagomiR-1207-5p induced
a sharp decrease in Bcl-2 and p-Akt expression, and an increase in
the Bax protein expression level. A notable elevation in the
expression of miR-1207-5p and a reduction in the expression of
LZTS1 were identified in TNBC tissues but not adjacent tissues and
Taxol non-responsive TNBC tissues but not responsive TNBC tissues.
Our study showed the novelty of the interaction of miR-1207-5p
regulating the LZTS1 gene expression and therefore, its role in
taxol sensitivity. Our data suggest that miR-1207-5p may be a
promising predictor of sensitivity towards Taxol in TNBC.
Materials and methods
Cell culture
Human normal breast epithelial MCF-10A cells, TNBC
cell lines (MDA-MB-231, MDA-MB-436 and MDA-MB-453) and 293 cells
were purchased from ATCC, authenticated by cytogenetic analysis and
were used within 6 months or stored in liquid nitrogen. Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) in an incubator at 37°C with 5% CO2.
Transient transfection
For cell transfection, MDA-MB-231 cells were seeded
onto 6-well plates at a density of 2×105 cells/well. In
brief, antagomiR-1207-5p (5′-CCCCUCCCAGCCUCCCUGCCA-3′, 100 nM;
Guangzhou RiboBio Co., Ltd., Guangzhou, China), an antisense-based
specific inhibitor against miR-1207-5p which was applied for
specific silencing of endogenous miR-1207-5p, or antagomiR-NC
(5′-UUCUCCGAACGUGUCACGU-3′, 100 nM; Guangzhou RiboBio Co., Ltd.)
was diluted in DMEM with Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The mixture was added into the 6-well
plates to obtain a final concentration of 20 nmol/L and incubated
for 48 h before the subsequent experiments.
Taxol treatment
Taxol (Selleck Chemicals, Shanghai, China) was
stored in DMSO as a 10 µmol/L stock solution. In brief, prior to
treatment with 10 nmol/L Taxol, 1×106 MDA-MB-231 cells
were first seeded onto 60-mm culture dishes and were cultured for
24 h. After incubation with Taxol for 3 days, fresh 10 nmol/L
Taxol-containing DMEM was added and incubated for another 2 days.
Finally, MDA-MB-231 cells were washed with PBS and were cultured in
drug-free DMEM. The medium was replaced every 2 days until the
commencement of the subsequent experiments.
Bioinformatics analysis
Bioinformatics analysis was performed using the
online software program TargetScan 6.2 (www.targetscan.org/).
Tissue specimens
Tissue specimens (tumor tissues and non-cancerous
tissues) were obtained from 30 TNBC patients who had undergone
surgery at Linfen People's Hospital. Non-cancerous tissues were at
least 2-cm distal to tumor margins. The definition of response to
Taxol-based chemotherapy in TNBC patients was based on the RECIST
criteria (14). Patients were
categorized into two groups: The Taxol responsive group and the
Taxol non-responsive group. Patients in the Taxol responsive group
achieved a complete response (CR), while patients in the Taxol
non-responsive group exhibited persistent disease (PR, SD and PD).
There were 18 TNBC patients in the Taxol responsive group and 12
patients in the Taxol non-responsive group. Written informed
consent was obtained from each patient. The study was conducted in
accordance with Declaration of Helsinki and was approved by the
Institutional Review Board of Linfen People's Hospital, China.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from TNBC tissues and TNBC
cells using TRIzol (Tiangen Biotech Co., Ltd., Beijing, China).
Briefly, RNA was reverse transcribed into cDNA using a Reverse
Transcription kit (Takara Biotechnology Co., Ltd., Dalian, China).
qPCR analyses were performed using Power SYBR Green (Takara
Biotechnology Co., Ltd.) in a 7500HT Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR data were
analyzed using the 2−ΔΔCq method. The thermocycling
conditions were as follows: 95°C for 5 min (pre-incubation), 30
cycles at 95°C for 30 sec (denaturation), 60°C for 1 min
(annealing), and 72°C for 30 sec (elongation). The expression of
GAPDH was used to standardize the amount of mRNA in each PCR tube.
U6 was used to standardize the amount of miRNA in each PCR tube.
Primers were as followed: LZTS1 forward,
5′-ACCTCTAGAAACCCAGAACTCA-3′ and reverse,
5′-TCCAGAAGAGCCCATATCACTA-3′; GAPDH forward,
5′-GCGCCCAATACGACCAA-3′ and reverse, 5′-CTCTCTGCTCCTCCTGTTC-3′;
miR-1207-5p forward, 5′-GCCAGATCTTGATTGACTTACAGCCCAGTT-3′ and
reverse, 5′-GCCGAATTCCACCTGTCTTTATTCCACCC-3′; U6 forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGT-3′.
Western blot analysis
Cell lysates were prepared using RIPA buffer (Roche,
Shanghai, China). Proteins were resolved by sodium dodecyl
sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) for the detection of proteins with primary
antibodies against AKT1/2/3 (1:500, sc-8312; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), p-AKT1/2/3 (Ser 473)-R
(1:500, sc-7985-R; Santa Cruz Biotechnology, Inc.), LZTS1 (1:1,000,
LBP62153; Novus Biologicals, LLC, Littleton, CO, USA), Bax
(1:1,000, 2772; Cell Signaling Technology, Inc., Danvers, MA, USA),
Bcl-2 (1:1,000, 3498 Cell Signaling Technology, Inc.) and GAPDH
(1:5,000, A5060; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
37°C overnight. Membranes were then incubated with goat anti-rabbit
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:5,000, ab97080; Abcam, Shanghai, China) at room temperature for
1 h. Protein bands were treated with an enhanced chemiluminescence
detection system (ECL; Bio-Rad Laboratories, Inc.), visualized with
the ChemiDoc XRS system (Bio-Rad Laboratories, Inc.) and analyzed
using Quality One 4.5.2 (Bio-Rad Laboratories, Inc.). Protein
expression levels were normalized to GAPDH.
Luciferase reporter assay
3′UTR of LZTS1 was amplified from cDNA of 293 cells.
