Introduction
A solitary fibrous tumor (SFT) is a rare mesenchymal
tumor, which can be found in different locations within the human
body (1). SFTs were first reported in
the pleura and subsequently in the peritoneum, thymus, orbit,
pericardium, meninges, spinal cord, and the parotid and thyroid
glands (2). A tumor of this type in
the liver is an extremely rare occurrence. The majority of SFTs are
considered benign, while <20% are reported to be malignant and
are accompanied by tumor invasion and metastasis (3). Surgical resection is the main approach
for SFT treatment. Nevertheless, in certain cases where vascular
structures are involved, conventional surgery may not be
practical.
Ex situ hepatectomy and liver
autotransplantation are novel methods for treating complicated
liver tumors that are unresectable by conventional approaches,
including liver transplantation, vascular reconstruction, organ
perfusion, extended hepatic resection and hemodynamic management,
which are considered to be some of the most complicated, difficult
and risky types of surgeries. In this maneuver, the whole liver is
removed and perfused with cold preservation solution. The tumor is
resected ex situ on the operating table and the remaining
liver is orthotopically implanted. The ex situ liver
resection was first described by Pichlmayr et al (4) as a novel surgical procedure to treat a
bilateral liver leiomyosarcoma. To date, only a few cases of ex
situ hepatectomy and liver autotransplantation have been
described worldwide (5,6). Furthermore, to the best of our
knowledge, there are no reports on adopting ex situ
hepatectomy and liver autotransplantation as an approach for
treating hepatic SFTs. The present case study reports the first
case of a giant SFT involving the inferior vena cava (IVC) of the
liver in a 32-year-old female who was treated with ex situ
hepatectomy and liver autotransplantation.
Case report
A 32-year-old female suffering from repeated
abdominal distension for 3 years was found to have an oversized
mass in the right hepatic lobe using a B-scan ultrasound, and was
admitted on July 7, 2017 to the Outpatient Department of the First
Affiliated Hospital, School of Medicine, Zhejiang University
(Hangzhou, China). A physical examination revealed a large mass in
the right hypochondrium, causing local discomfort. No aberration
was observed regarding the medical history of the patient. In
addition, no elevation of tumor markers, including α-fetoprotein
(AFP; ARCHITECT AFP Reagent kit; cat. no. 3P36-30; Abbott
Pharmaceutical Co., Ltd., Lake Bluff, IL, USA), carcinoembryonic
antigen (CEA; ARCHITECT CEA Reagent kit; cat. no. 7K68-32; Abbott
Pharmaceutical Co., Ltd.), cancer antigens (CA) 125 (ARCHITECT CA
125 II Reagent kit; cat. no. 2K45-35; Abbott Pharmaceutical Co.,
Ltd.) and 19-9 (ARCHITECT CA 19-9 XR Reagent kit; cat. no.
2K91-38; Abbott Pharmaceutical Co., Ltd.), was observed, as
detected using immunofluorescence assays, according to the
manufacturers protocols. Hepatitis virus markers were all negative,
as detected using an immunofluorescence assay (ARCHITECT Reagent
kits; cat. nos. 6C36-44, 8L44-30, 6C34-35, 6C32-20 and 7C18-34;
Abbott Pharmaceutical Co., Ltd.), according to the manufacturers
protocol. The patient underwent an enhanced abdominal computed
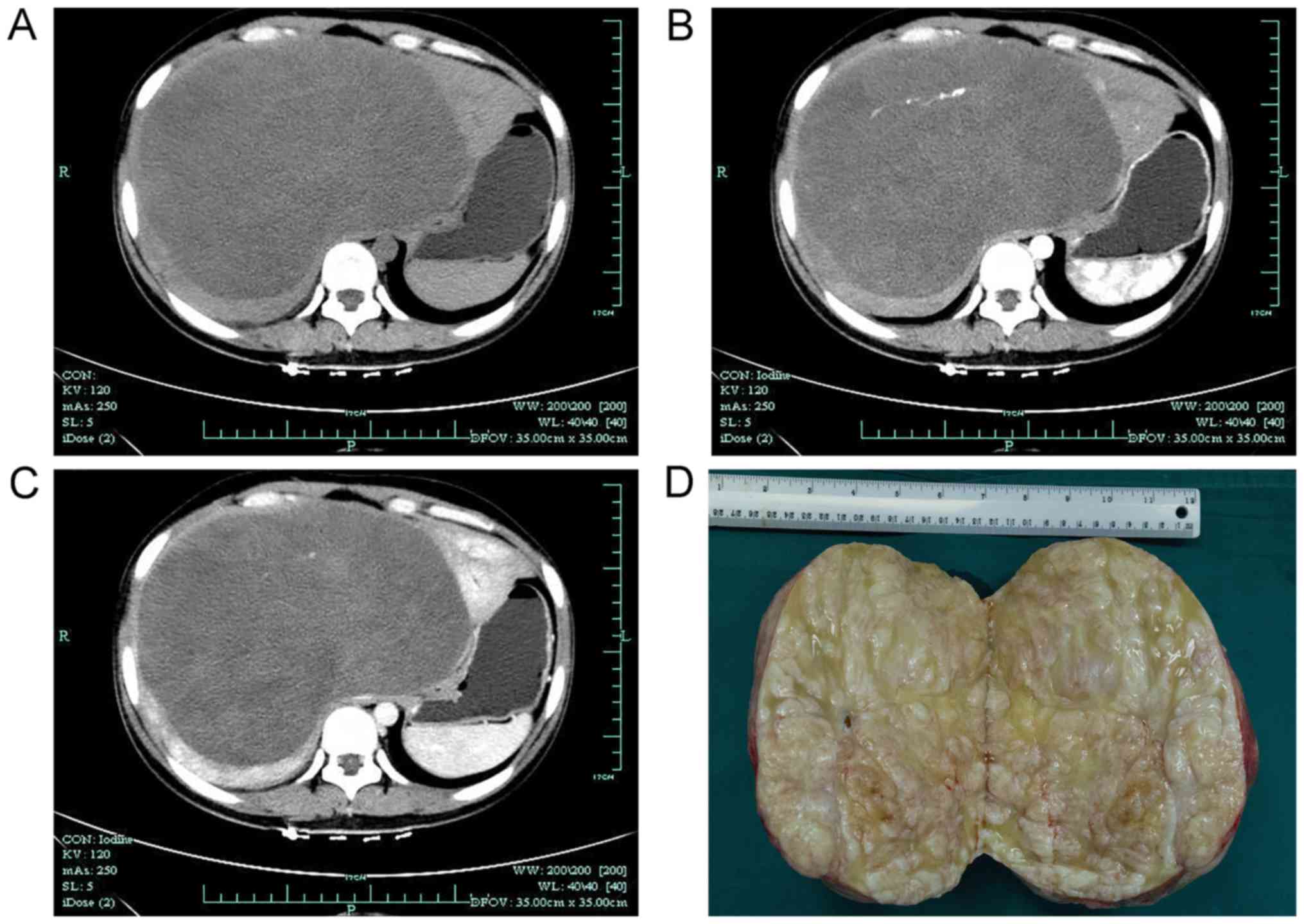
tomography (CT) scan, which revealed a giant, hypodense,
heterogeneous, cystic-solid lesion (20.0×16.0 cm), with irregular
contrast enhancement, causing compression of neighboring
structures. Dilation of the intrahepatic biliary ducts was also
noted (Fig. 1). A CT arteriography
scan showed that the mass was supplied with blood by the left and
right hepatic arteries. The right, middle and left hepatic veins
were compressed by the mass and there was indication of a filling
defect in the IVC. A magnetic resonance imaging scan was also
performed, revealing an oversized, heterogeneous liver mass
(21.2×19.7 cm). A heterogeneous high signal in the T2-weighted
image and a slightly low signal in the T1-weighted image were
observed. All results indicated that the lesion was malignant and a
mesenchymal tumor was suspected.
