Introduction
Ginseng has been used as a traditional medical herb
for thousands of years in Asian countries (1). Ginsenoside Rg3, one of the active
ingredients extracted from ginseng, has demonstrated potential
anticancer activity in multiple malignancy types (2). Previous research indicates that
ginsenoside Rg3 is able to induce cell apoptosis (3,4), attenuate
cell migration and invasion (5–7), and
enhance the sensitivity of cancer cells to chemotherapy (8,9). Similar
research has been performed in prostate cancer, a common malignancy
in elderly males (10). In such
studies, ginsenoside Rg3 was identified to attenuate cell migration
by inhibiting the expression of aquaporin 1 (11) and to enhance the antitumor effects of
docetaxel in prostate cancer cells (12). At a high dosage (250 µM), ginsenoside
Rg3 has also been revealed to induce cell apoptosis in the prostate
cancer cell line LNCaP (13).
However, to the best of our knowledge, the exact role of
ginsenoside Rg3 and the molecular mechanism involved in its effects
on prostate cancer cells remains to be fully understood.
Reactive oxygen species (ROS) consist of reactive
oxygen ions and peroxides. ROS are produced in normal metabolic
processes and at high concentrations they can cause detrimental
effects on biomolecules, including nucleic acids, proteins and
lipids (14). In several cancer cell
lines, ginsenoside Rg3 exerts its anticancer activity by modulating
the intracellular ROS level. However, the regulatory effects of
ginsenoside Rg3 on ROS levels are not consistent among different
cancer cell types. The accumulation of ROS induced by ginsenoside
Rg3 has been observed in hepatoma, breast cancer, glioblastoma,
leukemia and Jurkat cells, and has been identified to contribute to
cancer cell apoptosis (15–18). However, one study has demonstrated
that in Lewis lung carcinoma cells, ginsenoside Rg3 induces tumor
cell apoptosis by reducing intracellular ROS (3). These previous studies indicate that
ginsenoside Rg3 exhibits various effects on different types of
cancer cells through the modulation of ROS. However, to the best of
our knowledge, few studies have investigated the role of
ginsenoside Rg3 in modulating the levels of ROS in prostate cancer
cells.
The present study identified that ginsenoside Rg3
significantly increases the number of intracellular ROS in a
dose-dependent manner and subsequently induces cell cycle arrest
but not apoptosis in the prostate cancer cell line PC3.
Materials and methods
Cells and reagents
PC3, a prostate cancer cell line, was obtained from
the German Cancer Research Center (Heidelberg, Germany) and
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), at 37°C and 5%
CO2.
Drugs
Ginsenoside Rg3 (Tianjin YiFang S&T Co., Ltd.,
Tianjin, China) was dissolved in dimethylsulfoxide (DMSO) at 100
mM. N-acetyl-L-cysteine (NAC; Beyotime Institute of Biotechnology,
Haimen, China) was dissolved at 1 M in PBS.
Senescence-associated β-galactosidase
(SA-β-gal) staining
PC3 cells were cultured in six-well plates at a
density of 5×105 cells/well and treated with DMSO or 50
µM ginsenoside Rg3 for 48 h at 37°C in an atmosphere of 5%
CO2, followed by SA-β-gal staining using Senescence
β-Galactosidase Staining kit (Beyotime Institute of Biotechnology).
Images were captured using a phase-contrast microscope
(magnification, ×200). In total, six fields of view were randomly
selected in each well and the percentage of cells stained positive
was calculated. The data are expressed as the mean ± standard
deviation (SD).
Cell count
PC3 cells were digested with 0.5% trypsin (Yuanye
S&T, Shanghai, China) and suspended in RPMI-1640 medium. Cell
counts were performed using a hemocytometer and a light microscope
(magnification, ×100). All data are expressed as the mean of
triplicates ± SD.
Cell proliferation assays
PC3 cells were seeded in 24-well plates at a density
of 5×104 cells/well and on the following day they were
treated with DMSO or ginsenoside Rg3 (25, 50 or 100 µM) for 72 h at
37°C in an atmosphere of 5% CO2. Cell Counting Kit-8
(CCK8) solution (US Everbright, Inc., San Ramon, CA, USA) was added
to each well, followed by incubation at 37°C for 2 h, according to
the manufacturer's protocol. The absorbance of each well was
measured at 450 nm. All results are expressed as the mean of
triplicates ± SD.
Cell cycle analysis
PC3 cells were treated with DMSO or 50 µM
ginsenoside Rg3 for 48 h at 37°C in an atmosphere of 5%
CO2. A cell cycle and apoptosis analysis kit (Beyotime
Institute of Biotechnology) was used and flow cytometry assays were
performed as described previously (19).