Oligonucleotides that contained LZTS1 cDNA fragments, including
miR-1207-5p binding sites were amplified and cloned into pmirGLO
plasmids (Promega Corporation, Madison, WI, USA) to obtain
luciferase reporter plasmids, pmirGLO-LZTS1-WT. Mutant LZTS1
(pmirGLO-LZTS1-MUT) acted as a negative control and was obtained by
site-directed mutagenesis PCR with platinum pfx DNA polymerase,
according to the manufacturer's protocol. Cells (3×104)
were seeded onto 24-well plates and cultured for 24 h. Then,
pmirGLO-LZTS1-WT or pmirGLO-LZTS1-MUT and miR-1207-5p mimics
(5′-UGGCAGGGAGGCUGGGAGGGG-3′) or miR-NC mimics
(5′-UUCUCCGAACGUGUCACGU-3′) and 3 ng pRL-TK Renilla plasmid were
transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). At 48 h after transfection, a Dual
Luciferase Reporter Assay (Promega Corporation) was performed in a
luminometer, according to the manufacturer's protocol, to examine
the relative luciferase activity.
Flow cytometric analysis
Cell apoptosis was detected using an Annexin-V/Dead
Cell Apoptosis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. In brief, MDA-MB-231
cells were trypsinized and suspended in 1 × annexin binding buffer.
Subsequently, propidium iodide (PI) and Annexin V-FITC were added
to the cell suspension and cultured for 15 min. Stained cells were
analyzed using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
MTT assay
An MTT assay (Invitrogen; Thermo Fisher Scientific,
Inc.) was performed to evaluate the cell proliferation rate in each
group. In brief, MDA-MB-231 cells were washed with PBS,
trypsinized, and seeded onto 96-well plates. Then, MTT reagent (10
µl) was added and plates were incubated in an incubator at 37°C
with 5% CO2 until purple precipitate was observed.
Thereafter, 100 µl dimethylsulfoxide was added to dissolve the
formazan crystals and the plates were incubated at 37°C for 2 h in
the dark. Absorbance was read at 570 nm by a microplate reader.
Statistical analysis
Data were analyzed using SPSS version v.13 (SPPS,
Inc., Chicago, IL, USA) and are expressed as the mean ± standard
deviation. Comparisons between two groups were analyzed using
Student's t-test, and comparisons among multiple groups were
analyzed by one-way analysis of variance followed by
Student-Newman-Keuls test. The correlation between LZTS1 and
miR-1207-5pb was analyzed by Spearman's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated three times.
Results
miR-1207-5p expression was
significantly elevated in MDA-MB-231 cells
TNBC is the most aggressive breast cancer sub-type
with a high possibility of metastasis (3). Moreover, TNBC is unresponsive to any
hormonal treatment, making TNBC difficult to treat (4,5).
Therefore, there is an urgent need to identify novel therapeutic
targets for TNBC, our study focused only on TNBC, and we chose TNBC
MDA-MB-231, MDA-MB-436 and MDA-MB-453 cell lines for our study
instead of ER+, PR+/-, HER2- T47D or MCF7 cells despite of their
response to chemotherapy (15,16).
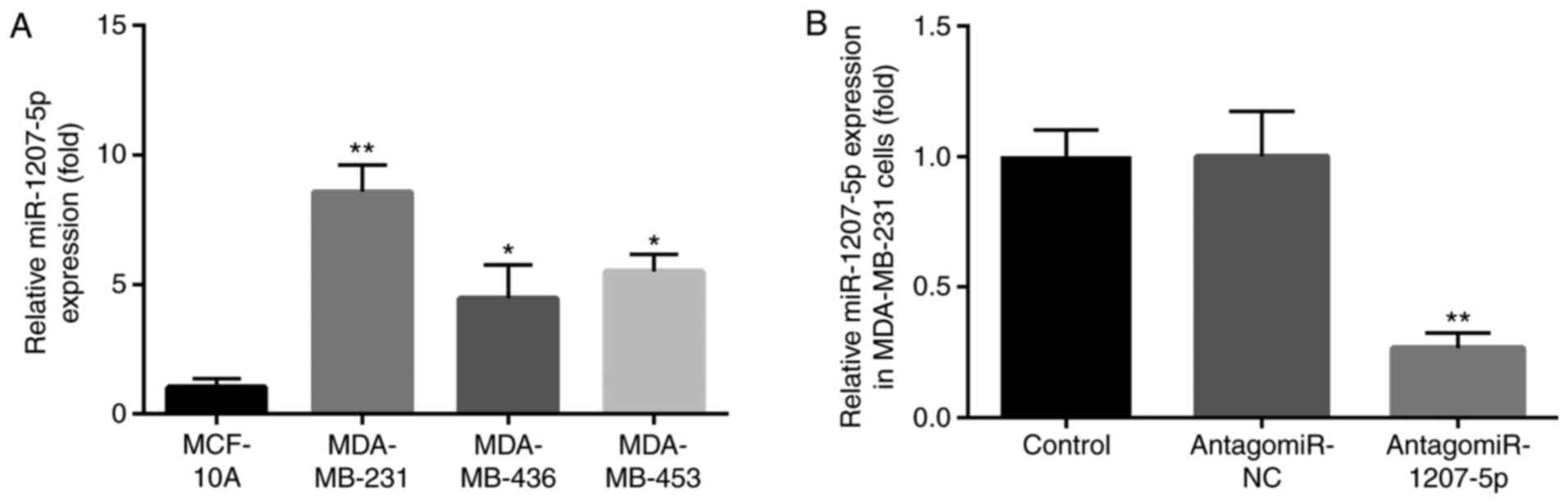
We evaluated the expression of miR-1207-5p in TNBC
MDA-MB-231, MDA-MB-436 and MDA-MB-453 cell line. Compared with
normal MCF-10A cells, miR-1207-5p expression was increased in
MDA-MB-436 and MDA-MB-453 cells (P<0.05). However, the greatest
increase in the miR-1207-5p expression level was in MDA-MB-231
cells (P<0.01; Fig. 1A).
Therefore, the MDA-MB-231 cells were selected for our research
model.
The effect of antagomiR-1207-5p on miR-1207-5p
expression was evaluated in MDA-MB-231 cells by RT-qPCR. There was
no significant difference in miR-1207-5p expression between the
control group and the antagomiR-NC group. However, the expression
level of miR-1207-5p was significantly decreased after treatment
with antagomiR-1207-5p, compared with expression in the
antagomiR-NC group (P<0.01; Fig.
1B).
Since miR-1207-5p was found to be up-regulated in
the MDA-MB-231 cells compared with expression in the MCF-10A cells,
this suggested an oncogenic role of miR-1207-5p in TNBC. Therefore,
using the online software programs TargetScan 6.2, we aimed to
identify target mRNAs of miR-1207-5p that functioned as tumor
suppressor factors during tumor progression.