In view of the young age of the patient, an ex
situ hepatectomy and liver autotransplantation were performed
on October 17, 2017. During the surgery, the second hepatic portal
was difficult to expose and the sternum was therefore removed with
the assistance of a cardiothoracic expert. An intra-operative
frozen section examination indicated the proliferation of fibrotic
tissue, and the tumor was considered to be benign. Consequently,
the tumor was resected on the bench. A 26-mm artificial blood
vessel was utilized to establish an end-to-side portocaval shunt
during the anhepatic period and the liver remnant was
autotransplanted in a piggyback fashion. The surgery was without
complications and lasted for 18 h, with an anhepatic period of 5
h.
The resected specimen was well-delimited and
measured 19.0×19.5×14.0 cm in size and 3.964 kg in weight. The
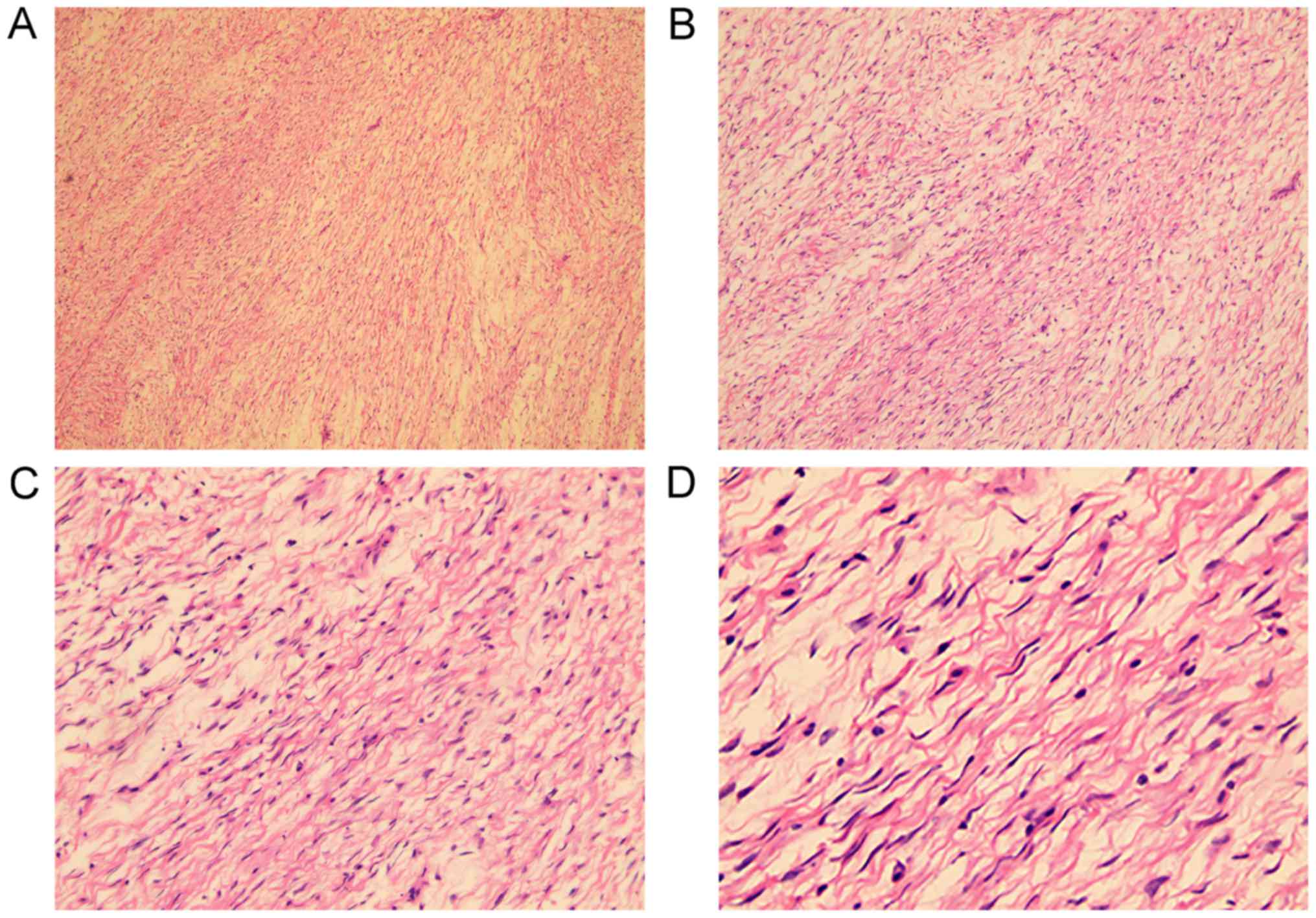
tumor presented with a grayish-white interlaced appearance in the
section plane, and was comprised of spindle-shaped cells
infiltrated with lymphocytes, plasmocytes and mastocytes (Fig. 2). The immunohistochemical (IHC)
examination was performed on paraffin-embedded specimens fixed in
4% buffered neutral formalin at 37°C for 24 h. Paraffin-embedded
sections (3 µm) were deparaffinized and and endogenous peroxidase
was inactivated with 15% H2O2-Methanol for 5
min at room temperature. The sections were then incubated with
primary antibodies at 37°C for 30 min, according to the
manufacturers protocols. Subsequently, the sections were incubated
with Dako REALREEnVision Detection system, Peroxidase/DAB,
Rabbit/Mouse (cat. no. K5007; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 30 min at room temperature. Results
revealed that the sections were positive for cluster of
differentiation 117 (CD117; cat. no. 790-2951; dilution 1:100;
Roche Diagnostics, Basel, Switzerland), CD34 (cat. no. M-0117;
dilution 1:50; Shanghai Changdao Biotechnology Co., Ltd., Shanghai,
China) and Caldesmon (cat. no. ZA-0535; dilution 1:100; OriGene
Technologies, Inc., Beijing, China). In addition focal expression
of smooth muscle actin (cat. no. ZM-0003; dilution 1:100; OriGene
Technologies, Inc.) and progestrone receptor (cat. no.
NCL-L-PGR-312; dilution 1:300; Leica Biosystems, Ltd., Milton
Keynes, UK) expression was detected. However, sections were
negative for S-100 protein (cat. no. ZA-0225; dilution 1:200;
OriGene Technologies, Inc.), desmin (cat. no. ZM-0091; dilution
1:100; OriGene Technologies, Inc.), deletions of G-rich-1 (cat. no.
ZM-0371; dilution 1:200; Zhongshanjinqiao Bio-Reagent Company),
cytokeratin (cat. no. M-0349; dilution 1:100; Shanghai Changdao
Biotechnology Co., Ltd.), β-catenin (cat. no. ZM-0442; dilution
1:100; OriGene Technologies, Inc.), anaplastic lymphoma kinase
(cat. no. ALK-L-CE-H; dilution 1:150; Leica Biosystems, Ltd.),
estrogen receptor (cat. no. NCL-L-ER-6F11; dilution 1:100; Leica
Biosystems, Ltd.), epithelial membrane antigen (cat. no. ZM-0095;
dilution 1:200; Zhongshanjinqiao Bio-Reagent Company), wilms tumor
type 1 (cat. no. ZM-0269; dilution 1:100; Zhongshanjinqiao
Bio-Reagent Company) and CD10 (cat. no. NCL-L-CD10-270; dilution
1:100; Leica Biosystems, Ltd.). The sections were evaluated under a
light microscope at ×200 magnification. The mean proliferative
index was 5% in the hypercellular areas, as determined by detection
of antigen KI-67 (Ki-67; cat. no. ZM-0166; dilution 1:100;
Zhongshanjinqiao Bio-Reagent Company). The Ki-67 proliferative
index was examined under a light microscope (BX53; Olympus
Corporation, Tokyo, Japan), and was evaluated by experienced
pathologists. A total of 500 tumor cells were counted both at the
center and at the periphery of the tumor. The examination was
repeated at least twice and the mean Ki-67 proliferative index was
calculated. At least 10 mm of the margins of the resected specimen
were tumor-free. Based on the pathological and IHC characteristics,
the lesion was identified as an SFT.
