Introduction
The ubiquitination pathway is an important way to
regulate protein levels in eukaryotic cells and serves an important
function in the post-translational modification of proteins
(1,2).
It is well-documented that the ubiquitination of a number of
proteins can be reversed by deubiquitinases (DUBs), thereby
affecting cell viability, signal transduction, and a number of
physiological and pathological processes (3). In total, ~100 DUBs of five associated
classes have been identified in the human genome (4). The human ubiquitin-specific protease 10
(USP10), also known as UBPO and located on chromosome 16q24.1,
belongs to the DUB family (5). An
increasing number of studies into the USP10 protein and its
tumor-associated molecular mechanism have been performed. It has
been identified that USP10 is associated with the deubiquitination
of certain proteins, including tumor protein p53, T-box protein 21,
proliferating-cell nuclear antigen (PCNA), Beclin1, sirtuin 6
(SIRT6) and inhibitor of nuclear factor κB kinase subunit γ
proteins (6–10), thus participating in cell
proliferation, differentiation and autophagy.
It has been identified that USP10 protein expression
was significantly decreased in human gastric cancer tissues and
cell lines compared with that in the normal gastric mucosa and
immortalized epithelial cell lines, which indicated that USP10 is a
tumor suppressor gene involved in the tumorigenesis of gastric
cancer (11). Furthermore, USP10
protein expression was negatively associated with the depth of
invasion, lymph node metastasis and Tumor-Node-Metastasis (TNM)
staging (12) of gastric carcinoma.
Survival analysis demonstrated that USP10 may serve as a novel
prognostic indicator predicting the outcome of gastric carcinoma
(11). It has been reported that
knockdown of USP10 in lung cancer cells causes increased cell
survival and decreased apoptosis upon treatment with a
DNA-methylating and antimetabolite agent (13). Thereafter, the aforementioned
phenotypes may be rescued by ectopic expression of MutS homolog 2
(MSH2). A recent study demonstrated that 50% (9/18) of patients
with non-small cell lung cancer (NSCLC) exhibited decreased
expression of USP10 in tumor tissues compared with that in the
respective adjacent normal lung tissues (14). However, the clinical sample size was
not large and the functional role of USP10 in NSCLC remains
unresolved. Therefore, it is necessary to use a large cohort of
patients with NSCLC to validate whether USP10 serves critical
functions in the tumorigenesis and progression of NSCLC.
MSH2, a crucial element of the highly conserved DNA
mismatch repair system, maintains genetic integrity by correcting
DNA replication errors (15). The
function of MSH2 is to recognize DNA mismatches and then assist in
recruiting DNA repair proteins to the mismatched site. A previous
study indicated that MSH2 is a promising marker of the benefit of
adjuvant cisplatin-based chemotherapy for NSCLC (16). Inhibition of protein kinase B activity
and MSH2 expression increased the tamoxifen-mediated cytotoxicity
in lung cancer A549 and H1703 cells (17). A previous study indicated that USP10
interacts with MSH2, and the major region of MSH2 responsible for
the interaction with USP10 is located in the N-terminal region,
whereas for USP10, the major region is a C-terminal hydrolase
domain, which interacts with MSH2 (13). Further study indicated that
USP10-mediated deubiquitination of MSH2 may partially account for
MSH2 stability. Furthermore, the researchers identified that the
protein level of MSH2 is positively associated with the USP10
protein level in a panel of lung cancer cell lines (17). However, whether the protein level of
MSH2 is positively correlated with the USP10 protein level in lung
cancer tissue samples also requires clarification. Therefore, the
aim of the present study was to investigate the potential
correlation between the USP10 and MSH2 proteins in NSCLC tissues.
In addition, USP10 expression levels in NSCLC and normal tissues
were investigated to determine whether USP10 is associated with
tumorigenesis and progression of NSCLC. Furthermore, the
association between USP10 or MSH2 expression and
clinicopathological features as well as prognosis was also
evaluated in patients with NSCLC.
Materials and methods
Tissue sample and data collection
A total of 148 patients with NSCLC (101 men and 47
women; age range, 40–76 years; mean age, 60.0±8.2 years) were
included in the present study. All cases were identified through
chest surgery and the Pathology Department (Remin Hospital of Wuhan
University, Wuhan, China), as well as through cancer registries at
Renmin Hospital of Wuhan University, between August 2013 and
September 2014. Following provision of written informed consent,
demographic and clinicopathological data (including age, sex, tumor
size, tumor differentiation and TNM stage) were collected at the
Renmin Hospital of Wuhan University, using a standard
interviewer-administered questionnaire and/or medical records. In
the present study, the TNM stage was confirmed according to the
Union for International Cancer Control (UICC) classification (8th
edition) (12). A total of 148
formalin-fixed paraffin-embedded NSCLC tumor tissues (54 cases of
squamous cell carcinoma, 82 cases of adenocarcinoma, 4 cases of
adenosquamous carcinoma, 3 cases of sarcomatoid carcinoma and 5
cases of large cell carcinoma) and 139 non-cancerous tissues (45
cases of bronchial epithelium and 94 cases of alveolar epithelium)
were selected. The tissue microarrays were prepared by Wuhan Iwill
Biological Technology Co., Ltd. (Wuhan, China).
For the survival analysis, a total of 56 patients,
who received the same surgical therapy excluding preoperative
chemotherapy or radiotherapy, were followed up. There were 26 cases
of squamous cell carcinoma, 27 cases of adenocarcinoma, 2 cases of
adenosquamous carcinoma and 1 case of large cell carcinoma. The
last follow-up day was set for March 2018, and the survival status
was confirmed by means of clinical records and patient or family
contact. The duration of overall survival was defined as extending
from the date of surgical treatment to the date of mortality or
last known date alive. The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University.
Immunohistochemistry (IHC)
IHC staining was performed on formalin-fixed
paraffin-embedded sections (4 µm) according to a standard protocol.
Briefly, sections were deparaffinized with xylene twice for 10 min
and rehydrated with 100% ethanol twice for 5 min, 95% ethanol twice
for 2 min and 85% ethanol for 2 min, then washed in deionized
H2O for 1 min at room temperature with stirring.
Subsequently, sections were treated with 3%
H2O2 at room temperature for 10 min and
subjected to antigen retrieval by citrate buffer (pH 6.0) at 98°C
for 15 min. Following blocking with 5% bovine serum albumin for 20
min at room temperature, the sections were incubated at 4°C
overnight with anti-USP10 (1:250; cat. no. ab109219; Abcam,
Cambridge, UK) and anti-MSH2, (cat. no. ZA-0622; ready-to-use;
OriGene Technologies, Inc., Beijing, China) primary antibodies. The
sections were incubated with biotinylated antibodies and
horseradish peroxidase-labeled streptavidin [Ready-to-use;
UltraSensitive™ SP (Mouse/Rabbit) IHC kit-9710; Fuzhou Maixin
Biotech Co., Ltd., China] for 15 min each at room temperature. The
reaction products were visualized with 3,3′-diaminobenzidine as a
chromogen followed by counterstaining with hematoxylin at room
temperature for 1 min. The sections without primary antibody served
as a negative control (18).