LZTS1 was a direct target for
miR-1207-5p
LZTS1 was previously found to be decreased in
cutaneous squamous cell carcinoma (17) and osteosarcoma (18), and was identified to suppress
colorectal cancer proliferation (19). Moreover, LZTS1 reduction conferred
Taxol resistance and was associated with a poor prognosis in
patients with breast cancer (20).
Additionally, loss of LZTS1 contributed to the lymph node
metastasis of breast invasive micropapillary carcinoma (21). Notably, LZTS1 was predicted to be a
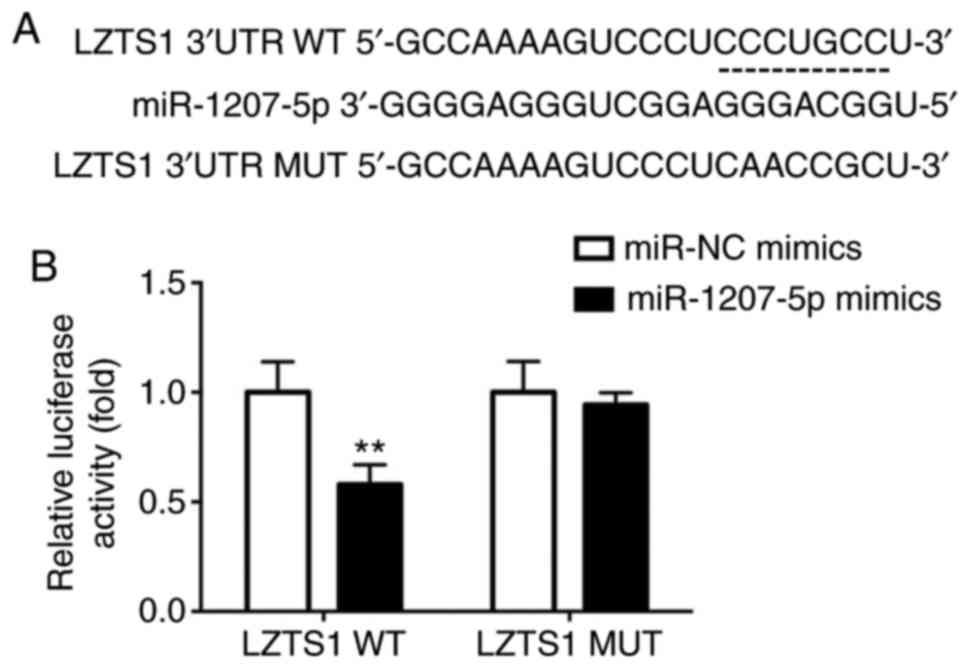
potential target for miR-1207-5p in our study, and the predicted
binding sites between LZTS1 and miR-1207-5p are presented in
Fig. 2A.
The interaction between LZTS1 and miR-1207-5p was
verified by luciferase reporter assay. There was a significant
reduction in luciferase activity in cells transfected with
pmirGLO-LZTS1-WT and miR-1207-5p mimics compared with activity in
cells transfected with pmirGLO-LZTS1-WT and miR-NC mimics
(P<0.01). Meanwhile, no significant difference was found between
cells transfected with pmirGLO-LZTS1-MUT and miR-NC mimics or
pmirGLO-LZTS1-MUT and miR-1207-5p mimics, as presented in Fig. 2B.
Based on these results, we inferred that miR-1207-5p
regulated LZTS1. However, antagomiR-1207-5p could not bind to
LZTS1, this luciferase reporter assay cannot demonstrate ‘direct
interaction’ of molecules, which was a limitation. The function of
miR-1207-5p and LZTS1 in the sensitivity of TNBC to Taxol has not
been studied previously. Therefore, the present study carried out
experiments to investigate this.
LZTS1 expression was repressed by
miR-1207-5p
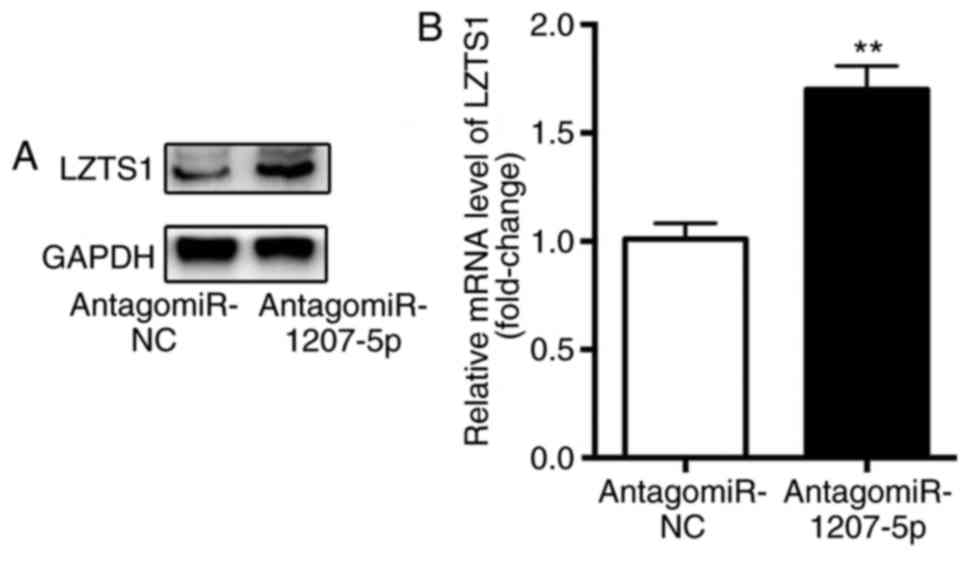
The influence of miR-1207-5p on the mRNA and protein
expression level of LZTS1 was evaluated by RT-qPCR and western
blotting, respectively. The results demonstrated that, compared
with cells in the antagomiR-NC group, there was a significantly
higher protein expression level (Fig.
3A) as well as mRNA level (P<0.01; Fig. 3B) of LZTS1 in cells transfected with
antagomiR-1207-5p. These results suggested that miR-1207-5p
inhibited the expression of LZTS1.