On post-operative day 9, the patient underwent
pleurocentesis and received drainage tubes due to moderate right
pleural effusion, which were successfully removed 16 days later.
The patient recovered fully and was discharged on post-operative
day 29. At the 1- and 3-month follow-ups, the patient had normal
liver function and there was no evidence of recurrence. Future
follow-up will be performed every 6 months.
Discussion
SFTs are mesenchymal neoplasms that typically occur
in the thoracic or pleural cavities. The SFTs found in the liver
may derive from the intra-hepatic connective tissue, for example
Glisson's capsule or conjunctive tissue, which are rare types of
liver neoplasm (7). A search of the
English literature on ‘Solitary Fibrous Tumour of the Liver’ was
conducted on PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Google
(https://www.google.com/). All published English
articles, case reports and literature reviews, and their reference
lists, were reviewed. The results indicated that only 88 cases of
SFTs of the liver have been reported since 1958 (7–71). The
main characteristics of these cases are listed in Table I. The mean age of onset was 57.1 years
(range, 16.0–87.0 years) and the lesion appeared more commonly in
female patients (ratio 1.4:1), with a mean tumor diameter of 18.2
cm (range, 1.5–30.0 cm). The median follow-up time for these cases
was 13.5 months. The prolonged course and biological features of
SFTs resulted in a relatively late age of onset and large lesion
size. The patient in the present study was a 32-year-old female
with a single lesion measuring ~19.0 cm; these characteristics were
consistent with the cases listed in Table
I.
 | Table I.Main characteristics of reported
liver solitary fibrous tumor cases. |
Table I.
Main characteristics of reported
liver solitary fibrous tumor cases.
| First author,
year | Age, years | Sex | Lobe | Size, cm | Hypo | Treatment | IHC | Follow-up | (Refs.) |
|---|
| Edmondson,
1958 | 16 | F | R | 23×17 | N | Resection | N/A | 24 months | (8) |
|
| N/A | N/A | R | 5×5 | N | Resection | N/A | N/A |
|
| Nevius and
Friedman, 1959 | 56 | M | R | 15×15 | Y | Radiation | N/A | Succumbed after 2
days | (9) |
| Ishak, 1976 | 62 | M | L | 24 | N | Resection | N/A | N/A | (10) |
|
| 62 | F | L | 23×20×13 | N | Resection | N/A | Succumbed during
surgery |
|
| Kim and Damjanov,
1983 | 27 | F | L | 27×23×15 | N | Resection | N/A | 6 months | (11) |
| Kottke-Marchant
et al, 1989 | 84 | F | L | 15×9×8 | N | Resection | V+ | 29 months | (12) |
| Kasano et
al, 1991 | 39 | F | L | 18×10×18 | N | Resection | N/A | 53 months | (13) |
| Barnoud et
al, 1996 | 50 | M | R | 17×15×11 | N | Resection | CD34+,
V+ | N/A | (14) |
| Levine and Rose,
1997 | 57 | M | L | 10×18×8 | N | Resection | CD34+,
V+ | 38 months | (15) |
| Guglielmi et
al, 1998 | 61 | F | R | 20×16×10 | Y | Resection | CD34+,
V+ | 72 months | (16) |
| Lecesne et
al, 1998 | 69 | F | L | N/A | N | Resection | CD34+,
V+ | 12 months | (17) |
| Bejarano et
al, 1998 | 49 | M | L | 17×12×10 | N | Resection | CD34+,
V+ | 15 months | (18) |
| Moran et al
1998 | 62 | F | N/A | 23×20×13 | N | Resection | CD34+,
V+ | N/A | (19) |
|
| 34 | F | N/A | 2×0.5 | N | Nil | N/A | Incidental
(autopsy) |
|
|
| 57 | F | N/A | 24×19×11 | N | Resection | CD34+,
V+ | N/A |
|
|
| 32 | M | N/A | 12×9×7 | N | Resection | CD34+,
V+ | N/A |
|
|
| 68 | F | N/A | 17×17 | N | Resection | CD34+,
V+ | Succumbed after 2
days |
|
|
| 83 | F | R | 18 | Y | Resection | CD34+,
V+ | Succumbed after 6
days |
|
|
| 72 | F | L | 9 | N | Resection | CD34+,
V+ | 12 months |
|
|
| 62 | M | L | 24 | N | Resection | CD34+,
V+ | N/A |
|
|
| 50 | F | N/A | 3×2×1.5 | N | Resection | CD34+,
V+ | N/A |
|
| Fuksbrumer et
al, 2000 | 40 | F | R | 14–17 | N | Resection | CD34+,
V+, Bcl-2+ | N/A | (20) |
|
| 71 | F | R | 14–17 | N | Resection | CD34+,
V+, Bcl-2+ | N/A |
|
|
| 80 | M | R | 14–17 | N | Nil | CD34+,
V+, Bcl-2+ | N/A |
|
| Yilmaz et
al, 2000 | 25 | F | R | 32×30 | N | Resection | V+ | 6 months | (21) |
| Lin et al,
2001 | 75 | M | R | 21×20×18 | Y | Resection |
CD34+ | 11 months | (22) |
| Gold et al,
2002 | N/A | N/A | N/A | N/A | N | N/A | N/A | N/A | (23) |
|
| N/A | N/A | N/A | N/A | N | N/A | N/A | N/A |
|
| Neeff et al,
2004 | 63 | F | R | 30×12×19 | N | Resection | CD34+,
V+ | 6 months | (24) |
| Chithriki et
al, 2004 | 76 | F | R | 20×15×16 | Y | Resection | CD34+,
Bcl-2+ | 11 months | (25) |
| Vennarecci et
al, 2005 | 65 | M | R | 30×28×14 | N | Resection | CD34+,
V+ | 30 months | (26) |
| Moser et al,
2005 | 73 | F | R | 35×20×15 | Y | Resection | CD34+,
V+, Bcl-2+ | N/A | (27) |
| Ji et al,
2006 | 42 | F | R | 6×5×5 | Y | Resection |
CD34+ | N/A | (28) |
| Lehmann et
al, 2006 | 63 | F | R | N/A | N | Resection |
CD34+ | 96 months | (7) |
| Nath et al,
2006 | 61 | F | R | 21×14.5×30 | N | Resection | CD34+,
V+ | 10 months | (29) |
| Terkivatan et
al, 2006 | 74 | M | L | 24×21×15 | N | Resection | CD34+,
CD99+, V+, Bcl-2+ | 12 months | (30) |
| Chan et al,
2007 | 70 | M | R | 27×24×12 | Y | Resection | CD34+,
CD99+, V+, Bcl-2+ | 9 months | (31) |
| Obuz et al,
2007 | 52 | M | L | 10×11×12 | N | Resection | CD34+,
V+ | 22 months | (32) |
| Perini et
al, 2008 | 40 | F | L | N/A | N | Resection | CD34+,
V+ | 49 months | (33) |
| Weitz et al,
2007 | N/A | N/A | N/A | N/A | N | Resection | N/A | N/A | (34) |
|
| N/A | N/A | N/A | N/A | N | Nil | N/A | N/A |
|
|
| N/A | N/A | N/A | N/A | N | Nil | N/A | N/A |
|
| Kandpal et
al, 2008 | 45 | F | R | N/A | N | Resection |
CD34+ | N/A | (35) |
| Fama et al,
2008 | 68 | M | R | N/A | Y | Resection | CD34+,
V+ | 25 months | (36) |
| Korkolis et
al, 2008 | 82 | F | L | 18×15×8 | N | Resection | CD34+,
V+, Bcl-2+, desmin+ | 21 months | (37) |
| Chen et al,
2008 | 71 | M | R | 8.7×5.5×8.5 | N | Resection | CD34+,
CD99+, Bcl-2+ | 9 months | (38) |
| El-Khouli et
al, 2008 | 68 | F | L, R | 15×10.5×13 | N | TACE | CD34+,
V+ | N/A | (39) |
| Hoshino et