Evaluation of IHC staining
The IHC staining results of USP10 and MSH2 in all
sections were evaluated by two pathologists (Dr Zhi Zeng and Dr
Yabing Huang, Department of Pathology, Renmin Hospital of Wuhan
University) unaware of the disease outcome. Expression levels were
ascertained according to the average score of the two observers'
evaluations. If the difference of score was ≥2, the final score was
determined by a third pathologist (Dr Jingping Yuan; Department of
Pathology, Renmin Hospital of Wuhan University). USP10 in the tumor
cells was mainly located in the cytoplasm and was present at a
different staining intensity; therefore, the USP10 expression
information in the tumor cells, scored by staining intensity was
analyzed according to a four-tier grading system (scored as 0,
absent; 1, weak; 2, moderate; and 3, strong staining intensity), as
in a previous study (11). The MSH2
protein was located in the cell nucleus, therefore the percentages
of nucleus-stain-positive cells were analyzed in the tumor tissues
as the IHC score, and were divided into four groups according to
the percentage of positive cells: 0 group, <10%; 1+ group,
10–25%; 2+ group, 26–50%; and 3+ group, >50%. For the analysis,
USP10 and MSH2 expression levels were divided into two groups:
Negative group (score, ≤1 or 1+) and positive group (score, >1
or 1+).
Analysis of publicly available
data
Datasets of patients with lung cancer and
corresponding clinical data were downloaded from the publicly
available Gene Expression Omnibus (GEO) datasets (www.ncbi.nlm.nih.gov/gds). The data were
retrieved from different GEO Series (GSE) and GEO Platform (GPL)
datasets. A total of nine independent datasets from GSE (GSE36471,
GSE44077, GSE32867, GSE31210, GSE46539, GSE67061, GSE33479,
GSE19804 and GSE43458) were used to analyze the expression level of
USP10 mRNA in lung cancer. The log2 intensity of
different probes was first transformed into original data used to
represent the expression level of USP10, and the y-axis of
the figures of different GSE datasets represents the unlogged data
of the gene expression intensity. P<0.05 was considered to
indicate a statistically significant difference, as presented in
Table I. Furthermore, UALCAN
(ualcan.path.uab.edu), an interactive web
portal for performing in-depth analyses of The Cancer Genome Atlas
(TCGA) gene expression data, was utilized to mine USP10 mRNA
expression between NSCLC and normal lung tissues. Kaplan-Meier
plots presenting the association of USP10/MSH2 mRNA expression and
patient survival were also obtained from TCGA database.
 | Table I.USP10 mRNA expression in normal
compared with cancer tissues of different Gene Expression Omnibus
datasets from different gene expression platforms. |
Table I.
USP10 mRNA expression in normal
compared with cancer tissues of different Gene Expression Omnibus
datasets from different gene expression platforms.
| GSE | Normal cases | Cancer cases | P-value | GPL | Probe |
|---|
| 36471 | 29 | 89 | 0.581 | 3720 | SNP A-1828377 |
| 44077 | 65 | 57 |
4.43×10−7 | 6244 | 7997633 |
| 32867 | 86 | 86 | 0.66 | 8490 | cg00200063 |
| 31210 | 20 | 226 | 0.001 | 570 | 209136_s_at |
| 46539 | 92 | 92 | 0.41 | 6883 | ILMN_1721116 |
| 67061 | 8 | 69 | 0.0028 | 6480 | A_23_P100196 |
| 33479 | 13 | 14 | 0.082 | 6480 | A_23_P100196 |
| 19804 | 60 | 59 | 0.5 | 570 | 209136_s_at |
| 43458 | 30 | 80 | 0.7 | 6244 | 7997633 |
Statistical analysis
All experimental data were analyzed using SPSS
statistical software (version 15.0; SPSS, Inc., Chicago, IL, USA).
χ2 and Fisher's exact tests were used to analyze the
statistical significance of the association between USP10
expression and the clinicopathological features. Student's t-test
was used to compare patients' age or tumor diameter between two
groups. The correlation between USP10 and MSH2 expression was
analyzed using Spearman's rank correlation analysis. For the
survival analysis, survival curves were obtained with the
Kaplan-Meier method and compared using the log-rank test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of USP10 protein between
NSCLC tissues and non-cancerous lung tissues
A total of 148 human NSCLC tissue samples were
analyzed, including 54 cases of squamous cell carcinoma, 82 cases
of adenocarcinoma, 4 cases of adenosquamous carcinoma, 3 cases of
sarcomatoid carcinoma and 5 cases of large cell carcinoma, and a
corresponding 139 non-cancerous lung tissues containing 45 cases of
bronchial epithelium and 94 cases of alveolar epithelium were also
analyzed. Representative IHC staining for USP10 protein expression
in different types of the aforementioned tissues are presented in
Fig. 1A-H. The positive rate of USP10
protein in different tissues, including bronchial epithelium,
alveolar epithelium, squamous cell carcinoma, adenocarcinoma,
adenosquamous carcinoma, sarcomatoid carcinoma and large cell
carcinoma was 97.78, 95.74, 35.18, 45.76, 0, 66.67 and 80%,
respectively (Fig. 1I). USP10 protein
expression was significantly decreased in the NSCLC tumor tissues,
including lung squamous cell carcinoma (P<0.001), lung
adenocarcinoma (P<0.001) and adenosquamous carcinoma
(P<0.001), compared with USP10 expression in normal lung tissues
(Fig. 1I).