ROS assays
PC3 cells were plated in 24-well plates at a density
of 5×104 cells/well and treated with DMSO or ginsenoside
Rg3 (25, 50 or 100 µM) at 37°C for 72 h in an atmosphere of 5%
CO2. 2,7-Dichlorodihydrofluorescein diacetate stain
(DCFH-DA) was diluted to 10 µM in serum-free RPMI-1640 medium and
was used to treat PC3 cells for 20 min at 37°C. Subsequently, cells
were washed with serum-free RPMI-1640 medium three times. An ROS
assay kit (Beyotime Institute of Biotechnology) was used to
evaluate ROS levels according to the manufacturer's protocol.
Images were captured using a fluorescence microscope
(magnification, ×100).
NAC treatment
PC3 cells were cultured in 24-well plates at a
density of 5×104 cells/well. The following day, the
cells were precultured with 10 mM NAC for 2 h, followed by
treatment with DMSO or 50 µM ginsenoside Rg3 for a further 0, 24,
48 and 96 h at 37°C in an atmosphere of 5% CO2. Cell
counts were performed with a hemocytometer and a light microscope
(magnification, ×100). All data are expressed as the mean of
triplicates ± SD.
Statistical analysis
All statistical analysis was performed using SPSS
software (version 23.0; IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± SD. Analysis of variance (ANOVA) was
performed to analyze the data. One-way ANOVA was used for complete
random designed data and repeated measures ANOVA was used for
repeated measured designed data. A Fisher's least significant
difference test was used to perform multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
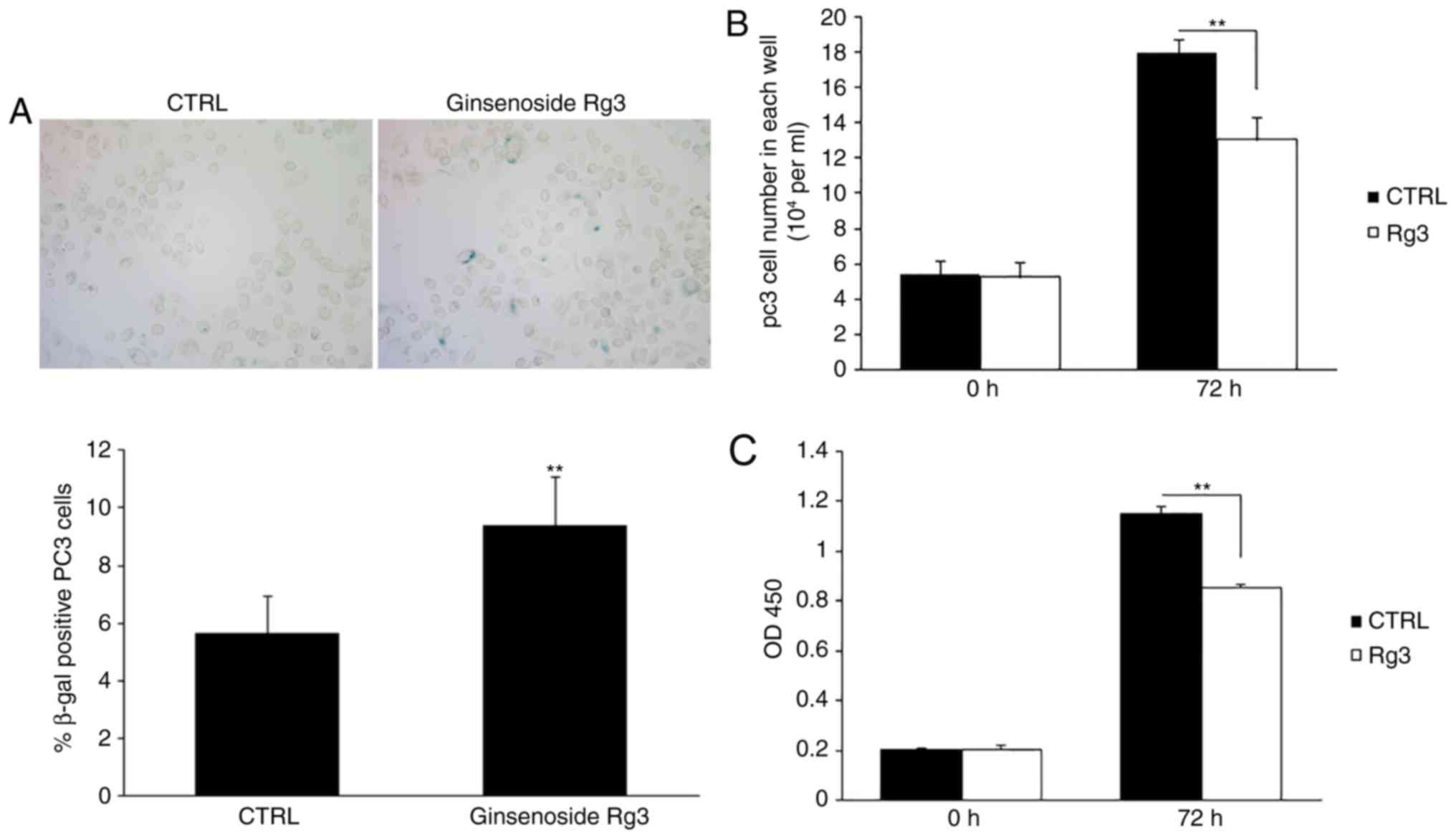
Ginsenoside Rg3 inhibits PC3 cell
proliferation in vitro
PC3 prostate cancer cells were seeded in six-well
plates and treated with DMSO or 50 µM ginsenoside Rg3 for 48 h.