al, 2009 | 30 | F | R | 6.7×4.5×4 | N | Nil | CD34+,
Bcl-2+ | 6 months | (40) |
| Novais et
al, 2010 | 34 | F | R | 25×23×13 | N | Resection | CD34+,
V+ | 24 months | (41) |
| Brochard et
al, 2010 | 54 | M | R | 17 | N | Resection | CD34+,
V+, desmin+, actin+ | 72 months | (42) |
| Haddad et
al, 2010 | 62 | M | L | N/A | N | Resection |
CD34+ | N/A | (43) |
|
| 45 | F | R | 7.4×5.9×5.4 | N | Resection | CD34+,
V+, Bcl-2+ | N/A |
|
| Park et al,
2011 | 51 | F | L | N/A | N | Resection | N/A | N/A | (44) |
| Peng et al,
2011 | 24 | F | R | 30×17×15 | N | Resection | CD34+,
V+, Bcl-2+ | Succumbed after 16
months | (45) |
| Sun et al,
2011 | 59 | M | L | 9×7×6 | N | Resection | CD34+,
CD99+, V+, Bcl-2+ | 24 months | (46) |
| Patra et al,
2012 | 34 | F | L | 14.5×10×8 | N | Resection | CD34+,
V+, Bcl-2+ | 48 months | (47) |
| Radunz et
al, 2012 | 85 | F | L | N/A | Y | Resection | CD34+,
Bcl-2+ | N/A | (48) |
| Belga et al,
2012 | 66 | F | R | N/A | N | Resection |
CD34+ | 30 months | (49) |
| Morris et
al, 2012 | 23 | F | R | 27× 23.5×4 | N | Resection | CD34+,
V+, Bcl-2+ | 10 months | (50) |
| Beyer et al,
2012 | 46 | M | RLig | 21×7 | N | HRT, chemo therapy,
resection |
CD34+ | 10 months | (51) |
| Soussan et
al, 2013 | 64 | M | L | N/A | N | Resection | CD34+,
Bcl-2+ | N/A | (52) |
| Liu et al,
2013 | 42 | M | L | 1.5×1×1 | N | Resection | CD34+,
Bcl-2+ | N/A | (53) |
| Jakob et al,
2013 | 62 | F | L | N/A | N | Resection | CD34+,
CD99+, Bcl-2+ | N/A | (54) |
| Debs et al,
2014 | 65 | M | L | N/A | N | Resection | CD34+,
CD99+, Bcl-2+ | 12 months | (55) |
|
| 87 | F | R | 14.6×12.3×17 | N | Nil | N/A | 10 months |
|
| Guray-Durak et
al, 2013 | 38 | F | L | 8×6×2 | N | Resection | CD34+,
CD99+, SMA+ | N/A | (56) |
| Vythianathan and
Jim, 2013 | 78 | M | L | 17×13 | N | Resection | CD34+,
CD99+, V+, Bcl-2+ | N/A | (57) |
| Song et al,
2014 | 49 | M | L, R | 7.6×5×4.8 | N | Resection | CD34+,
V+, Bcl-2+ | 3 months | (58) |
| Texeira et
al, 2014 | 68 | F | L | 7.5×6.5×5.5 | N | Resection | CD34+,
V+ | 28 months | (59) |
| Du et al,
2015 | 55 | F | L | 11×17×15 | Y | Resection | CD34+,
Bcl-2+ | 60 months | (60) |
| Beltran, 2015 | 58 | M | L | 15×9×6 | N | Resection | CD34+,
V+ | 36 months | (61) |
| Bejarano-Gonzalez
et al, 2015 | 79 | F | R | 15 | N | TACE,
resection | CD34+,
V+, Bcl-2+ | 31 months | (62) |
| Feng et al,
2015 | 51 | M | R | 2.3×0.3 | N | Resection | CD34+,
Bcl-2+ | 11 months | (63) |
|
| 49 | M | L | 8.7 | N | Resection | CD34+,
V+, Bcl-2+ | 17 months |
|
|
| 51 | F | R | 8.4 | N | Resection, adjuvant
chemotherapy | CD34+,
V+, Bcl-2+ | 31 months |
|
|
| 52 | F | R | 12 | N | Resection, MWA | CD34+,
V+ | 37 months |
|
| Silvanto et
al, 2015 | 65 | M | L | 18 | N | Resection | CD34+,
CD99+, Bcl-2+ | 16 months | (64) |
| Kueht et al,
2015 | 40 | M | L | 4.7×4×4 | N | Resection | CD34+,
CD99+, V+, Bcl-2+ | N/A | (65) |
| Maccio et
al, 2015 | 74 | F | R | 24×16 | N | Resection | CD34+,
V+, Bcl-2+, STAT6+ | Succumbed after 15
months | (66) |
|
| 80 | F | R | 19×15 | N | Chemotherapy | CD34+,
V+, Bcl-2+, STAT6+ | Succumbed after 4
months |
|
|
| 65 | M | R | 3×2 | N | Chemotherapy | CD34+,
V+, Bcl-2+, STAT6+ | Succumbed after 5
months |
|
| Makino et
al, 2015 | 55 | M | R | 8.6×6.3 | N | Resection | CD34+,
CD99+, Bcl-2+ | 11 months | (67) |
| Dey et al,
2016 | 56 | F | R | 23×22×10 | N | Resection | CD34+,
V+, Bcl-2+ | 6 months | (68) |
| Degnan et
al, 2016 | 39 | M | L | 20×18×15 | Y | Resection | CD34+,
V+, Bcl-2+, STAT6+ | 6 months | (69) |
| Macak et al,
2016 | 64 | N/A | R | 20×14.6×19 | Y | Resection,
TACE | NSE+,
CD34+, V+, Bcl-2+,
STAT6+ | N/A | (70) |
| Chen and Slater,
2017 | 61 | M | R | 15×11.5×7.5 | N | Resection | CD34+,
CD99+, Bcl-2+ | 74 months | (71) |
| Present case | 32 | F | L, R | 19×19.6×14 | N | Ex situ hepatectomy
and liver autotransplantation | CD34+,
CD117+, Caldesmon+, SMA+,
PR+ | 3 month | − |
Due to the lack of overt or specific symptoms,
patients with SFTs are usually diagnosed coincidentally during a
check-up or at a late stage when masses are large enough to produce
discomfort by invading or compressing adjacent structures. In the
present case, the patient had complained of repeated abdominal
distension for 3 years due to the compression of neighboring
structures by the lesion. With the exception of the presence of
non-islet cell tumor hypoglycemia syndrome observed in 13 cases
(9,16,17,19,22,25,28,31,36,48,60,69,70),
the results of laboratory tests for routine biochemical parameters,
including aminotransferases or tumor markers, appear normal or
slightly, non-specifically altered (33), as was the case with the present
patient. Although imaging tests can provide physical
characteristics of lesions for clinical analysis, including
location and size, the radiological features of an SFT lack
specificity, suggesting that none of the aforementioned tests are
conclusive.
The gold standard for diagnosing an SFT remains as
histopathological analysis combined with IHC examination.
Fine-needle aspiration cytology (FNAC) is commonly performed to
acquire lesion tissue for pathological diagnosis prior to surgery.
Nevertheless, FNAC may be misleading or inconclusive pertaining to
the diagnosis of an SFT (37).
Percutaneous liver biopsy may not provide the definitive diagnosis,
while increasing the risk of tumor growth and implantation
(53). Histopathological findings in
the present case were typical for an SFT: Diffusive proliferation
of spindle-shaped mesenchymal cells with oval-fusiform nuclei along
with fibrocollagenous stroma; no prominent cellular atypia,
infiltrative margins or other indicative evidence of malignancy;
and tumor cells of all reported cases with an SFT, including the
present case, were positive for CD34, which may differentiate an
SFT from other spindle cell neoplasms (12). CD99, B-cell lymphoma 2 or vimentin
have also shown to be positive in almost half the cases (14–19–21,24–27,29–33,36–48,50,52–71).