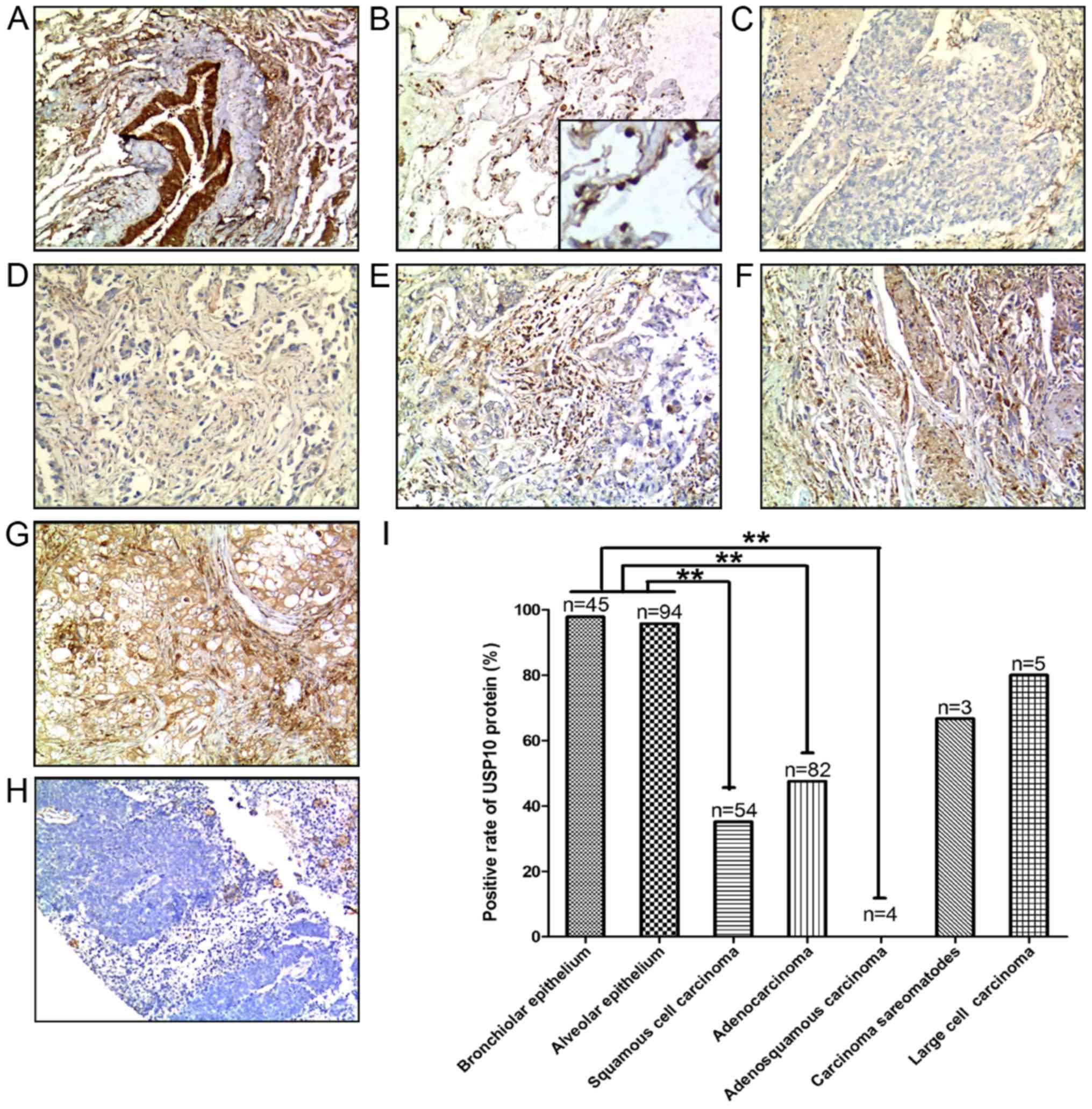 | Figure 1.Positive expression of USP10 protein
in representative normal and NSCLC tissues: (A) Bronchial
epithelium, (B) alveolar epithelium (C) squamous cell carcinoma,
(D) adenocarcinoma, (E) adenosquamous carcinoma, (F) sarcomatoid
carcinoma, (G) large cell carcinoma and (H) negative control.
3,3′-Diaminobenzidene staining (brown), nuclear counterstaining
(hematoxylin). Original magnification, ×100. (I) Positive rate of
USP10 protein between normal and lung cancer tissues. Statistical
analysis was performed by χ2 test. **P<0.01. NSCLC,
non-small cell lung cancer; USP10, ubiquitin-specific protease
10. |
Expression of USP10 mRNA between NSCLC
tissues and non-cancerous lung tissues
USP10 mRNA expression was investigated using
bioinformatics analysis of the GEO datasets and TCGA database. A
total of nine independent GSE datasets was selected (Fig. 2A-I). In total, six of the GSE datasets
indicated that there was no significant difference in USP10 mRNA
expression between NSCLC and normal lung tissues. Of the GSE
datasets, two indicated significantly increased expression of USP10
in NSCLC tissues (P<0.05; Fig. 2B
and Fig. 2D), and one indicated
significantly decreased expression in NSCLC tissues (P<0.05;
Fig. 2F); however, the increase or
decrease was <1.5-fold in these three GSE datasets. The
different probes and GPL used for the corresponding GSE are
presented in Table I. Furthermore,
the P-values and scatter plots between normal and NSCLC groups and
the total cases in each group are presented in Table I and Fig.
2A-I, respectively. There was no significant difference in
USP10 mRNA expression between the lung squamous cell carcinoma or
adenocarcinoma NSCLC subtypes and normal lung tissues from TCGA
database (Fig. 2J and K).
Collectively, bioinformatics analysis did not reveal a significant
difference in USP10 mRNA expression between NSCLC tissues and
normal lung tissues.
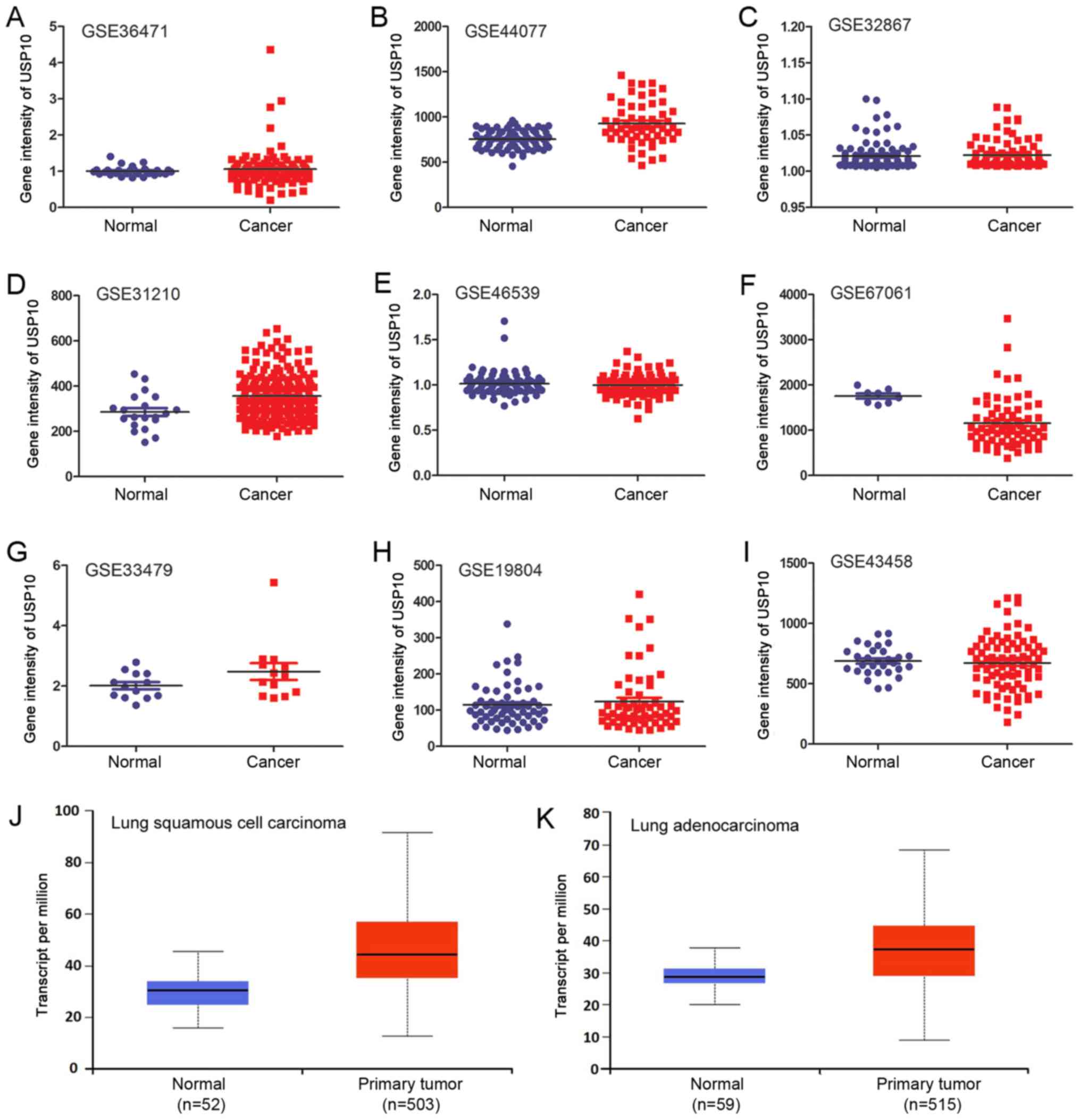 | Figure 2.USP10 mRNA expression in normal and
NSCLC tissues of (A) GSE36471, (B) GSE44077, (C) GSE32867, (D)
GSE31210, (E) GSE46539, (F) GSE67061, (G) GSE33479, (H) GSE19804
and (I) GSE43458 datasets from the GEO database. Relative
expression of USP10 between normal lung tissue and (J) lung
squamous cell carcinoma or (K) lung adenocarcinoma from The Cancer
Genome Atlas database. NSCLC, non-small cell lung cancer; USP10,
ubiquitin-specific protease 10; GEO, Gene Expression Omnibus; GSE,
GEO Series. |
Correlation between USP10 and MSH2
protein expression
From the IHC staining of NSCLC cells, positive
expression of USP10 was observed primarily in the cytoplasm,
whereas the MSH2 protein was present primarily in the nucleus.