AntagomiR-1207-5p enhanced the
Taxol-induced reduction in cell proliferation and increase in cell
apoptosis
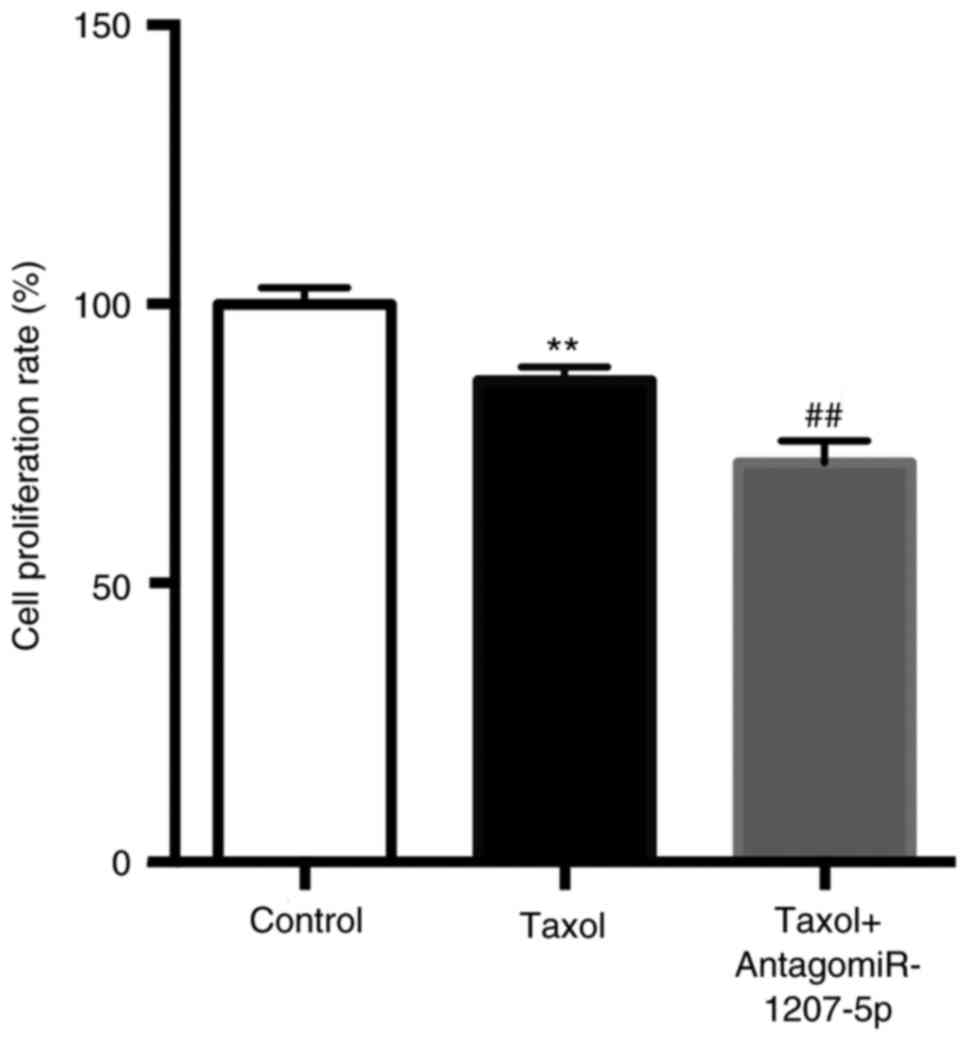
As presented in Fig.
4, compared with the control group, Taxol significantly reduced
the cell proliferation rate (P<0.01), which was further reduced
by the co-administration of Taxol and antagomiR-1207-5p
(P<0.01).
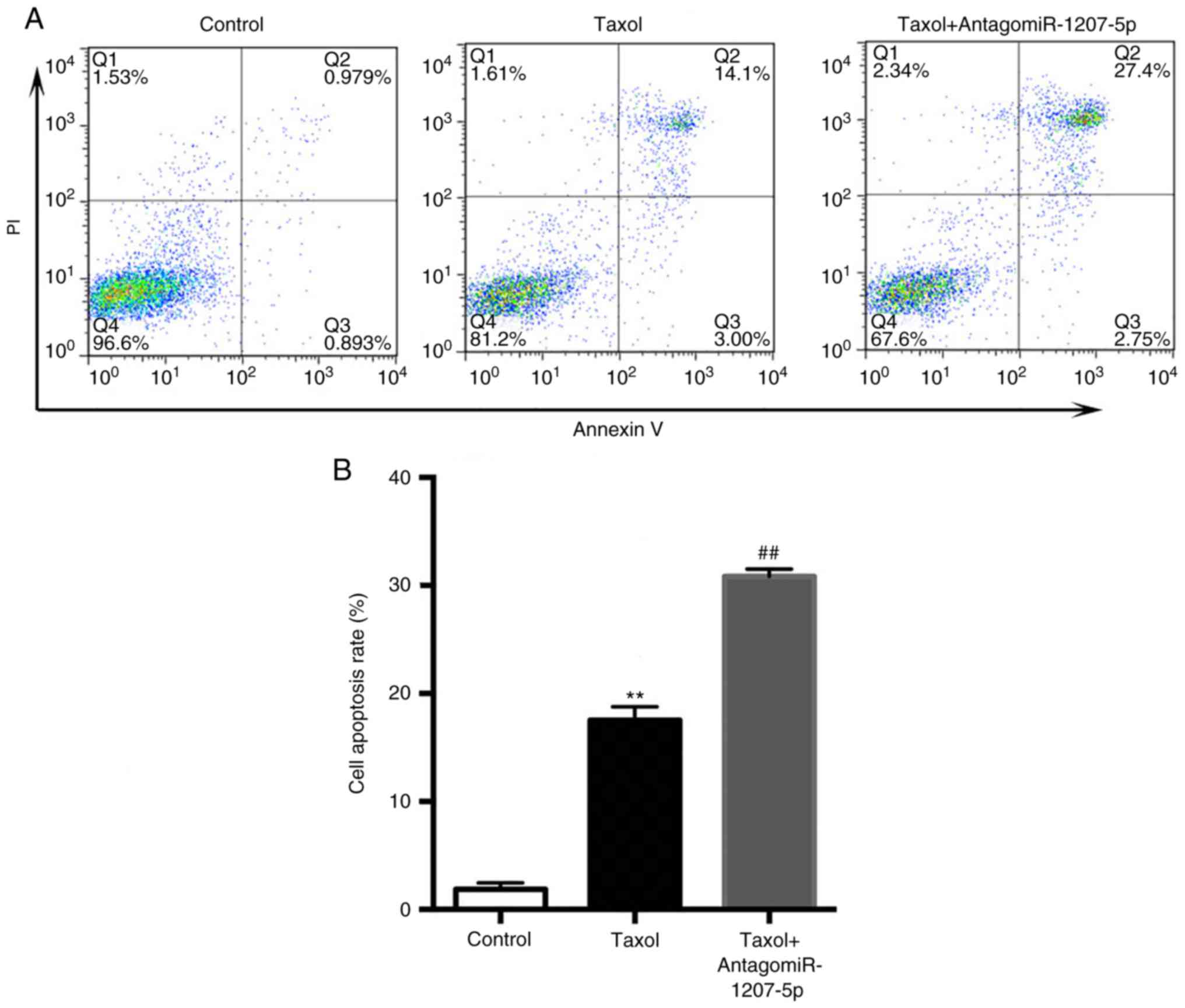
As presented in Fig. 5A
and B, compared with the control group, Taxol significantly
increased the cell apoptosis rate (P<0.01), which was further
increased by the co-administration of Taxol and antagomiR-1207-5p
(P<0.01).