Radical surgical resection is the main treatment
approach. Among the reported cases listed in Table I, 85.2% (75/88) patients received
radical surgical resection and on average had ≥3 segments removed
owing to the size of the SFTs. Other treatments, such as
chemotherapy, which was adopted recently in two patients listed in
Table I, resulted in only 4–5 months
survival following treatment. Therefore, Makino et al
(67) suggested that due to the
unclear effects of other treatments, including chemotherapy and
radiotherapy, hepatic resection with clear margins is highly
recommended in patients with large SFTs, considering the risk of
urgent symptoms and the potential for malignancy. Compared with the
previously reported cases, the tumor reported in the present study
was difficult to resect using the conventional approach due to the
advanced extent of the lesion therefore, novel techniques may be
required.
Even with the vast improvement of surgical
techniques, hepatic lesions abutting hepatic veins or the
confluence of the IVC, as well as large centrally located lesions,
fail to be managed by conventional surgery. In such cases, liver
transplantation may be indicated (26). However, no liver transplantations have
been performed to date for treating SFTs. In the present case, the
second hepatic portal was compressed by the neoplasm, causing
obstruction of the outflow tract and liver congestion. Full
exposure of the second hepatic portal was achieved only after the
sternum was removed. The vascular reconstruction and extended
hepatic resection were extremely difficult to achieve by in
situ surgical resection. Given the young age of the patient,
the alternative of ex situ hepatectomy and liver
autotransplantation was implemented. The surgery was performed
successfully and the patient recovered without further
complications. To the best of our knowledge, this is the first case
report describing the adoption of ex situ hepatectomy and
liver autotransplantation to treat a liver SFT, otherwise
unresectable by standard surgery.
The ex situ hepatectomy and liver
autotransplantation was initially performed by Pichlmayr et
al (4) in 1988 for treating liver
tumors involving major vascular structures. The procedure has
progressed and developed over the past 30 years, and has been
successfully applied for the treatment of several types of lesions,
including hepatocellular carcinoma (72,73), hilar
cholangiocarcinoma (74), giant
hepatic hemangiomas (75) and
metastatic colon cancer (72,73,76). The
reported cases are listed in Table
II. The median follow-up and survival times for these cases
were 13 and 25 months, respectively. In addition, 63.6% (28/44)
patients receiving ex situ hepatectomy and liver
autotransplantation were <55 years old, as was the patient
reported in the present case. The common indications for this
method are major vein involvement and extensive resection area,
both of which were noted in the present case. Compared with
conventional liver resection, ex situ hepatectomy and liver
autotransplantation markedly improve the rate of successful
resection and clear margins of the otherwise unresectable lesions.
There is no long waiting time for a suitable allograft, as an
autologous liver is implanted. In addition, the total cost is lower
and no post-operative immunosuppressants are required for patients
receiving ex situ hepatectomy and liver autotransplantation,
compared with an allograft liver transplantation (5).
 | Table II.Data on reported ex situ liver
autotransplantation cases. |
Table II.
Data on reported ex situ liver
autotransplantation cases.
| First author,
year | Age, years | Sex | Diagnosis | Characteristics of
lesion/contraindication to traditional resection | Follow-up | (Refs.) |
|---|
| Pichlmayr et
al, 1988 | 40 | N/A | Metastatic
leiomyosarcoma | Bilateral liver
metastases | N/A | (4) |
| Yagyu et al,
1994 | N/A | N/A | Intrahepatic
cholangiocarcinoma | Involved the
confluence of the 3 main hepatic veins and the retrohepatic
IVC | No recurrence at 8
months | (78) |
| Hemming and
Cattral, 1999 | 50 | M | Colorectal liver
metastases | A single lesion in
the caudate lobe involved the origin of all 3 hepatic veins | N/A | (76) |
| Lodge et al,
2000 | 55 | F | Colorectal liver
metastases | Involvement of IVC
and segments 1, 2, 3, 4a and 8 | Post-operative
chemotherapy, 30 months survival | (79) |
|
|
|
| Colorectal liver
metastases | Involvement of
segments 2 and 4–8 | Succumbed from
complications of renal and respiratory failure on post-operative
day 15 |
|
|
|
|
| Colorectal liver
metastases | 17×15 cm mass
involving segments 1, 2 and 4–8 | Alive at 5
months |
|
|
|
|
| Colorectal liver
metastases | 17×13 cm mass
involving segments 1, 2, 4–8 | Alive at 5
months |
|
| Oldhafer et
al, 2000 | 40 | F | Metastatic
leiomyosarcoma | Involvement of
segments 2, 3 and 5–8 | Succumbed at 36
months due to tumor recurrence | (72) |
|
| 46 | F | Metastatic colon
cancer | Involvement of
segments 5–7 | Succumbed at 13
months due to tumor recurrence |
|
|
| 52 | M | Klatskin's
tumor | Required right
hepatic trisegmentectomy | Succumbed at 13
months due to tumor recurrence |
|
|
| 58 | M | Metastatic colon
cancer | Metastatic colon
cancer infiltrating the IVC | Succumbed at 44
days due to sepsis and hepatic insufficiency |
|
|
| 30 | F | Focal nodular
hyperplasia | Large FNH in
segment 4 with compression | Alive at 9 years of
the IVC |
|
|
| 57 | M | Metastatic colon
cancer | Infiltration of RHV
requiring extended left hemihepatectomy | Succumbed at 21
months due to tumor recurrence |
|
|
| 48 | F | Klatskin's
tumor | Klatskin's tumor
requiring extended left hemihepatectomy | Succumbed on
post-operative day 50 due to sepsis |
|
|
| 62 | M | Klatskin's
tumor | Tumor invading left
and right portal veins | Succumbed on
post-operative day 113 due to sepsis |
|
|
| 55 | F | Klatskin's
tumor | Klatskin's tumor
requiring right hepatic trisegmentectomy | Succumbed on
post-operative day 35 due to sepsis |
|
|
| 35 | M | HCC | HCC requiring
resection of segments 1–4 | Lost to follow-up
and wedge 6 |
|
|
| 43 | M | HCC | HCC requiring
resection of segments 5 and 6 | Succumbed at 25
months due to tumor recurrence |
|
|
| 67 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1-4b and wedge 6–7 | Succumbed at 2
months due to intracerebral bleed |
|
|
| 53 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1 and 4–8 | Succumbed at 2
months due to sepsis |
|
|
| 54 | F | Focal nodular
hyperplasia | Lesions requiring
resection of segments 1, 4, 5 and 7 | Alive at 5
years |
|
|
| 55 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1 and 4 | Succumbed at 15
months due to tumor recurrence |
|
|
| 55 | F | Metastatic
leiomyosarcoma | Lesion requiring
resection of segments 2-4b and wedge 5 | Succumbed at 13
months due to tumor recurrence |
|
|
| 52 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1, 5 and 8 | Succumbed at 36
months due to tumor recurrence |
|
|
| 40 | F | Metastatic
leiomyosarcoma | Lesions requiring
resection of segments 1, 4–8 and wedge 2–3 | Succumbed at 18
months due to tumor recurrence |
|
|
| 52 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1 and partial 4 | Succumbed on
post-operative day 14 due to pneumonia |
|
|
| 52 | M |
Cholangiocarcinoma | Lesion requiring
resection of segments 1–5 and wedge 8 | Succumbed on
post-operative day 41 due to sepsis |
|
|
| 39 | M | HCC | Lesion requiring
resection of segments 1–4 and partial 8 | Succumbed on
post-operative day 23 due to sepsis |
|
|
| 71 | M | Metastatic colon
cancer | Lesions requiring
resection of segments 1 and 5–8 | Alive after 13
months |
|
| Chui et al,
2003 | 26 | F |
Cholangiocarcinoma | 2.3-cm hilar mass
involving the portal vein confluence and right hepatic artery | Alive with no
recurrence on MRI at 17 months | (80) |
| Gruttadauria et
al, 2005 | 41 | M |
Cholangiocarcinoma | 14×12 cm mass
encompassing segments 4a, 4b and 5 with RHA involvement | Recurrence at 10