Representative IHC staining specimens for different scores of USP10
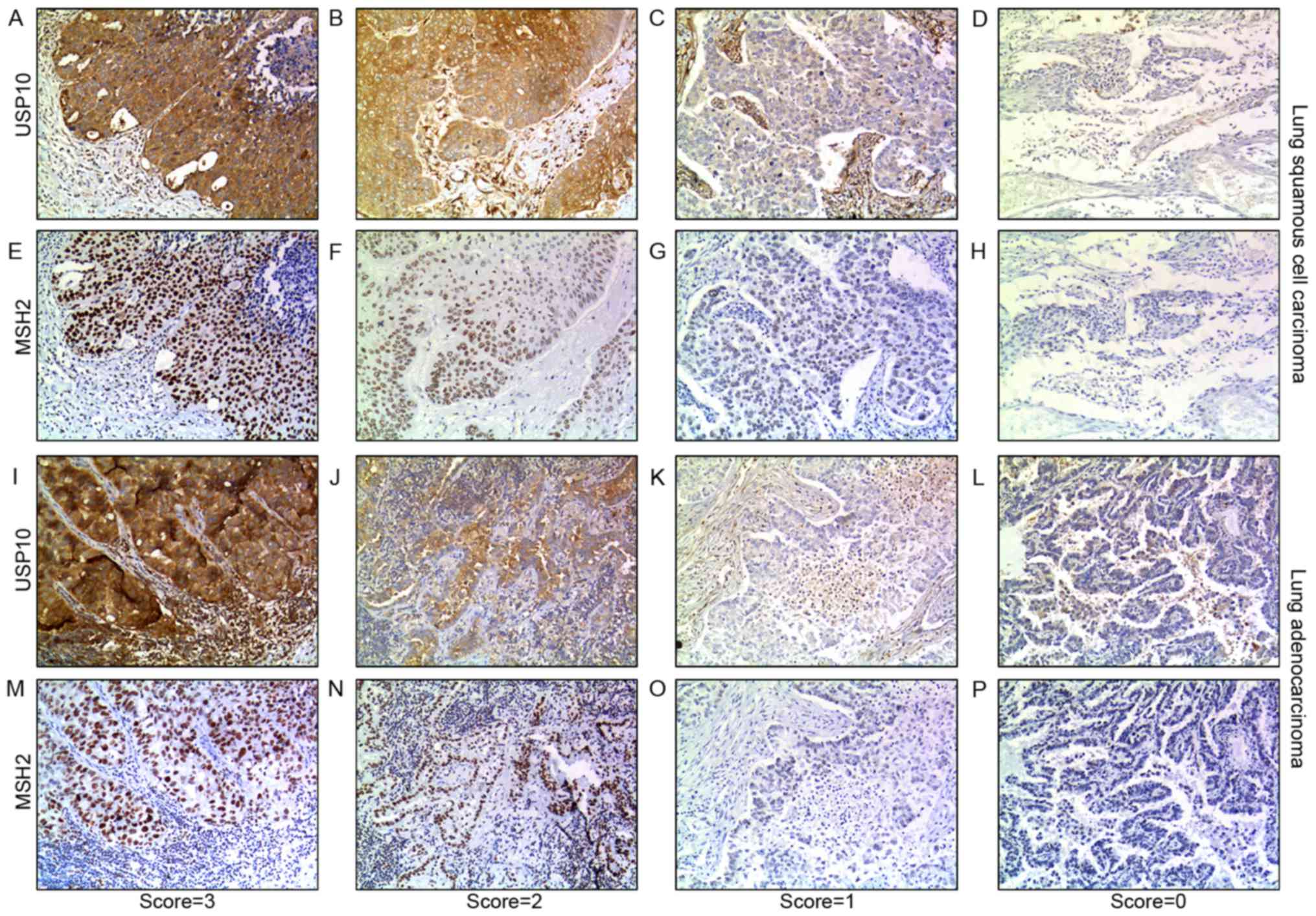
or MSH2 protein expression are presented in Fig. 3, of which IHC staining specimens in
Fig. 3A and E, B and F, C and G, D and H,
I and M, J and N, K and O, and L and P were in the same field
from the same slice. As presented in Fig.