SA-β-gal staining revealed that ginsenoside Rg3 significantly
increased the percentage of positively stained cells (Fig. 1A). The cells were then cultured in
24-well plates at 5×104 cells/well, followed by
treatment with DMSO or 50 µM ginsenoside Rg3 for 72 h. The number
of cells in each well was counted and cell proliferation was
evaluated using a CCK8 assay. The results demonstrated that
ginsenoside Rg3 exhibited significant inhibitory effects on cell
proliferation in vitro compared with DMSO (Fig. 1B and C).
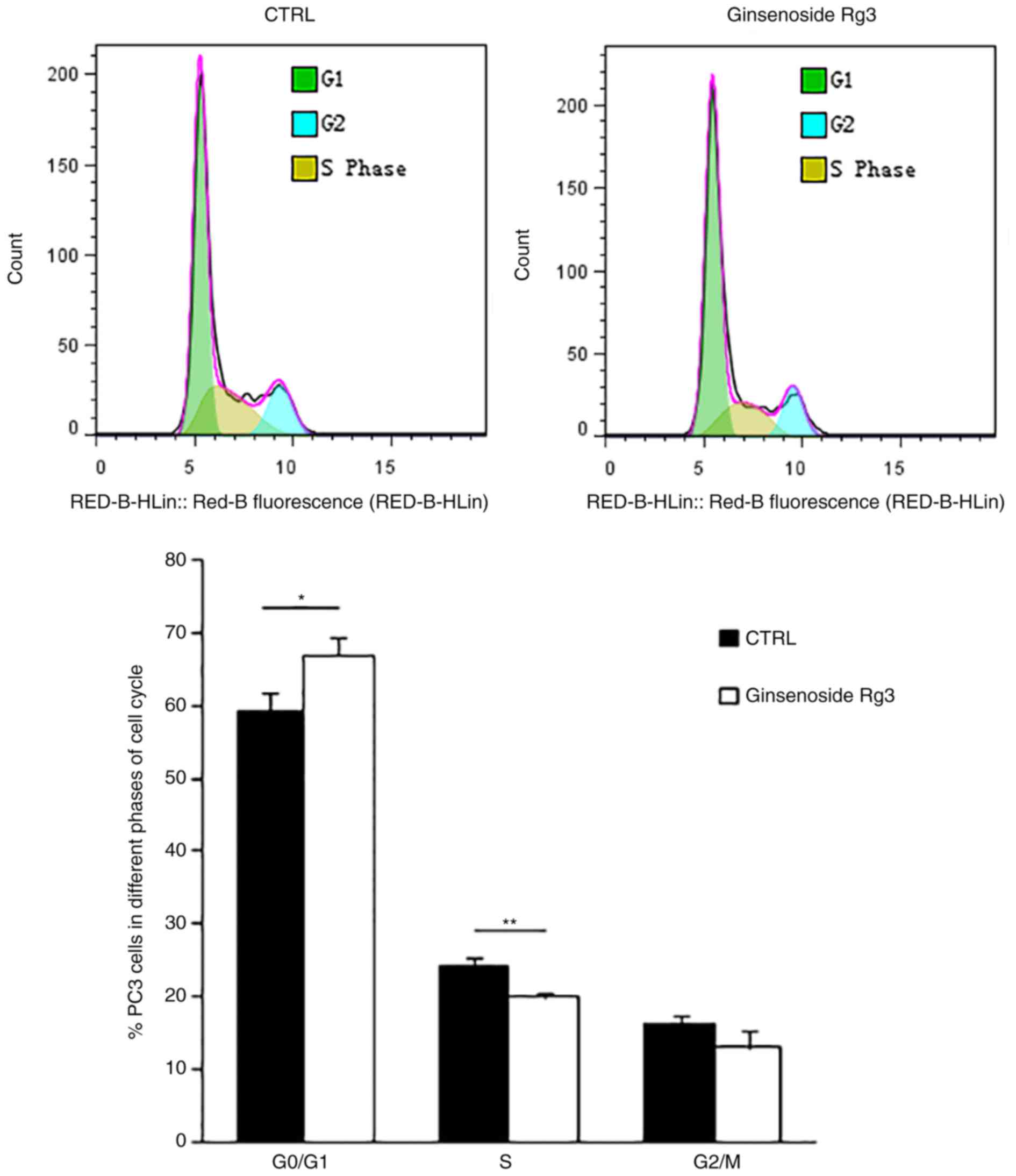
Ginsenoside Rg3 inhibits the G1/S
transition in the PC3 cell cycle
To further study the molecular mechanism involved in
the inhibition of cell proliferation by ginsenoside Rg3, flow
cytometry analysis was performed to examine the cell cycle of PC3
cells treated with DMSO or 50 µM ginsenoside Rg3 for 48 h.
Ginsenoside Rg3 significantly induced cell cycle arrest in the
G0/G1 phase and significantly decreased the
percentage of cells in the S phase (Fig.
2). These results indicate that treatment with ginsenoside Rg3
inhibits cell cycle transition from the G1 phase to the
S phase in PC3 cells. However, apoptosis of PC3 cells induced by
ginsenoside Rg3 was not observed in the current study according to
the results of flow cytometry assays (data not shown).