months and 13 months, receiving radiation; still alive at 23
months | (81) |
|
| 58 | M | Leiomyosarcoma of
the IVC | Third attempt at
resection | Discharged on
post-operative day 10; long-term outcome N/A |
|
| Ikegami et
al, 2007 | 39 | F | Giant hepatic
hemangiomas | 4 large hemangiomas
in segments 1–3, caudal 4 and 6–7 | Normal liver
function with a regenerated liver graft at 8 months | (75) |
| Sugimachi et
al, 2010 | 17 | F | HCC | 18×12 cm lesion
involving IVC, RHV and first left branch of portal vein | Alive 28 months
after resection; no post-operative chemotherapy | (82) |
| Gringeri et
al, 2012 | 38 | F | Hepatic metastasis
from | 2.5 cm lesion in
left lobe and 2.7-cm lesion in pancreatoblastoma | Alive after 8
months right lobe involving MHV and RHV | (83) |
| Zhang et al,
2012 | 60 | F | Hepatic
hemangiomas | Lesions requiring
resection of segments 4–8 | Alive at 22
months | (74) |
|
| 64 | M |
Cholangiocarcinoma | Lesions requiring
resection of segments 1–4 | Alive at 17
months |
|
|
| 55 | M |
Cholangiocarcinoma | Lesions requiring
resection of segments 1, 5, 7 and 8 | Succumbed on
post-operative day 1 due to liver and renal failure |
|
| Wen et al,
2013 | 67 | M | HCC | 18×12 cm lesion at
the confluence of the LHV, MHV and IVC | Alive at 28 months,
no recurrence | (73) |
|
| 71 | M | HCC | 18×13 cm lesion at
the confluence of the V7/RHV into the IVC | Alive at 26 months,
recurrence treated with RFA |
|
|
| 60 | M | HCC | 5.8×6.8 cm lesion
centrally located, involving the RHV and PV | Alive at 23 months,
recurrence treated with TACE |
|
| Baker et al,
2015 | 66 | F | Extensive
cholangiocarcinoma | Lesions involving
all 3 hepatic veins | Alive at 3
months | (6) |
| Vicente et
al, 2017 | 51 | M |
Cholangiocarcinoma | Lesions involving
retrohepatic vena cava together with the 3 hepatic veins | Alive at 36
months | (77) |
| Present case | 32 | F | Solitary fibrous
tumor | Lesions involving
the right and left hepatic arteries | Alive at 3
month | N/A |
The successful establishment of venous reflux and
hypothermic perfusion of the isolated liver are essential for
securing a successful surgery and a good prognosis (77). As the liver resection and trimming
surgery are time-consuming, the prolonged anhepatic phase can lead
to hemodynamic or internal environmental disturbances. Therefore,
in the present case, an artificial blood vessel was used to
establish an end-to-side portocaval shunt in order to maintain the
venous return of the intestinal tract and lower limbs during the
anhepatic period. Compared with autologous vessels, including the
great saphenous vein, use of an artificial blood vessel minimizes
the invasiveness of the procedure for the patient. As the shunt is
temporary, the potential tissue rejection is of no concern.
The commonly encountered complications of ex
situ hepatectomy and liver autotransplantation include bile
leakage, bleeding, pulmonary infection, pneumonedema, hydrothorax
and renal insufficiency, with a total incidence of 58.1%. The
overall mortality rate within 90 days after surgery is 19.5%
(5). In the present study, the
patient presented with moderate right pleural effusion following
surgery, and recovered fully following pleurocentesis and drainage.
No other severe complications were encountered up to 3 months after
surgery.
In conclusion, this is the first report that
describes the successful adoption of ex situ hepatectomy and
liver autotransplantation for treating an otherwise unresectable
giant SFT. The uneventful surgical course and post-operative period
suggested that this procedure was suitable for managing an
unresectable liver neoplasm. Furthermore, the artificial blood
vessels may provide a reliable source for establishing an
end-to-side portocaval shunt during the anhepatic period.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81372626 and 81572975), the
Key Research and Development Project of Science and Technology
Department of Zhejiang, China (grant no. 2015C03053), and the
Zhejiang Provincial Program for the Cultivation of High-level
Innovative Health Talents (2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and WW conceived and designed the study. ZS, YD,
YJ, QZ, ZL, JX, JD, SY and WW performed the surgery and provided
the patient care. QZ, ZL, JX and JD performed the literature review
and collected the substantial data. ZS and YJ analyzed the data. ZS
and YD wrote and revised the manuscript. ZS, YD and YJ organized
the data and figures. SY and WW revised the final version of
manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital, School of Medicine, Zhejiang
University.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salas S, Resseguier N, Blay JY, Le Cesne
A, Italiano A, Chevreau C, Rosset P, Isambert N, Soulie P, Cupissol
D, et al: Prediction of local and metastatic recurrence in solitary
fibrous tumor: Construction of a risk calculator in a multicenter
cohort from the French Sarcoma Group (FSG) database. Ann Oncol.
28:1979–1987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Amico FE, Ruffolo C, Romano M, DI
Domenico M, Sbaraglia M, Dei Tos AP, Garofalo T, Giordano A, Bassi
I and Massani M: Rare neoplasm mimicking neuoroendocrine pancreatic
tumor: A case report of solitary fibrous tumor with review of the
literature. Anticancer Res. 37:3093–3097. 2017.PubMed/NCBI
|
|
3
|
Robinson LA: Solitary fibrous tumor of the
pleura. Cancer Control. 13:264–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pichlmayr R, Bretschneider HJ, Kirchner E,
Ringe B, Lamesch P, Gubernatis G, Hauss J, Niehaus KJ and
Kaukemüller J: Ex situ operation on the liver. A new possibility in
liver surgery. Langenbecks Arch Chir. 373:122–126. 1988.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuxun T, Aini A, Li YP, Apaer S, Zhang H,
Li T, Aji T, Yimiti Y, Zhao JM, Shao YM and Wen H: Systematic
review of feasibility, safety and efficacy of ex situ liver
resection and autotransplantation. Zhonghua Yi Xue Za Zhi.
96:2251–2257. 2016.(In Chinese). PubMed/NCBI
|
|
6
|
Baker MA, Maley WR, Needleman L and Doria
C: Ex vivo resection of hepatic neoplasia and autotransplantation:
A case report and review of the literature. J Gastrointest Surg.
19:1169–1176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lehmann C, Mourra N, Tubiana JM and Arrivé
L: Solitary fibrous tumor of the liver. J Radiol. 87:139–142.
2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edmondson HA: Tumors of the liver and
intrahepatic bile ductsAtlas of Tumor Pathology, Fascicle 25.
Washington: Armed Forces Institute of Pathology; 1958
|
|
9
|
Nevius DB and Friedman NB: Mesotheliomas
and extraovarian thecomas with hypoglycaemic and nephritic
syndromes. Cancer. 12:1263–1269. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishak KG: Mesenchymal tumors of the
liverHepatocellular Carcinoma. Okusa K and Peters RL: John Wiley
and Sons; New York, USA: pp. 247–307. 1976
|
|
11
|
Kim H and Damjanov I: Localised fibrous
mesothelioma of the liver. Report of a giant tumour studied by
light and electron microscopy. Cancer. 52:1662–1665. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kottke-Marchant K, Hart WR and Broughan T:
Localized fibrous tumor (localized fibrous mesothelioma) of the
liver. Cancer. 64:1096–1102. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasano Y, Tanimura H, Tabuse K, Nagai Y,
Mori K and Minami K: Giant fibrous mesothelioma of the liver. Am J
Gastroenterol. 86:379–380. 1991.PubMed/NCBI
|
|
14
|
Barnoud R, Arvieux C, Pasquier D, Pasquier
B and Letoublon C: Solitary fibrous tumour of the liver with CD34
expression. Histopathology. 28:551–554. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine TS and Rose DS: Solitary fibrous
tumour of the liver. Histopathology. 30:396–397. 1997.PubMed/NCBI
|
|
16
|
Guglielmi A, Frameglia M, Iuzzolino P,
Iuzzolino P, Martignoni G, De Manzoni G, Laterza E, Veraldi GF and
Girlanda R: Solitary fibrous tumor of the liver with CD 34
positivity and hypoglycaemia. J Hepatobiliary Pancreat Surg.