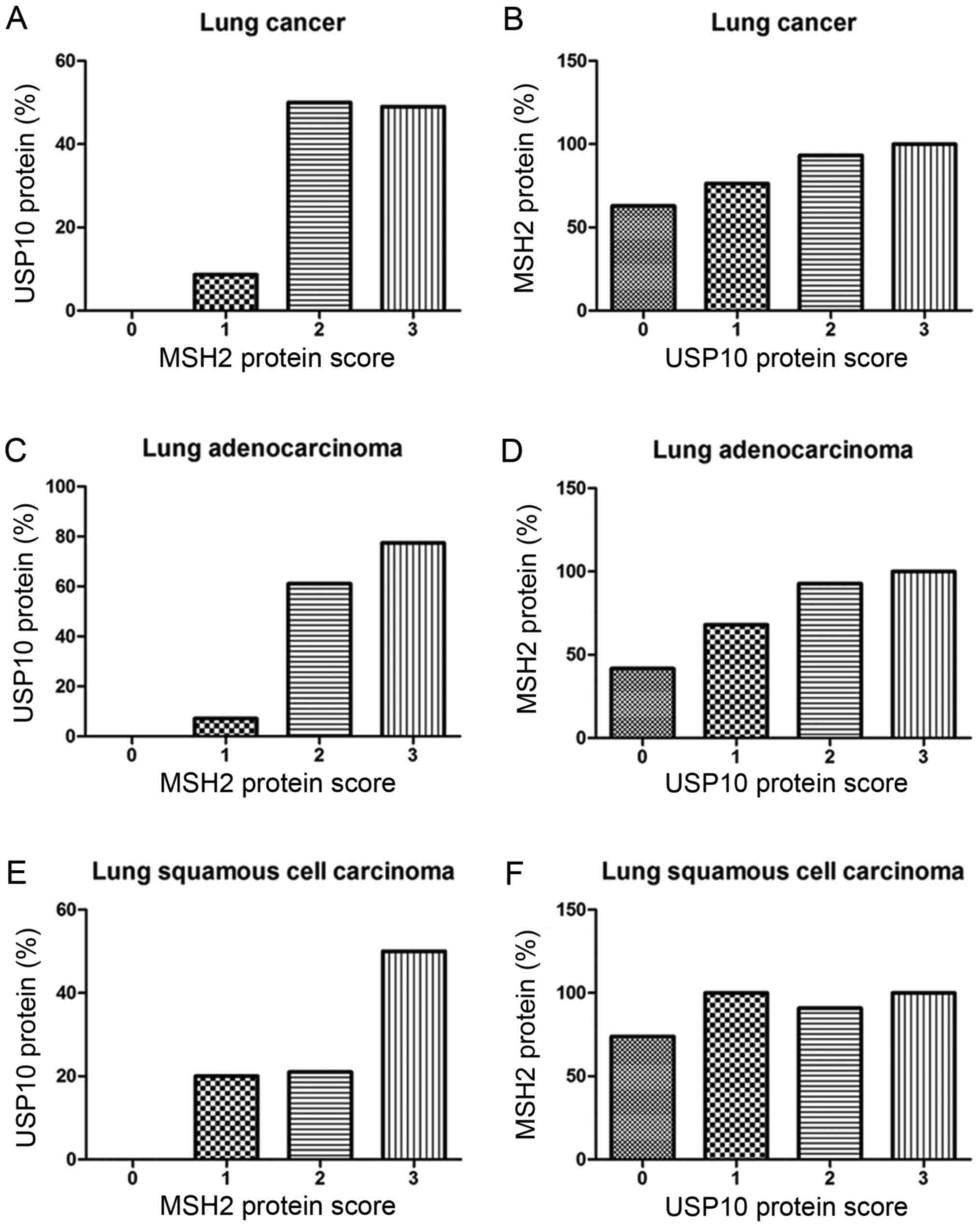
4, the expression of MSH2 was positively correlated with the
expression of USP10 (r=0.25, P=0.004). The positive expression rate
of MSH2 in NSCLC was 61.90, 76.32, 93.33 and 100% with a USP10
protein score of 0, 1 2 and 3, respectively, whereas the positive
expression rate of USP10 in NSCLC was 0, 8.67, 50.00 and 49.02%
with an MSH2 protein score of 0, 1+, 2+ to 3+ (Fig. 4A and B). Similarly, further systematic
analysis of the association between USP10 and MSH2 revealed the
same trend in squamous cell carcinoma and adenocarcinoma,
respectively (Fig. 4C-F). In lung
adenocarcinoma, the positive expression rate of MSH2 was 41.67,
68.00, 92.86 and 100% with a USP10 protein score of 0, 1, 2 and 3,
whereas the positive expression rate of USP10 was 0, 7.14, 61.11
and 77.42% with an MSH2 protein score of 0, 1+, 2+ and 3+ (Fig. 4C and D). In lung squamous cell
carcinoma, the positive expression rate of MSH2 was 73.91, 100.00,
90.91 and 100.00% with a USP10 protein score of 0, 1, 2 and 3,
whereas the positive expression rate of USP10 was 0, 20.00, 21.05
and 50.00% with an MSH2 protein score of 0, 1+, 2+ and 3+ (Fig. 4E and F).
Association of the USP10 or MSH2
proteins in tumor cells with clinicopathological features of
NSCLC
The association between USP10 protein cytoplasmic
staining in tumor cells and the traditional clinicopathological
features for the 148 NSCLC samples was analyzed further, as
presented in Table II. There was no
significant association of USP10 protein expression with the
clinicopathological features, including age, sex, tumor size, TNM
stage and tumor cell differentiation (P>0.05; Table II). Previous studies indicated that
MSH2 was positively associated with the expression of USP10
(13); thus, the association between
MSH2 protein nuclear staining in tumor cells and the traditional
clinicopathological features for the 148 NSCLC samples was
statistically analyzed further (Table
II). Additionally, there was no significant association of MSH2
protein expression with the clinicopathological features, including
age, sex, tumor size, TNM stage and tumor cell differentiation
(P>0.05; Table II).
 | Table II.Correlation between USP10/MSH2
expression and clinicopathological features of NSCLC. |
Table II.
Correlation between USP10/MSH2
expression and clinicopathological features of NSCLC.