Ginsenoside Rg3 increases ROS levels
in PC3 cells in a dose-dependent manner
Oxidative stress acts as a pivotal modulator in the
proliferation and apoptosis of cancer cells, and an imbalance in
the production and scavenging of ROS triggers the progression of
cancer (20). In the current study,
different doses of ginsenoside Rg3 (0, 25, 50 and 100 µM) were used
to treat PC3 cells cultured in 24-well plates at 5×104
cells/well for 72 h. Compared with the control group, cell counting
and CCK8 analysis demonstrated that 50 and 100 µM ginsenoside Rg3
significantly inhibited cell proliferation. In addition, compared
with 25 µM ginsenoside Rg3 treatment, 50 and 100 µM ginsenoside Rg3
exhibited significant inhibitory effects on PC3 cell proliferation
(Fig. 3A and B). In addition, DCFH-DA
staining was performed to evaluate ROS levels and an accumulation
of intracellular ROS was observed in PC3 cells, suggesting a
potential association between ginsenoside Rg3-induced cell cycle
arrest and increased levels of ROS (Fig.
3C).
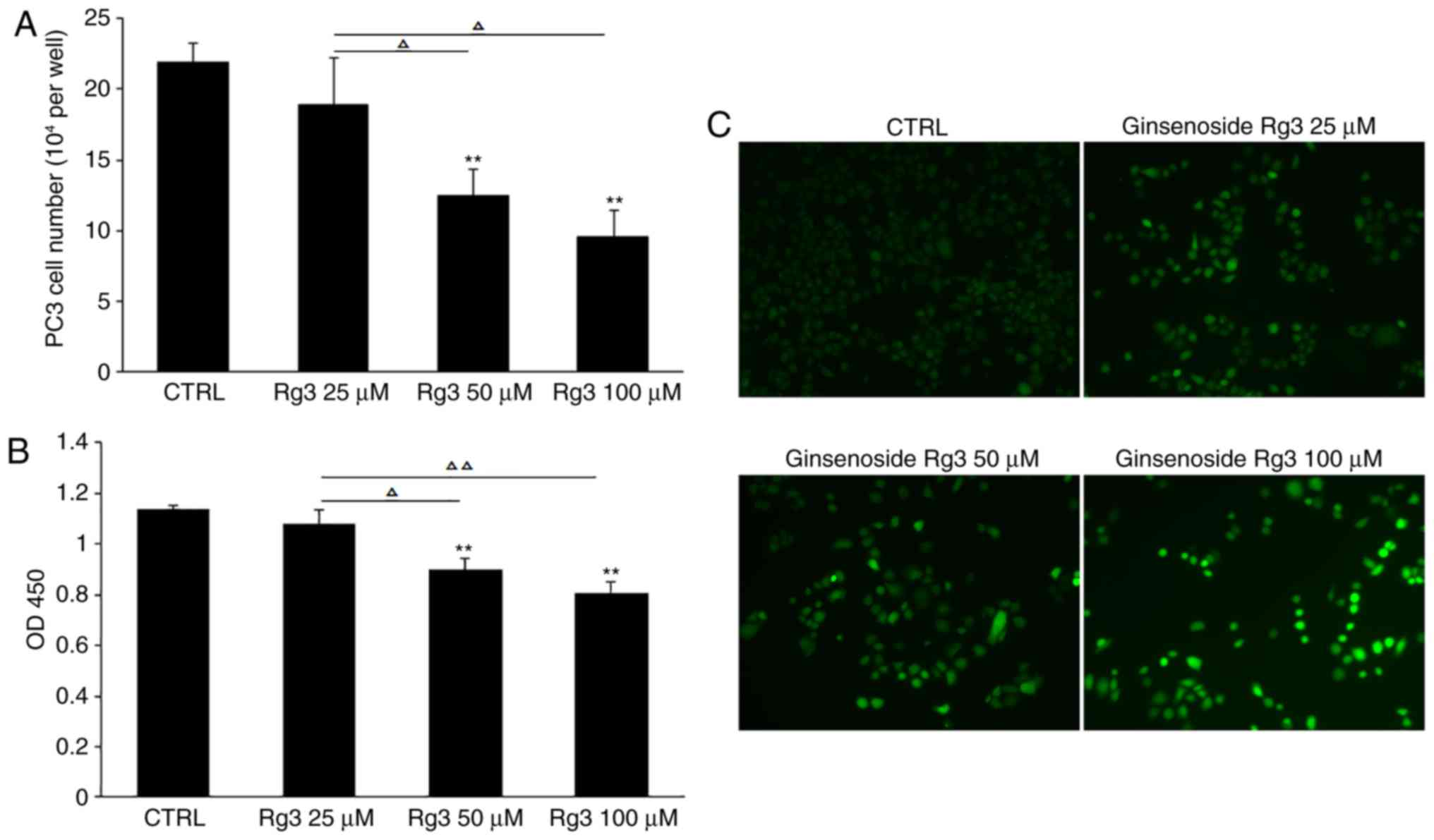 | Figure 3.Ginsenoside Rg3 inhibits cell
proliferation and induces the accumulation of ROS in PC3 cells in a
dose-dependent manner. (A) PC3 cells were treated with various
doses of ginsenoside Rg3 (0, 25, 50 and 100 µM) for 72 h, followed