5:212–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lecesne R, Drouillard J, Le Bail B, Saric
J, Balabaud C and Laurent F: Localised fibrous tumor of the liver:
Imaging findings. Eur Radiol. 8:36–38. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bejarano PA, Blanco R and Hanto DW:
Solitary fibrous tumor of the liver. A case report, river of the
literature and differential diagnosis of spindle cell lesions of
the liver. Int J Surg Pathol. 6:93–100. 1998. View Article : Google Scholar
|
|
19
|
Moran CA, Ishak KG and Goodman ZD:
Solitary fibrous tumor of the liver: A clinicopathologic and
immunohistochemical study of nine cases. Ann Diagn Pathol. 2:19–24.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuksbrumer MS, Klaimstra D and Panicek DM:
Solitary fibrous tumor of the liver: Imaging findings. AJR Am J
Roentgenol. 175:1683–1687. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yilmaz S, Kirimlioglu V, Ertas E,
Hilmioglu F, Yildirim B, Katz D and Mizrak B: Giant solitary
fibrous tumor of the liver with metastasis to the skeletal system
successfully treated with trisegmentectomy. Dig Dis Sci.
45:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin YT, Lo GH, Lai KH, Tsai CC, Pan HB,
Tseng HH and Lo YS: Solitary fibrous tumor of the liver. Zhonghua
Yi Xue Za Zhi (Taipei). 64:305–309. 2001.PubMed/NCBI
|
|
23
|
Gold JS, Antonescu CR, Hajdu C, Ferrone
CR, Hussain M, Lewis JJ, Brennan MF and Coit DG: Clinicopathologic
correlates of solitary fibrous tumors. Cancer. 94:1057–1068. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neeff H, Obermaier R, Technau-Ihling K,
Werner M, Kurtz C, Imdahl A and Hopt UT: Solitary fibrous tumour of
the liver: Case report and review of the literature. Langenbecks
Arch Surg. 389:293–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chithriki M, Jaibaji M and Vandermolen R:
Solitary fibrous tumor of the liver with presening symptoms of
hypoglycemic coma. Am Surg. 70:291–293. 2004.PubMed/NCBI
|
|
26
|
Vennarecci G, Ettorre GM, Giovannelli L,
Del Nonno F, Perracchio L, Visca P, Corazza V, Vidiri A, Visco G
and Santoro E: Solitary fibrous tumor of the liver. J Hepatobiliary
Pancreat Surg. 12:341–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moser T, Nogueira TS, Neuville A, Riehm S,
Averous G, Weber JC and Veillon F: Delayed enhancement pattern in a
localised fibrous tumor of the liver. AJR Am J Roentgenol.
184:1578–1580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji Y, Fan J, Xu Y, Zhou J, Zeng HY and Tan
YS: Solitary fibrous tumor of the liver. Hepatobiliary Pancreat Dis
Int. 5:151–153. 2006.PubMed/NCBI
|
|
29
|
Nath DS, Rutzick AD and Sielaff TD:
Solitary fibrous tumor of the liver. AJR Am J Roentgenol.
187:187–190. 2006. View Article : Google Scholar
|
|
30
|
Terkivatan T, Kliffen M, de Wilt JH, van
Geel AN, Eggermont AM and Verhoef C: Giant solitary fibrous tumour
of the liver. World J Surg Oncol. 4:812006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan G, Horton PJ, Thyssen S, Lamarche M,
Nahal A, Hill DJ, Marliss EB and Metrakos P: Malignant
transformation of a solitary fibrous tumor of the liver and
intractable hypoglycaemia. J Hepatobiliary Pancreat Surg.
14:595–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Obuz F, Secil M, Sagol O, Karademir S and
Topalak O: Ultrasonography and magnetic resonance imaging findings
of solitary fibrous tumor of the liver. Tumori. 93:100–102. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perini MV, Herman P, D'Albuquerque LA and
Saad WA: Solitary fibrous tumor of the liver: Report of a rare case
and review of the literature. Int J Surg. 6:396–399. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weitz J, Klimstra DS, Cymes K, Jarnagin
WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH
and Dematteo RP: Management of primary liver sarcomas. Cancer.
109:1391–1396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kandpal H, Sharma R, Gupta SD and Kumar A:
Solitary fibrous tumour of the liver: A rare imaging diagnosis
using MRI and diffusion-weighted imaging. Br J Radiol.
81:e282–e286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Famà F, Le Bouc Y, Barrande G, Villeneuve
A, Berry MG, Pidoto RR and Marc Saint O: Solitary fibrous tumour of
the liver with IGF-II-related hypoglycaemia. A case report.
Langenbecks Arch Surg. 393:611–616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korkolis DP, Apostolaki K, Aggeli C,
Plataniotis G, Gontikakis E, Volanaki D, Sebastiadou M,
Dimitroulopoulos D, Xinopoulos D, Zografos GN and Vassilopoulos PP:
Solitary fibrous tumor of the liver expressing CD34 and vimentin: A
case report. World J Gastroenterol. 14:6261–6264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JJ, Ong SL, Richards C, Garcea G,
Pollard C, Berry D and Dennison A: Inaccuracy of fine-needle biopsy
in the diagnosis of solitary fibrous tumour of the liver. Asian J
Surg. 31:195–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El-Khouli RH, Geschwind JF, Bluemke DA and
Kamel IR: Solitary fibrous tumor of the liver: Magnetic resonance
imaging evaluation and treatment with transarterial
chemoembolisation. J Comput Assist Tomogr. 32:769–771. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hoshino M, Nakajima S, Futagawa Y, Fujioka
S, Okamoto T and Yanaga K: A solitary fibrous tumor originating
from the liver surface. Clin J Gastroenterol. 2:320–324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Novais P, Robles-Medranda C, Pannain VL,
Barbosa D, Biccas B and Fogaca H: Solitary fibrous liver tumor: Is
surgical approach the best option? J Gastrointestin Liver Dis.
19:81–84. 2010.PubMed/NCBI
|
|
42
|
Brochard C, Michalak S, Aubé C, Singeorzan
C, Fournier HD, Laccourreye L, Calès P and Boursier J: A not so
solitary fibrous tumor of the liver. Gastroenterol Clin Biol.
34:716–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haddad A, Karras R, Fraiman M and Mackey
R: Solitary fibrous tumor of the liver. Am Surg. 76:E78–E79.
2010.PubMed/NCBI
|
|
44
|
Park HS, Kim YK, Cho BH and Moon WS:
Pedunculated hepatic mass. Liver Int. 31:5412011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng L, Liu Y, Ai Y, Liu Z, He Y and Liu
Q: Skull base metastases from a malignant solitary fibrous tumor of
the liver. A case report and literature review. Diagn Pathol.
6:1272011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun K, Lu JJ, Teng XD, Ying LX and Wei JF:
Solitary fibrous tumor of the liver: A case report. World J Surg
Oncol. 9:372011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Patra S, Vij M, Venugopal K and Rela M:
Hepatic solitary fibrous tumor: A report of a rare case. Indian J
Pathol Microbiol. 55:236–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Radunz S, Baba HA and Sotiropoulos GC:
Large tumor of the liver and hypoglycemic shock in an 85-year-old
patient. Gastroenterology. 142:e10–e11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Belga S, Ferreira S and Lemos MM: A rare
tumor of the liver with a sudden presentation. Gastroenterology.