|
| USP10 protein
expression | MSH2 protein
expression |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | Negative, n | Positive, n
(%) |
P-valuea | Negative, n | Positive, n
(%) |
P-valuea |
|---|
| Sex |
|
Male | 58 | 43 (42.57) | 0.81 | 13 | 77 (85.56) | 0.085 |
|
Female | 26 | 21 (44.68) |
| 12 | 33 (73.33) |
|
| T stage |
| T1 | 31 | 29 (48.33) | 0.601 | 9 | 43 (82.69) | 0.553 |
| T2 | 35 | 23 (39.66) |
| 15 | 39 (72.22) |
|
| T3 | 9 | 4 (30.77) |
| 2 | 9 (81.82) |
|
| T4 | 9 | 8 (47.06) |
| 3 | 15 (83.33) |
|
| N stage |
| N0 | 58 | 45 (43.69) | 0.868 | 17 | 79 (82.29) | 0.974 |
|
N1/N2/N3 | 26 | 19 (42.22) |
| 7 | 32 (82.05) |
|
| M stage |
| M0 | 82 | 64 (43.84) | − | 29 | 103 (78.03) | − |
|
M1/M2/M3 | 2 | 0 (0) |
| 0 | 3 (100) |
|
| TNM |
| I | 47 | 35 (42.68) | 0.394 | 14 | 62 (81.58) | 0.186 |
| II | 13 | 15 (53.57) |
| 3 | 18 (85.71) |
|
|
III/IV | 24 | 14 (36.84) |
| 12 | 26 (68.42) |
|
|
Differentiationb |
|
Well | 18 | 13 (41.94) | 0.739 | 8 | 19 (70.37) | 0.287 |
|
Moderate | 35 | 30 (46.15) |
| 11 | 48 (81.36) |
|
|
Poor | 24 | 15 (46.15) |
| 5 | 31 (86.11) |
|
| Age,
years | 59.76±8.34 | 60.22±8.04 | 0.738c | 58.59±8.2 | 59.86±8.06 | 0.454c |
|
Diameter, cm | 4.47±2.03 | 4.09±2.32 | 0.29c | 4.38±1.93 | 4.44±2.35 | 0.895c |
Correlation between USP10 or MSH2
protein expression and survival of patients with NSCLC
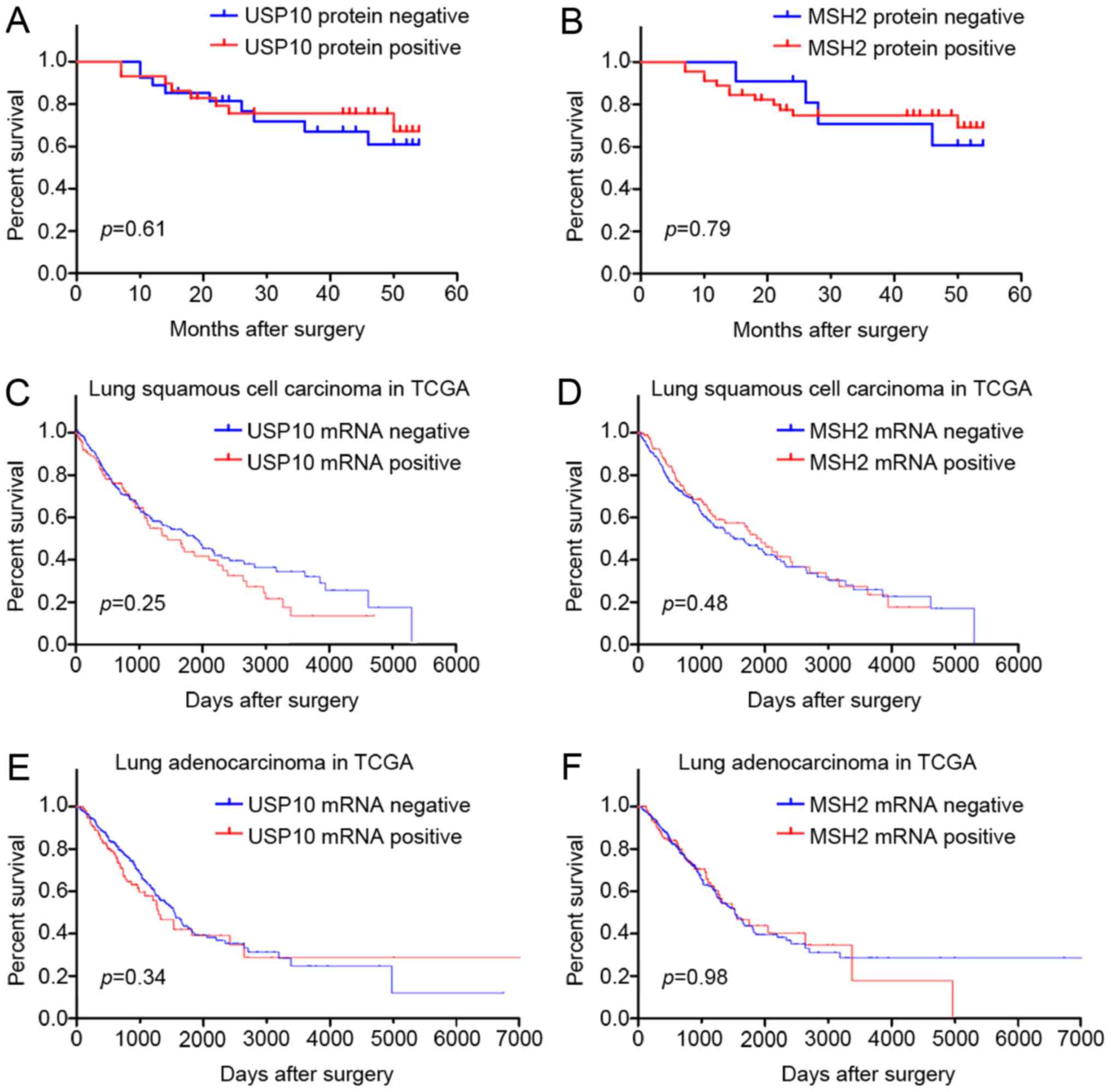
The prognostic value of USP10 or MSH2 protein for
NSCLC in 56 patients was assessed using a postoperative follow-up.
Kaplan-Meier analysis and log-rank testing revealed no significant
difference between the USP10 or MSH2 protein negative expression
group and the positive expression group (P>0.05; Fig. 5A and B). Furthermore, survival
analysis from TCGA database also indicated that USP10 and MSH2 mRNA
expression was not associated with survival of patients with lung
squamous cell carcinoma and adenocarcinoma (Fig. 5C-F). Therefore, the survival analysis
indicated that expression of USP10 and MSH2 may not serve as a
biomarker for the tumor progression of NSCLC.
Discussion
Lung cancer is a common malignant disease and the
leading cause of cancer-associated mortality globally (19). NSCLC is the primary type of lung
cancer, accounting for between 80 and 85% of cases (20), and is the leading cause of mortality
caused by lung cancer encompassing lung adenocarcinomas, squamous
cell lung carcinomas, large cell carcinomas, sarcomatoid carcinoma
and adenosquamous carcinoma (21).
Surgical treatment is generally considered curative for early-stage
NSCLC, however, most patients present with advanced stages at
diagnosis (22). Furthermore, the
5-year survival rate is only 16%, and patients have a high
incidence of recurrence (23).
Therefore, it is important to identify novel biomarkers for the
early stages of NSCLC.
USP10 is involved in a number of biological
processes by stabilizing several proteins (6,7) that serve
direct or indirect functions in tumorigenesis and in the
progression of several tumors. Yuan et al (3) reported that USP10, as a novel regulator
of p53, provides an alternative mechanism of p53 inhibition in
cancer. A number of studies also suggested increased USP10
expression in certain types of breast cancer (24) and glioblastoma (25). Lin et al (9) identified that the protein expression
levels of two interacting proteins (USP10 and SIRT6) were
downregulated in human colon cancer, which also indicated that
gene-dysregulated USP10 function promotes tumorigenesis through
SIRT6 degradation. Yang et al (8) reported that PCNA stability was regulated
by USP10 and promoted cell viability by upregulating melanoma
antigen family D1 in esophageal tumor tissues. A previous study
indicated that a positive USP10 immunoreaction may be useful in
distinguishing adrenal cortical tumors from pheochromocytomas
(18). It was further demonstrated
that USP10 could be used as an independent prognostic predictor for
patients with gastric carcinoma (11). However, there is limited information
about the functions of USP10 in human patients with NSCLC.