by cell counting. (B) Cell proliferation was measured by Cell
Counting Kit-8 assay. (C) 2,7-Dichlorodihydrofluorescein diacetate
staining was performed to evaluate the level of ROS. Images were
captured using a fluorescence microscope. Magnification, ×100. All
data were obtained from three independent experiments and are
presented as the mean ± standard deviation. **P<0.01 vs. CTRL,
ΔP<0.05, ΔΔP<0.01. ROS, reactive oxygen
species; Rg3, ginsenoside Rg3; CTRL, control; OD, optical density;
CTRL, control. |
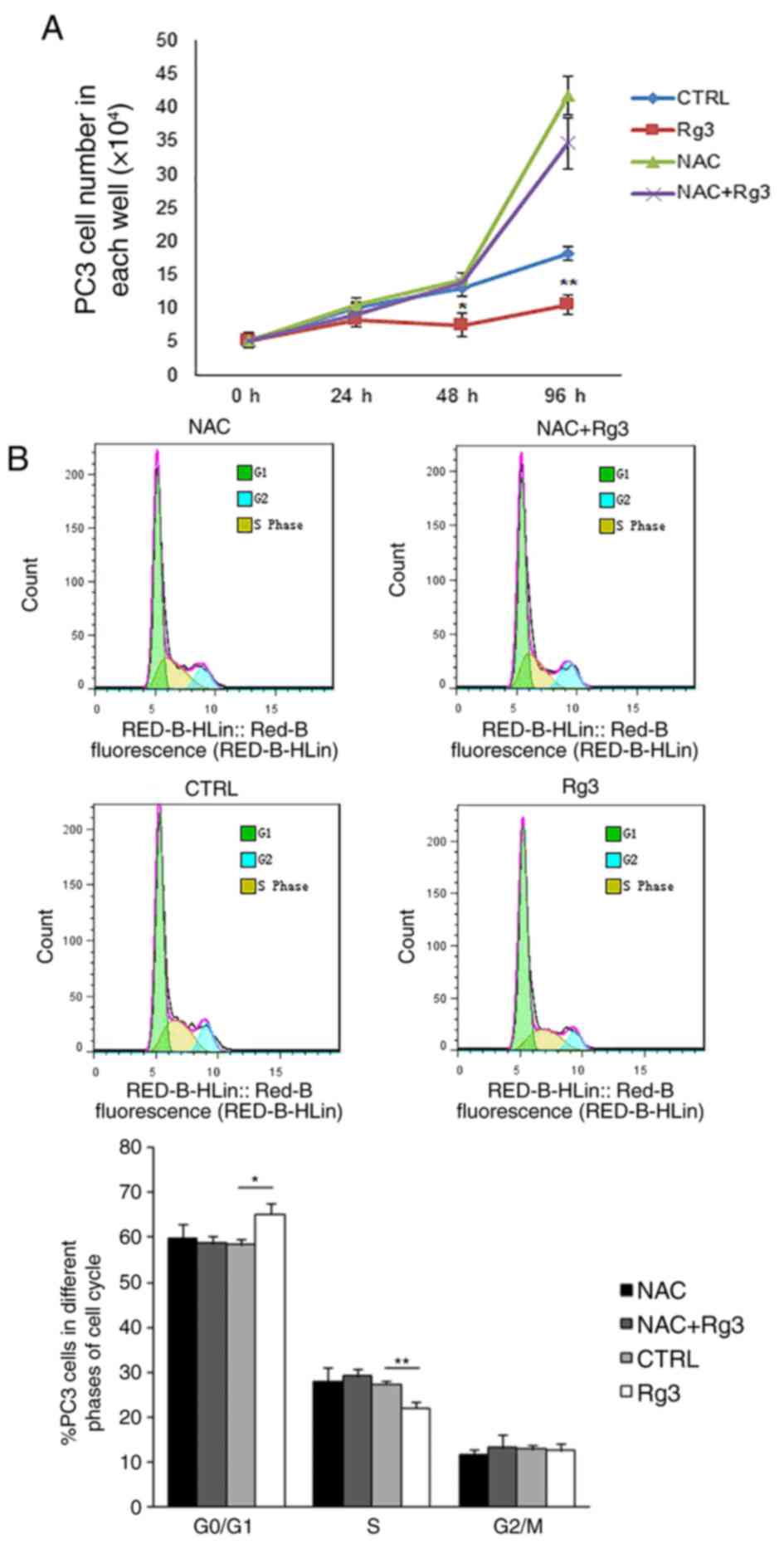
Elimination of intracellular ROS with
NAC can block ginsenoside Rg3-induced cell cycle arrest in PC3
cells
To investigate the effect of intracellular ROS
accumulation on the arrest of cell proliferation induced by
ginsenoside Rg3, PC3 cells were precultured with 10 mM NAC for 2 h,
followed by treatment with DMSO or 50 µM ginsenoside Rg3 for a
further 0, 24, 48 and 96 h. Cell counting revealed that the
elimination of intracellular ROS by NAC significantly blocked the
ginsenoside Rg3-induced proliferation inhibition in PC3 cells
(Fig. 4A). Flow cytometry analysis
was also performed 48 h following treatment with DMSO or
ginsenoside Rg3 in PC3 cells. Pretreatment with NAC decreased the
cell cycle arrest caused by ginsenoside Rg3 and reestablished the
transition of PC3 cells from the G1 phase to the S
phase. The results indicated that compared with the control group,
ginsenoside Rg3 significantly increased the proportion of cells in
the in G0/G1 phase and decreased the
proportion of cells in the S phase. However, no significant
differences were identified in the proportion of cells in the
G0/G1 phase or the S phase when treated with
NAC or NAC+Rg3 compared with the control (Fig. 4B).
Discussion
Oxidative stress is a pivotal factor associated with
the pathology of prostate cancer (20). A previous study has identified that
the accumulation of ROS induced by a redox disorder can contribute
to carcinogenesis resulting from epigenetic regulation and
macromolecular damage, including DNA instability (21). Therefore, certain antioxidants are
assumed to have therapeutic value in the treatment of prostate
cancer (22). The Selenium and
Vitamin E Cancer Prevention Trial study was performed with a large
sample size and long duration to evaluate the benefits of selenium
and vitamin E supplementation in the treatment of prostate cancer,
however, the study failed to identify any benefits (23). Therefore, previous studies suggest
that antioxidant therapy in cancer treatment requires further
assessment.