These results regarding the effects of miR-1207-5p
on cancer cell proliferation and apoptosis were consistent with
those of a previous report (12).
AntagomiR-1207-5p enhanced
Taxol-induced Bax upregulation, and Bcl-2 and p-Akt
downregulation
According to the aforementioned findings, we
inferred that miR-1207-5p affected cell proliferation and apoptosis
after Taxol treatment. However, the molecules that were regulated
by miR-1207-5p were unknown. Therefore, we investigated pathways or
molecules that were associated with cell proliferation and
apoptosis. We found that the PI3K/Akt signaling pathway is
important in regulating cell proliferation, migration, apoptosis,
and angiogenesis (22,23); meanwhile, the Bcl-2 protein family
including Bax (pro-apoptosis) and Bcl-2 (anti-apoptosis), takes
part in the apoptotic process (24).
Therefore, we wanted to investigate whether expression of Bax,
Bcl-2 and p-Akt could be influenced by miR-1207-5p after
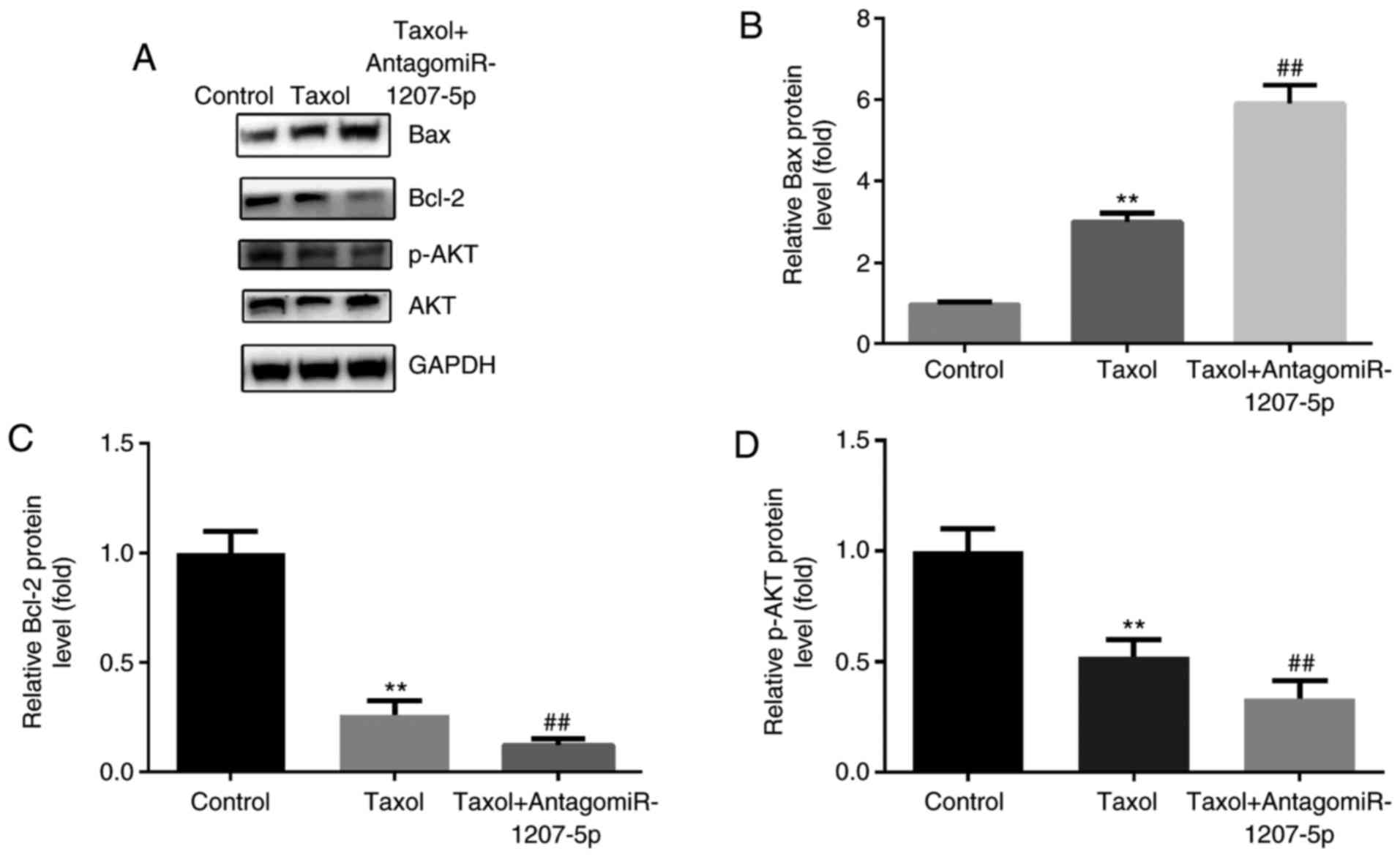
administration of Taxol. We detected the protein expression of
p-Akt, Bax and Bcl-2 after antagomiR-1207-5p treatment in
MDA-MB-231 cells. The results indicated that, compared with the
control group, Taxol induced notable up-regulation of Bax, and
downregulation of Bcl-2 and p-Akt (P<0.01), which was further
induced by the co-administration of antagomiR-1207-5p (P<0.01;
Fig. 6A-D).