Therefore, the aim of the present study was to clarify whether
USP10 is involved in the tumorigenesis and progression of
NSCLC.
The majority of lung squamous cell carcinoma
originates from the bronchial epithelium, whereas type II alveolar
cells and Clara cells are regarded as the possible origin of lung
adenocarcinoma (26–28). Therefore, bronchial epithelium and
alveolar epithelium samples were used in the present study as
well-directed normal lung tissue controls.
In 2004, the World Health Organization published a
new classification of lung tumors, of which the incidence of the
four main types of lung cancer are as follows: 31.5% for lung
adenocarcinoma, 29.4% for lung squamous carcinoma, 17.8% for small
cell lung cancer, and 9.2% for large-cell lung cancer (29). Therefore, the clinical NSCLC samples
enrolled in the present study were predominantly from lung
adenocarcinoma and lung squamous cell carcinoma, and only these
types of NSCLC sample were analyzed to determine the significant
differences between NSCLC and non-cancerous lung tissues. In the
IHC staining, USP10 was localized primarily to the cytoplasm in
NSCLC tissues as well as in gastric carcinoma tissues (11). In the 148 patients with NSCLC enrolled
in the present study, the positive rate of USP10 was 35.18 and
45.76% in squamous cell carcinoma and adenocarcinoma, respectively.
By contrast, USP10 was highly expressed in bronchial epithelium and
alveolar epithelium, with a positive rate of 97.78 and 95.74%
(P<0.01), indicating that the expression of USP10 protein is
significantly downregulated in the majority of NSCLC tumor tissues
compared with non-cancerous lung tissues. This result was
consistent with that of a recent study performed by Cao et
al (14), which demonstrated that
50% (9/18) of patients have decreased expression of USP10 in NSCLC
tumor tissue compared with that in the respective adjacent normal
lung tissue. Several differences between the present study and the
study of Cao et al (14) must
be emphasized. First, an IHC assay was utilized in the present
study to detect the USP10 protein expression, whereas a western
blot assay was used in the study of Cao et al (14). In the present study, the protein
expression level could be determined and the protein localization
was also identified. Secondly, in the present study, the clinical
sample size was enlarged to analyze the USP10 expression in NSCLC
tissues. A total of 148 human NSCLC tissue samples and 139
non-cancerous lung tissues were analyzed in the present study,
whereas only 18 cases were analyzed in the study of Cao et
al (14). Finally, the present
study emphasized elucidating the critical roles of USP10 in NSCLC;
however, Cao et al (14)
focused on the roles of eukaryotic translation initiation factor 4γ
1 (EIF4G1) in NSCLC, and identified that USP10 could act as a
negative regulator of EIF4G1. Therefore, combining the two
investigations, it is more convincing to conclude that USP10 is
downregulated in NSCLC clinical tissue samples and may participate
in the tumorigenesis of NSCLC.
To explore whether the mRNA level of USP10 is
downregulated in human NSCLC tissues, the expression of USP10 mRNA
was further analyzed in human normal lung and NSCLC tissues using
bioinformatics analysis in the GEO datasets and TCGA database. A
total of nine independent GSE datasets with different numbers of
clinical samples and supplied by different investigators were
selected to evaluate the difference in USP10 mRNA expression
between normal lung tissues and NSCLC tissues. Different GPLs and
the corresponding probe substrates were selected for the USP10
gene, which guaranteed the accuracy of the comprehensive results.
Generally, the result of bioinformatics analysis in the GEO
datasets and TCGA database revealed no significant difference in
USP10 mRNA expression between normal lung tissues and NSCLC
tissues. However, 3/9 GSE results exhibited a significant
difference. Nevertheless, the increase and decrease were
<1.5-fold in these GSE datasets. Therefore, bioinformatics
analysis did not identify a significant difference in USP10 mRNA
expression between normal lung tissues and NSCLC tissues, which
indicated that the mechanism of downregulation of USP10 protein in
the NSCLC tissues may occur at the post-transcriptional level, not
at the transcriptional level. It is speculated that microRNA (miR)
is involved in this process, as it was reported that miR-191 may
inhibit protein levels of USP10 in pancreatic cancer (30). Additionally, it was further verified
that H19-derived miR-675 is targeted at the 3′-untranslated region
(UTR) of USP10 in C-kit(+) cardiac progenitor cells (31). Thus, the expression of USP10 protein
will be downregulated if one of the microRNAs targets the 3′-UTR of
USP10 mRNA, which requires investigation in a future study.
Previous research indicated that USP10 stabilizes
and deubiquitinates MSH2 in vitro and in vivo
(13). Similarly, Lin et al
(9) also reported that USP10, as one
of the SIRT6-interacting proteins, suppresses SIRT6 ubiquitination
to protect SIRT6 from proteasomal degradation. Guturi et al
(32) demonstrated that USP10
functions as a DUB that negatively regulates DNA topoisomerase II α
ubiquitylation and limits its chromatin association in human breast
cancer cell lines. A positive correlation was previously identified
between S100A12 and USP10 protein in gastric carcinoma (33). In the present study, on the basis of
previous research of the underlying molecular mechanism of
interaction between USP10 and MSH2 in NSCLC cell lines (13), human clinical NSCLC tissue samples
were investigated to clarify the association of these two proteins.
A cohort of human NSCLC tissue samples was assessed to clarify
whether there was a positive correlation between USP10 and MSH2 by
evaluating the positive staining of USP10 and MSH2 in human NSCLC
tissue samples utilizing IHC assays, which was consistent with the
previous in vitro results (13). This result may indicate that USP10
stabilizes MSH2 in lung cancer tissues.
USP10 serves crucial functions in the tumor
biological process, which is critically involved in the control of
cell viability, differentiation and apoptosis (3,13).
Increased USP10 expression has been detected in certain breast
cancer (24) and glioblastoma
(25) samples, and USP10
overexpression has been identified to be associated with a poor
prognosis in patients with glioblastoma. However, USP10 has been
identified to be underexpressed in a number of types of cancer,
including gastric carcinoma (11).
Furthermore, it was also demonstrated that the downregulated
expression of USP10 indicates a worse outcome and decreased
survival time in patients with gastric carcinoma, which may be used
as an independent predictor of the prognosis of gastric carcinoma
(11). Therefore, whether USP10 is
irregularly expressed in the development and progression of NSCLC
was the primary focus of the present study. The results of the
present study failed to validate the clinical outcome and
prognostic value of USP10 in the patients with NSCLC. No
correlation was identified between USP10 protein expression and
clinicopathological features, including age, sex, tumor size, TNM
stage and tumor cell differentiation, indicating that USP10 may not
participate in the tumor progression of NSCLC.