143:e14–e15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morris R, McIntosh D, Helling T and Martin
JN Jr: Solid fibrous tumor of the liver: A case in pregnancy. J
Matern Fetal Neonatal Med. 25:866–868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beyer L, Delpero JR, Chetaille B, Sarran
A, Perrot D, Moureau-Zabotto L, Guiramand J and Bertucci F:
Solitary fibrous tumor in the round ligament of the liver: A
fortunate intraoperative discovery. Case Rep Oncol. 5:187–194.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Soussan M, Felden A, Cyrta J, Morère JF,
Douard R and Wind P: Case 198: Solitary fibrous tumor of the liver.
Radiology. 269:304–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Q, Liu J, Chen W, Mao S and Guo Y:
Primary solitary fibrous tumors of liver: A case report and
literature review. Diagn Pathol. 8:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jakob M, Schneider M, Hoeller I, Laffer U
and Kaderli R: Malignant solitary fibrous tumor involving the
liver. World J Gastroenterol. 19:3354–3357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Debs T, Kassir R, Amor IB, Martini F,
Iannelli A and Gugenheim J: Solitary fibrous tumor of the liver:
Report of two cases and review of the literature. Int J Surg.
12:1291–1294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Durak Güray M, Sağol Ö, Tuna B, Ertener Ö,
Ünek T, Karademır S and Dıcle O: Cystic solitary fibrous tumor of
the liver: A case report. Turk Patoloji Derg. 29:217–220.
2013.PubMed/NCBI
|
|
57
|
Vythianathan M and Yong J: A rare primary
malignant solitary fibrous tumour of the liver. Pathology.
45:86–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Song L, Zhang W and Zhang Y: (18)F-FDG
PET/CT imaging of malignant hepatic solitary fibrous tumor. Clin
Nucl Med. 39:662–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Teixeira F Jr, de Freitas Perina AL, de
Oliveira Mendes G, de Andrade AB and da Costa FP: Fibrous solitary
tumour of the liver. J Gastrointest Canc. 45 Suppl 1:S216–S217.
2014. View Article : Google Scholar
|
|
60
|
Du EH, Walshe TM and Buckley AR: Recurring
rare liver tumor presenting with hypoglycemia. Gastroenteroloy.
148:e11–e13. 2015. View Article : Google Scholar
|
|
61
|
Beltrán MA: Solitary fibrous tumor of the
liver: A review of the current knowledge and report of a new case.
J Gastrointest Canc. 46:333–342. 2015. View Article : Google Scholar
|
|
62
|
Bejarano-Gonzalez N, Garcia-Borobia FJ,
Romaguera-Monzonis A, García-Monforte N, Falcó-Fagés J, Bella-Cueto
MR and Navarro-Soto S: Soliary fibrous tumor of the liver. Case
report and review of the literature. Rev Esp Enferm Dig.
107:633–639. 2015.PubMed/NCBI
|
|
63
|
Feng LH, Dong H, Zhu YY and Cong WM: An
update on primary hepatic solitary fibrous tumor: An examination of
the clinical and pathological features of four case studies and a
literature review. Pathol Res Pract. 211:911–917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Silvanto A, Karanjia ND and Bagwan IN:
Primary hepatic solitary fibrous tumor with histologically benign
and malignant areas. Hepatobiliary Pancreat Dis Int. 14:665–668.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kueht M, Masand P, Rana A, Cotton R and
Goss J: Concurrent hepatic hemangioma and solitary fibrous tumor:
Diagnosis and management. J Surg Case Rep. 2015:rjv0892015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maccio L, Bonetti LR, Siopis E and
Palmiere C: Malignant metastasizing solitary fibrous tumors of the
liver: A report of three cases. Pol J Pathol. 66:72–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Makino Y, Miyazaki M, Shigekawa M, Ezaki
H, Sakamori R, Yakushijin T, Ohkawa K, Kato M, Akasaka T, Shinzaki
S, et al: Solitary fibrous tumor of the liver from development to
resection. Intern Med. 54:765–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dey B, Gochhait D, Kaushal G, Barwad A and
Pottakkat B: Solitary fibrous tumor of the liver: A rare tumor in a
rarer location. Rare Tumors. 8:64032016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Degnan AJ, Lee KK, Minervini MI and
Borhani AA: Metastatic extrapleural malignant solitary fibrous
tumor presenting with hypoglycemia (Doege-Potter syndrome). Radiol
Case Rep. 12:113–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mačák J, Buzrla P, Dvořáčková J, Prokop P
and Jalůvka F: Hypoglycemia in a solitary fibrous tumor of the
liver. Cesk Patol. 52:41–44. 2016.(In Czech). PubMed/NCBI
|
|
71
|
Chen N and Slater K: Solitary fibrous
tumour of the liver-report on metastasis and local recurrence of a
malignant case and review of literature. World J Surg Oncol.
15:272017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Oldhafer KJ, Lang H, Schlitt HJ, Hauss J,
Raab R, Klempnauer J and Pichlmayr R: Long-term experience after ex
situ liver surgery. Surgery. 127:520–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wen PH, Lin KH, Chen YL, Hsieh CE, Ko CJ
and Kuo SJ: Extracorporeal hepatic resection and
autotransplantation using temporary portocaval shunt provides an
improved solution for conventionally unresectable HCC. Dig Dis Sci.
58:3637–3640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang KM, Hu XW, Dong JH, Hong ZX, Wang
ZH, Li GH, Qi RZ, Duan WD and Zhang SG: Ex-situ liver surgery
without veno-venous bypass. World J Gastroenterol. 18:7290–7295.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ikegami T, Soejima Y, Taketomi A,
Kayashima H, Sanefuji K, Yoshizumi T, Harada N, Yamashita Y and
Maehara Y: Extracorporeal hepatic resection for unresectable giant
hepatic hemangiomas. Liver Transpl. 14:115–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hemming AW and Cattral MS: Ex vivo liver
resection with replacement of the inferior vena cava and hepatic
vein replacement by transposition of the portal vein. J Am Coll
Surg. 189:523–526. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vicente E, Quijano Y, Ielpo B, Duran H,
Diaz E, Fabra I, Malavé L, Ferri V, Lazzaro S, Kalivaci D and
Caruso R: Ex Situ Hepatectomy and Liver Autotransplantation for
Cholangiocarcinoma. Ann Surg Oncol. 24:39902017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yagyu T, Shimizu R, Nishida M, Nakashima
K, Uchiyama T and Suzuki T: Reconstruction of the hepatic vein to
the prosthetic inferior vena cava in right extended hemihepatectomy
with ex situ procedure. Surgery. 115:740–744. 1994.PubMed/NCBI
|
|
79
|
Lodge JP, Ammori BJ, Prasad KR and Bellamy
MC: Ex vivo and in situ resection of inferior vena cava with
hepatectomy for colorectal metastases. Ann Surg. 231:471–479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chui AK, Rao AR, Wong J, Mann D, Leung KF
and Lau WY: Ex Situ Ex Vivo liver resection, partial liver
autotransplantation for advanced Hilar cholangiocarcinoma: A case
report. Transplant Proc. 35:402–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gruttadauria S, Marsh JW, Bartlett DL,
Gridelli B and Marcos A: Ex Situ resection techniques and liver
autotransplantation: Last resource for otherwise unresectable
malignancy. Dig Dis Sci. 50:1829–1835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sugimachi K, Shirabe K, Taketomi A,
Soejima Y, Yoshizumi T, Yamashita Y, Umeda K, Morita K and Maehara
Y: Successful curative extracorporeal hepatic resection for
far-advanced hepatocellular carcinoma in an adolescent patient.
Liver Transpl. 16:685–687. 2010.PubMed/NCBI
|
|
83
|
Gringeri E, Polacco M, D'Amico FE, Bassi
D, Boetto R, Tuci F, Bonsignore P, Noaro G, D'Amico F, Vitale A, et
al: Liver autotransplantation for the treatment of unresectable
hepatic metastasis: An uncommon indication-a case report.
Transplant Proc. 44:1930–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|