These results suggested that miR-1207-5p functioned
through regulation of apoptosis-related pathways and molecules via
regulating the expression of LZTS1. The role of miR-1207-5p in
regulating the expression of LZTS1 was evidenced by the results as
follows: 1. Luciferase assay; 2. the elevated mRNA/protein levels
of LZTS1 after AntagomiR-1207-5p treatment.
miR-1207-5p was negatively correlated
with LZTS1 in TNBC patients
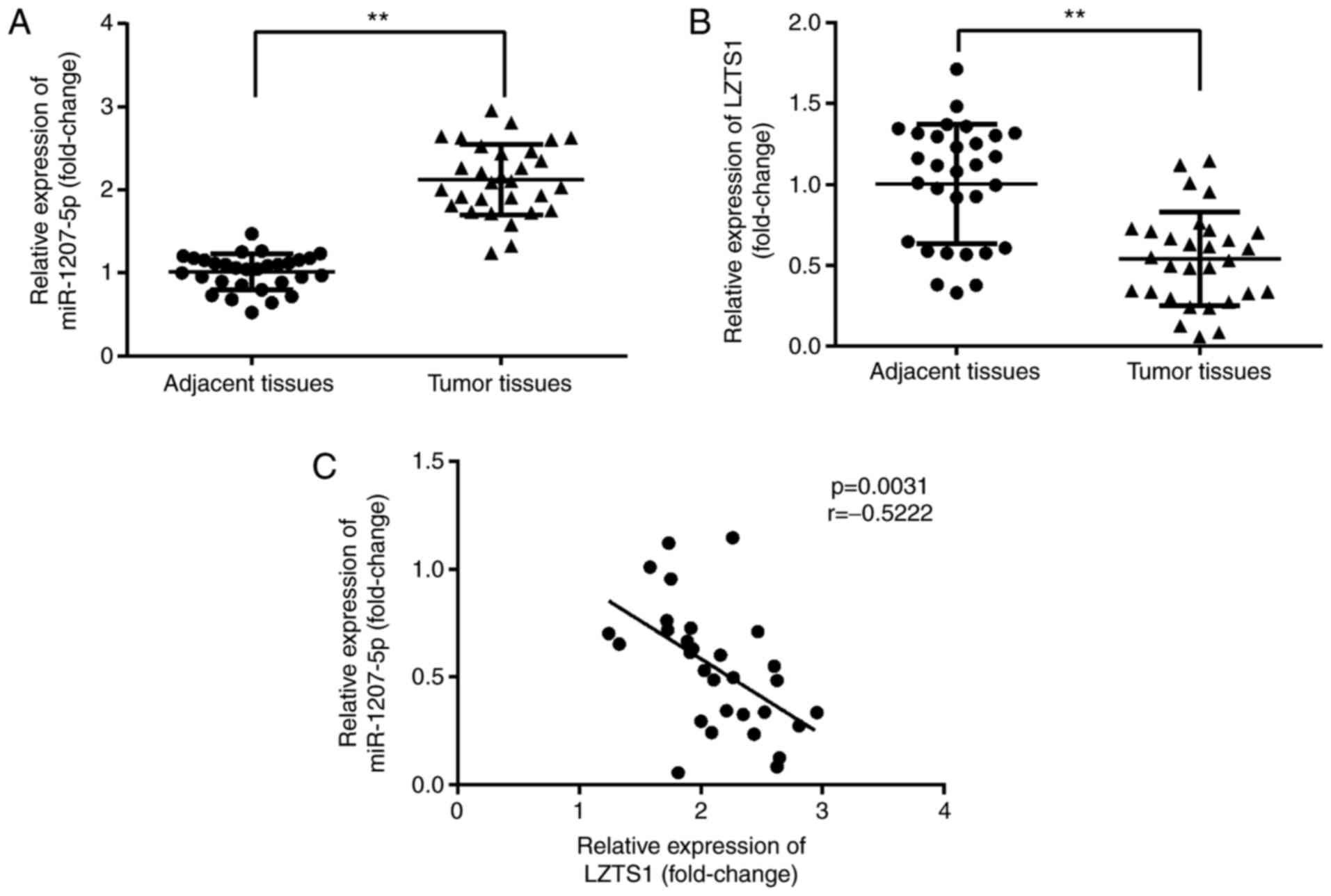
We investigated whether there were differences in
miR-1207-5p and LZTS1 expression levels between the adjacent normal
tissues and tumor tissues from TNBC patients using RT-qPCR. As
shown in Fig. 7A and B, miR-1207-5p
expression was significantly increased (P<0.01), while LZTS1
expression was significantly decreased (P<0.01) in tumor tissues
compared with normal adjacent tissues. Moreover, there was a
negative correlation between miR-1207-5p and LZTS1 expression
(P=0.0031, r=−0.5222; Fig. 7C).