Antioxidants may provide a feasible strategy for
cancer treatment in the early stage of disease, however, they may
not provide a feasible strategy in the later stages, particularly
in cases involving high levels of ROS that are below the toxic
threshold. At the later stages of disease, decreased levels of ROS
may have no effects on the progression of cancer or may even
exhibit procarcinogenic effects (24).
The current study identified that increased levels
of ROS inhibited the proliferation of PC3 cells. However, using a
different prostate cancer cell line, DU145, it was revealed that
ginsenoside Rg3 increased the intracellular level of ROS but
enhanced cell viability, instead of inducing cell proliferation
arrest or cell death (data not shown). This result may partially be
due to a distinct tolerance of ROS in the two cell lines. A
previous study compared the responses of PC3 cells and DU145 cells
to ionizing radiation; this revealed that DU145 cells exhibit a
higher resistance to radiation due to lower basal levels of ROS, a
higher glutathione (GSH) content and a higher GSH/glutathione
disulfide ratio, suggesting DU145 cells possess a greater tolerance
to radiation-induced ROS compared with PC3 cells (25). A recent study also identified that
phosphatase and tensin homolog (PTEN)-deficient cancer cells with
upregulated Akt exhibit high intracellular ROS levels and are more
sensitive to ROS-induced cell death compared with PTEN wild-type
cell lines (26). This may partially
explain the different responses of the PTEN wild-type cell line
DU145 and the PTEN-deficient cell line PC3 to ginsenoside
Rg3-induced ROS accumulation (27).
Therefore, ginsenoside Rg3 should be used with caution in the
treatment of prostate cancer as the drug may serve either a
pro-cancer or anticancer role depending on the subtype of the
disease.
In the current study, ginsenoside Rg3 increased the
levels of ROS in prostate cancer cells in a dose-dependent manner.
A previous study has demonstrated that ginsenoside Rg3
downregulates ROS levels and inhibits cellular senescence in
prostatic stromal cells (28). This
suggests that ginsenoside Rg3 may conversely regulate ROS levels in
prostate cancer cells and stromal cells. The molecular mechanism
involved requires further investigation and the complex effects of
ginsenoside Rg3 on both cancer cell types and the tumor
microenvironment should be carefully considered before this agent
is utilized in cancer treatment.
In summary, the current study revealed that
ginsenoside Rg3 induces intracellular ROS accumulation in the
prostate cancer cell line PC3 and subsequently inhibits cell
proliferation via ROS-induced cell cycle arrest.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Tianjin Municipal Committee for Health and Family Planning (grant
no. 2017111), the Natural Science Foundation in Tianjin (grant no.
16JCQNJC10700) and the National Natural Science Foundation of China
(grant no. 81703828).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
FZ and YP conceived and designed the experiments.
YP, RZ, XY and NK performed the experiments. HY, LB, YS and ZZ
analyzed the data. HY and ZZ wrote the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vayghan HJ, Ghadimi SS and Nourazarian AR:
Preventive and therapeutic roles of ginseng-focus on colon cancer.
Asian Pac J Cancer Prev. 15:585–588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun HY, Lee JH, Han YS, Yoon YM, Yun CW,
Kim JH, Song YS and Lee SH: Pivotal roles of ginsenoside rg3 in
tumor apoptosis through regulation of reactive oxygen species.
Anticancer Res. 36:4647–4654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X,
Xiang S, Li H, Jiang L, Tan Z, et al: 20(S)-ginsenoside Rg3
promotes senescence and apoptosis in gallbladder cancer cells via
the p53 pathway. Drug Des Devel Ther. 9:3969–3987. 2015.PubMed/NCBI
|
|
5
|
Zheng X, Chen W, Hou H, Li J, Li H, Sun X,
Zhao L and Li X: Ginsenoside 20(S)-Rg3 induced autophagy to inhibit
migration and invasion of ovarian cancer. Biomed Pharmacother.
85:620–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Junmin S, Hongxiang L, Zhen L, Chao Y and
Chaojie W: Ginsenoside Rg3 inhibits colon cancer cell migration by
suppressing nuclear factor kappa B activity. J Tradit Chin Med.
35:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian L, Shen D, Li X, Shan X, Wang X, Yan
Q and Liu J: Ginsenoside Rg3 inhibits epithelial-mesenchymal
transition (EMT) and invasion of lung cancer by down-regulating
FUT4. Oncotarget. 7:1619–1632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Tian L, Khan MN, Zhang L, Chen Q,
Zhao Y, Yan Q, Fu L and Liu J: Ginsenoside Rg3 sensitizes hypoxic
lung cancer cells to cisplatin via blocking of NF-κB mediated
epithelial-mesenchymal transition and stemness. Cancer Lett.