Elevated miR-1207-5p and reduced LZTS1
in Taxol non-responsive TNBC tissues
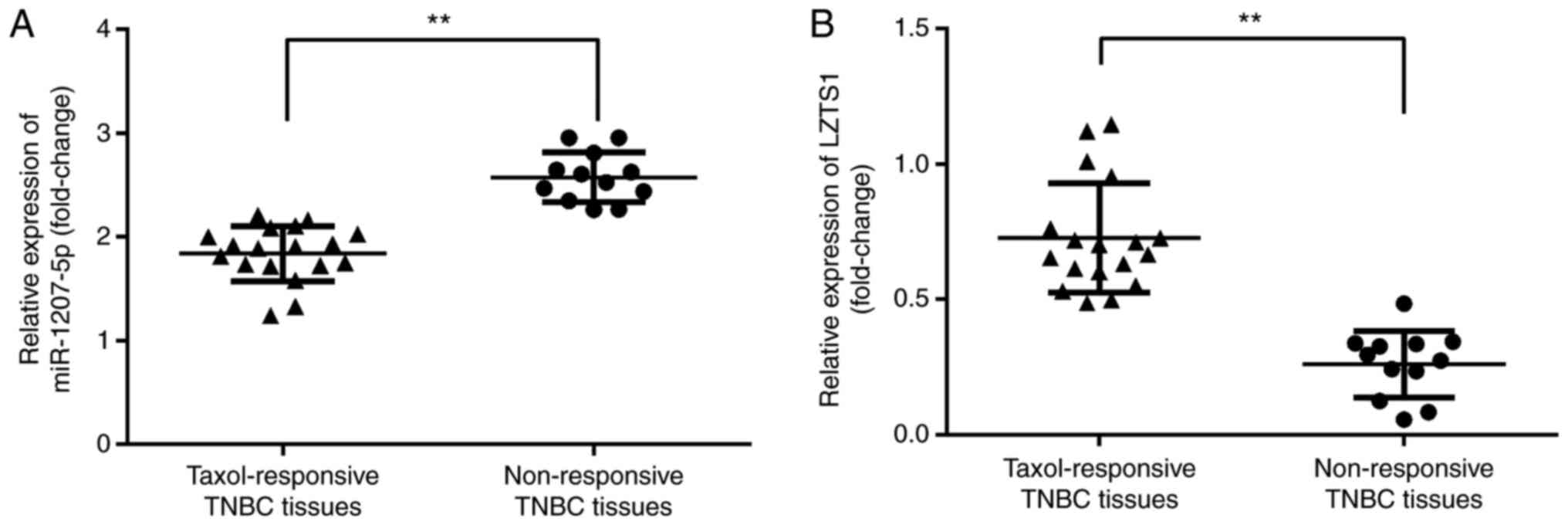
Among the 30 TNBC patients, we found that there were
18 TNBC patients who responded to Taxol and 12 patients who were
non-responsive to Taxol. Furthermore, we evaluated the expression
of miR-1207-5p and LZTS1 in Taxol-responsive and Taxol
non-responsive TNBC tissues. The results demonstrated that,
compared with Taxol-responsive TNBC tissues, there was a
significant elevation in the expression of miR-1207-5p (P<0.01;
Fig. 8A) and a significant reduction
in the expression of LZTS1 (P<0.01; Fig. 8B) in the Taxol non-responsive TNBC
tissues.
Discussion
Due to the lack of ER, PR and HER2 expression, TNBC
is unresponsive to any hormonal treatment (2,5). Although
there have been multiple effective treatment strategies developed
for breast cancer, TNBC patients are commonly treated with
chemotherapy agents, such as Taxol (6), resistance to which makes TNBC patients
more susceptible to relapse (7).
Therefore, there is an urgent requirement to identify novel
therapeutic targets.
Decreases in tumor suppressor miRNAs or increases in
onco-miRNAs are known to be involved in the pathogenesis of human
cancer (25). MiRNAs also have the
potential to be therapeutic targets (10).
MiR-1207 was identified to be up-regulated in
younger breast cancer patients (11)
and colorectal cancer patients (13),
and overexpression of miR-1207 also promoted the cancer stem
cell-like traits of ovarian cancer (12). These reports suggest a potential
oncogenic role of miR-1207 in cancer. Moreover, a previous report
about the effects of miR-1207-5p expression in peripheral blood on
cisplatin-based chemosensitivity in primary gallbladder carcinoma
patients, indicating the lower miR-1207-5p expression in effective
group than in the ineffective group after chemotherapy (26), suggesting its role in
chemosensitivity. Therefore, the present study first detected the
expression level of miR-1207-5p in normal MCF-10A cells and TNBC
MDA-MB-436, MDA-MB-453 and MDA-MB-231 cell lines, and found that,
compared with normal MCF-10A cells, there was a higher miR-1207-5p
expression level in the TNBC cell lines, with the highest level
being observed in the MDA-MB-231 cells. This suggested an oncogenic
role for miR-1207-5p in TNBC. Therefore, we aimed to identify the
target mRNAs that functioned as tumor suppressor factors during
tumor progression for miR-1207-5p using the online software
programs TargetScan 6.2.
LZTS1 was found to be decreased in cutaneous
squamous cell carcinoma (17) and
osteosarcoma (18), suggesting a
tumor suppressor role for LZTS1 in cancer. Interestingly, the
tumor-suppressor gene LZTS1 suppressed colorectal cancer cell
proliferation through inhibition of the Akt/mTOR signaling pathway
(19), and inhibited hepatocellular
carcinoma cell proliferation through inhibition of the PI3K/Akt
signaling pathway (27). LZST1
reduction was correlated with a poor prognosis, increased cell
motility/invasion and epithelial-to-mesenchymal transition of
breast carcinoma (28). Meanwhile,
LZST1 reduction conferred Taxol resistance and was associated with
a poor prognosis in breast cancer (20). However, there was no report about the
relationship between miR-1207-5p and LZTS1. In the present study,
LZTS1 was first predicted and verified to be a potential target for
miR-1207-5p. The function of miR-1207-5p and LZTS1 in the
sensitivity of TNBC cells to Taxol treatment has not yet been
elucidated. Therefore, we carried out experiments to investigate
this.
Compared with the control group, Taxol dramatically
reduced the cell proliferation rate and increased the cell
apoptosis rate in MDA-MB-231 cells. These effects were further
promoted by the co-administration of Taxol and antagomiR-1207-5p.
Accordingly, we inferred that miR-1207-5p affected cell
proliferation and apoptosis after Taxol treatment. However, it was
unknown which molecules could be regulated by miR-1207-5p or
LZTS1.
We investigated signaling pathways or molecules that
were correlated with cell proliferation and apoptosis. LZTS1
suppressed the proliferation of colorectal cancer and
hepatocellular carcinoma cells by inhibiting the Akt/mTOR signaling
pathway (19) and the PI3K/Akt
signaling pathway (27),
respectively. Bax and Bcl-2 are involved in the apoptotic process
(24). Therefore, we tested the
protein levels of Bax and Bcl-2, as well as the activation level of
p-Akt by western blotting. Up-regulation of Bax, and downregulation
of Bcl-2 and p-Akt, was observed after transfection of
antagomiR-1207-5p compared with the control and Taxol groups. This
suggested that miR-1207-5p functioned through regulating
apoptotic-related pathways and molecules via regulating the
expression of LZTS1.
A significant increase in miR-1207-5p expression and
a decrease in LZTS1 expression were observed in TNBC tissues
compared with normal adjacent tissues. Additionally, there was a
negative correlation between miR-1207-5p and LZTS1 expression.
Moreover, in comparison with Taxol-responsive TNBC tissues, there
was a notable elevation in miR-1207-5p expression and a reduction
in LZTS1 expression in non-responsive TNBC tissues. Compare with
the literature that previously reported LZTS1 gene as tumor
suppressor gene related to paclitaxel resistant in cancer therapy
in, our results was consistent with it (20).
In conclusion, miR-1207-5p may be a promising
predictor of sensitivity to Taxol in TNBC. Our study showed the
novelty of the interaction of miR-1207-5p regulating the LZTS1 gene
expression and therefore, its role in taxol sensitivity.
Unfortunately, there are limitations of our study: 1. The absence
of data on breast cancer cell lines that are not of the triple
negative subtype; 2. the use of a single TNBC cell line; 3.
miR-1207-5p may target not only LZTS1 but also other molecules
critical for MDA-MB-231 cell proliferation, apoptosis and TNBC
development. Comprehensive gene expression analysis of these cells
with or without miR-1207-5p inhibitor should be performed in future
studies to understand its real effects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, ZN and LL performed the experiments and analyzed
the data. QG, HL and XY conducted the experiments. XZ conceived the
study, analyzed the data and prepared the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient. The present study was conducted in accordance with
Declaration of Helsinki and was approved by the Institutional
Review Board of Linfen People's Hospital (Shanxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there were no competing
interests.
References
|
1
|
American Cancer Society: Breast Cancer
Facts & Figures 2013–2014. American Cancer Society; Atlanta,
GA: 2013
|
|
2
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heitz F, Harter P, Lueck HJ,
Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A and
du Bois A: Triple negative and HER2-overexpressing breast cancers
exhibit an elevated risk and an earlier occurrence of cerebral
metastases. Eur J Cancer. 45:2792–2798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zasadil LM, Andersen KA, Yeum D, Rocque
GB, Wilke LG, Tevaarwerk AJ, Raines RT, Burkard ME and Weaver BA:
Cytotoxicity of paclitaxel in breast cancer is due to chromosome
missegregation on multipolar spindles. Sci Transl Med.
6:229ra432014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jamdade VS, Sethi N, Mundhe NA, Kumar P,
Lahkar M and Sinha N: Therapeutic targets of triple-negative breast
cancer: A review. Br J Pharmacol. 172:4228–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim VN and Nam JW: Genomics of microRNA.
Trends Genet. 22:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Zhang W, Shen Y and Guan X:
Identification of dysregulated microRNAs in triple-negative breast
cancer (review). Int J Oncol. 46:927–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghelani HS, Rachchh MA and Gokani RH:
MicroRNAs as newer therapeutic targets: A big hope from a tiny
player. J Pharmacol Pharmacother. 3:217–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peña-Chilet M, Martínez MT, Pérez-Fidalgo
JA, Peiró-Chova L, Oltra SS, Tormo E, Alonso-Yuste E,
Martinez-Delgado B, Eroles P, Climent J, et al: MicroRNA profile in
very young women with breast cancer. BMC Cancer. 14:5292014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu G, Liu A, Zhu J, Lei F, Wu S, Zhang X,
Ye L, Cao L and He S: MiR-1207 overexpression promotes cancer stem
cell-like traits in ovarian cancer by activating the Wnt/β-catenin
signaling pathway. Oncotarget. 6:28882–28894. 2015.PubMed/NCBI
|
|
13
|
Nagy ZB, Barták BK, Kalmár A, Galamb O,
Wichmann B, Dank M, Igaz P, Tulassay Z and Molnár B: Comparison of
circulating miRNAs expression alterations in matched tissue and
plasma samples during colorectal cancer progression. Pathol Oncol
Res. 4–Oct;2017.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United States, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perez-Neut M, Rao VR and Gentile S:
hERG1/Kv11.1 activation stimulates transcription of p21waf/cip in
breast cancer cells via a calcineurin-dependent mechanism.
Oncotarget. 7:58893–58902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singel SM, Cornelius C, Batten K, Fasciani
G, Wright WE, Lum L and Shay JW: A targeted RNAi screen of the
breast cancer genome identifies KIF14 and TLN1 as genes that
modulate docetaxel chemosensitivity in triple-negative breast
cancer. Clin Cancer Res. 19:2061–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olasz EB, Seline LN, Schock AM, Duncan NE,
Lopez A, Lazar J, Flister MJ, Lu Y, Liu P, Sokumbi O, et al:
MicroRNA-135b regulates leucine zipper tumor suppressor 1 in
cutaneous squamous cell carcinoma. PLoS One. 10:e01254122015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Z and Wang T: miR-214 promotes the
proliferation and invasion of osteosarcoma cells through direct
suppression of LZTS1. Biochem Biophys Res Commun. 449:190–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou W, He MR, Jiao HL, He LQ, Deng DL,
Cai JJ, Xiao ZY, Ye YP, Ding YQ, Liao WT and Liu SD: The
tumor-suppressor gene LZTS1 suppresses colorectal cancer
proliferation through inhibition of the AKT-mTOR signaling pathway.
Cancer Lett. 360:68–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lovat F, Ishii H, Schiappacassi M, Fassan
M, Barbareschi M, Galligioni E, Gasparini P, Baldassarre G, Croce
CM and Vecchione A: LZST1 downregulation confers paclitaxel
resistance and is associated with worse prognosis in breast cancer.
Oncotarget. 5:970–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang XX, Liu BB, Wu X, Su D, Zhu Z and Fu
L: Loss of leucine zipper putative tumor suppressor 1 (LZTS1)
expression contributes to lymph node metastasis of breast invasive
micropapillary carcinoma. Pathol Oncol Res. 21:1021–1026. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baselga J: Targeting the
phosphoinositide-3 (PI3) kinase pathway in breast cancer.
Oncologist. 1 16 Suppl:S12–S19. 2011. View Article : Google Scholar
|
|
23
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen ED, Liu B, Yu XS, Xiang ZF and Huang
HY: The effects of miR-1207-5p expression in peripheral blood on
cisplatin-bsed chemosensitivity of primary gallbladder carcinoma.
Onco Targets Ther. 9:3633–3642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Y and Liu X: The tumor-suppressor gene
LZTS1 suppresses hepatocellular carcinoma proliferation by
impairing PI3K/Akt pathway. Biomed Pharmacother. 76:141–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang XX, Zhu Z, Su D, Lei T, Wu X, Fan Y,
Li X, Zhao J and Fu L, Dong JT and Fu L: Down-regulation of leucine
zipper putative tumor suppressor 1 is associated with poor
prognosis, increased cell motility and invasion, and
epithelial-to-mesenchymal transition characteristics in human
breast carcinoma. Hum Pathol. 42:1410–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|