415:73–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Yuan Z, Sun Y, Bu Y, Li W and Fei
Z: Ginsenoside Rg3 enhances the anti-proliferative activity of
erlotinib in pancreatic cancer cell lines by downregulation of
EGFR/PI3K/Akt signaling pathway. Biomed Pharmacother. 96:619–625.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnett CM, Nielson CM, Shannon J, Chan
JM, Shikany JM, Bauer DC, Hoffman AR, Barrett-Connor E, Orwoll E
and Beer TM: Serum 25-OH vitamin D levels and risk of developing
prostate cancer in older men. Cancer Causes Control. 21:1297–1303.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan XY, Guo H, Han J, Hao F, An Y, Xu Y,
Xiaokaiti Y, Pan Y and Li XJ: Ginsenoside Rg3 attenuates cell
migration via inhibition of aquaporin 1 expression in PC-3M
prostate cancer cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB and Hong JT: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu WK, Xu SX and Che CT:
Anti-proliferative effect of ginseng saponins on human prostate
cancer cell line. Life Sci. 67:1297–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanz A: Mitochondrial reactive oxygen
species: Do they extend or shorten animal lifespan? Biochim Biophys
Acta. 1857:1116–1126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim BM, Kim DH, Park JH, Na HK and Surh
YJ: Ginsenoside Rg3 induces apoptosis of human breast cancer
(MDA-MB-231) cells. J Cancer Prev. 18:177–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi YJ, Lee HJ, Kang DW, Han IH, Choi BK
and Cho WH: Ginsenoside Rg3 induces apoptosis in the U87MG human
glioblastoma cell line through the MEK signaling pathway and
reactive oxygen species. Oncol Rep. 30:1362–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia T, Wang YN, Zhou CX, Wu LM, Liu Y,
Zeng QH, Zhang XL, Yao JH, Wang M and Fang JP: Ginsenoside Rh2 and
Rg3 inhibit cell proliferation and induce apoptosis by increasing
mitochondrial reactive oxygen species in human leukemia jurkat
cells. Mol Med Rep. 15:3591–3598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Du X, Yang R, Liu J, Xu D, Shi J,
Chen L, Shao R, Fan G, Gao X, et al: The prevention and treatment
effects of tanshinone IIA on oestrogen/androgen-induced benign
prostatic hyperplasia in rats. J Steroid Biochem Mol Biol.
145:28–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Udensi UK and Tchounwou PB: Oxidative
stress in prostate hyperplasia and carcinogenesis. J Exp Clin
Cancer Res. 35:1392016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paschos A, Pandya R, Duivenvoorden WC and
Pinthus JH: Oxidative stress in prostate cancer: Changing research
concepts towards a novel paradigm for prevention and therapeutics.
Prostate Cancer Prostatic Dis. 16:217–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohsenzadegan M, Seif F, Farajollahi MM
and Khoshmirsafa M: Anti-oxidants as chemopreventive agents in
prostate cancer: A gap between preclinical and clinical studies.
Recent Pat Anticancer Drug Discov. 13:224–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramamoorthy V, Rubens M, Saxena A and
Shehadeh N: Selenium and vitamin E for prostate
cancer-justifications for the SELECT study. Asian Pac J Cancer
Prev. 16:2619–2627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Assi M: The differential role of reactive
oxygen species in early and late stages of cancer. Am J Physiol
Regul Integr Comp Physiol. 313:R646–R653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jayakumar S, Kunwar A, Sandur SK, Pandey
BN and Chaubey RC: Differential response of DU145 and PC3 prostate
cancer cells to ionizing radiation: Role of reactive oxygen
species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta.
1840:485–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nogueira V, Patra KC and Hay N: Selective
eradication of cancer displaying hyperactive Akt by exploiting the
metabolic consequences of Akt activation. Elife. 7:e322132018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Isebaert SF, Swinnen JV, McBride WH and
Haustermans KM: Insulin-like growth factor-type 1 receptor
inhibitor NVP-AEW541 enhances radiosensitivity of PTEN wild-type
but not PTEN-deficient human prostate cancer cells. Int J Radiat
Oncol Biol Phys. 81:239–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng Y, Zhang R, Kong L, Shen Y, Xu D,
Zheng F, Liu J, Wu Q, Jia B and Zhang J: Ginsenoside Rg3 inhibits
the senescence of prostate stromal cells through down-regulation of
interleukin 8 expression. Oncotarget. 8:64779–64792.
2017.PubMed/NCBI
|