Multiple DNA repair pathways have previously been
confirmed to be associated with tumor prognosis, drug efficacy or
chemotherapeutic resistance. MSH2 in the mismatch repair pathway is
associated with DNA repair following platinum insult. However, this
protein was not identified to be associated with
clinicopathological features of lung cancer, including smoking
history, sex, age and TNM stage (34). A previous study failed to identify a
correlation between MSH2 expression and prognosis in either lung
squamous cell carcinoma or adenocarcinoma, which may be attributed
to the limited number of clinical samples analyzed (34). Consistent with this study, the present
study also failed to reveal a correlation between NSCLC prognostic
value and MSH2 protein expression. Notably, a recent study
indicated that MSH2/breast cancer early onset 1 (BRCA1) expression
may act as a DNA-repair signature predicting survival in patients
with early-stage lung cancer (35).
The difference from that study is that the patients enrolled were
all post-chemotherapy and in early Stage I and II. Furthermore, the
two proteins were used together to predict the outcome, and only
high BRCA1 and low MSH2 expression significantly predicted an
improved overall survival time (35).
In summary, more data are needed to support the use of MSH2 to
predict NSCLC prognoses.
In conclusion, USP10 protein expression was
downregulated in clinical NSCLC tissue samples compared with
non-cancerous lung tissues, whereas USP10 mRNA exhibited no
significant difference between normal lung tissues and NSCLC
tissues. It was identified that USP10 protein expression was
positively correlated with MSH2 in a large cohort of clinical NSCLC
tissue samples. However, USP10 and MSH2 were not associated with
clinicopathological features of NSCLC, including age, sex, tumor
size, TNM stage and tumor cell differentiation. Furthermore, USP10
and MSH2 mRNA and protein expression were not associated with the
prognosis of NSCLC. Thus, the detection of USP10 may be used as a
biomarker to distinguish the tumorigenesis of NSCLC.
Acknowledgements
The authors would like to thank Dr Xu Zhang and Dr
Liwen Yao (Renmin Hospital of Wuhan University) for collecting the
samples.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81602535, 81704023 and
81803789), the scientific research project of Integrated
Traditional Chinese and Western Medicine from the Health and Family
Planning Commission of Hubei Province (2017–23) and the guidance funding of Renmin
Hosipital of Wuhan University (RMYD2018M79).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and DL performed the experiments and wrote the
paper; HY and TY collected the samples and performed statistical
analysis; YH and JY performed bioinformatics analysis; LG and JY
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan, China).
Patients provided written informed consent for the use of their
tissues in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hicke L and Dunn R: Regulation of membrane
protein transport by ubiquitin and ubiquitin-binding proteins. Annu
Rev Cell Dev Biol. 19:141–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Love KR, Catic A, Schlieker C and Ploegh
HL: Mechanisms, biology and inhibitors of deubiquitinating enzymes.
Nat Chem Biol. 3:697–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan J, Luo K, Zhang L, Cheville JC and
Lou Z: USP10 regulates p53 localization and stability by
deubiquitinating p53. Cell. 140:384–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soncini C, Berdo I and Draetta G: Ras-GAP
SH3 domain binding protein (G3BP) is a modulator of USP10, a novel
human ubiquitin specific protease. Oncogene. 20:3869–3879. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L,
Cai Y, Norberg HV, Zhang T, Furuya T, et al: Beclin1 controls the
levels of p53 by regulating the deubiquitination activity of USP10
and USP13. Cell. 147:223–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan L, Chen Z, Wang L, Chen C, Li D, Wan
H, Li B and Shi G: Deubiquitination and stabilization of T-bet by
USP10. Biochem Biophys Res Commun. 449:289–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Q, Ou C, Liu M, Xiao W, Wen C and Sun
F: NRAGE promotes cell proliferation by stabilizing PCNA in a
ubiquitin-proteasome pathway in esophageal carcinomas.
Carcinogenesis. 35:1643–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q,
Kong S, Ye J, Gao B and Fang D: USP10 antagonizes c-Myc
transcriptional activation through SIRT6 stabilization to suppress
tumor formation. Cell Rep. 5:1639–1649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu J, Shi Y, Xue J, Miao R, Huang S, Wang
T, Wu J, Fu M and Wu ZH: USP10 inhibits genotoxic NF-κB activation
by MCPIP1-facilitated deubiquitination of NEMO. EMBO J.
32:3206–3219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS,
Yang GF, Wang P and Fu GH: Prognostic significance of USP10 as a
tumor-associated marker in gastric carcinoma. Tumour Biol.
35:3845–3853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koul R, Rathod S, Dubey A, Bashir B and
Chowdhury A: Comparison of 7th and 8th editions of the UICC/AJCC
TNM staging for non-small cell lung cancer in a non-metastatic
North American cohort undergoing primary radiation treatment. Lung
Cancer. 123:116–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Hu C, Tong D, Xiang S, Williams
K, Bai W, Li GM, Bepler G and Zhang X: Ubiquitin-specific peptidase
10 (USP10) deubiquitinates and stabilizes MutS Homolog 2 (MSH2) to
regulate cellular sensitivity to DNA damage. J Biol Chem.
291:10783–10791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Wei M, Li B, Liu Y, Lu Y, Tang Z,
Lu T, Yin Y, Qin Z and Xu Z: Functional role of eukaryotic
translation initiation factor 4 gamma 1 (EIF4G1) in NSCLC.
Oncotarget. 7:24242–24251. 2016.PubMed/NCBI
|
|
15
|
Heinen CD: Translating mismatch repair
mechanism into cancer care. Curr Drug Targets. 15:53–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamal NS, Soria JC, Mendiboure J,
Planchard D, Olaussen KA, Rousseau V, Popper H, Pirker R, Bertrand
P, Dunant A, et al: MutS homologue 2 and the long-term benefit of
adjuvant chemotherapy in lung cancer. Clin Cancer Res.
16:1206–1215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ko JC, Chiu HC, Syu JJ, Chen CY, Jian YT,
Huang YJ, Wo TY, Jian YJ, Chang PY, Wang TJ and Lin YW:
Down-regulation of MSH2 expression by Hsp90 inhibition enhances
cytotoxicity affected by tamoxifen in human lung cancer cells.
Biochem Biophys Res Commun. 456:506–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Z, Zhou Z, Zhan N, Yuan J, Ye B, Gu
L, Wang J, Jian Z and Xiong X: USP10 expression in normal adrenal
gland and various adrenal tumors. Endocr Pathol. 26:302–308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Politi K and Herbst RS: Lung cancer in the
era of precision medicine. Clin Cancer Res. 21:2213–2220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang H and Shrager JB: CRISPR/Cas-mediated
genome editing to treat EGFR-mutant lung cancer: A personalized
molecular surgical therapy. EMBO Mol Med. 8:83–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCloskey P, Balduyck B, Van Schil PE,
Faivre-Finn C and O'Brien M: Radical treatment of non-small cell
lung cancer during the last 5 years. Eur J Cancer. 49:1555–1564.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santarpia M, Rolfo C, Peters GJ, Leon LG
and Giovannetti E: On the pharmacogenetics of non-small cell lung
cancer treatment. Expert Opin Drug Metab Toxicol. 12:307–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing
T, Dong L, Tang E and Yang H: Over-expression of genes and proteins
of ubiquitin specific peptidases (USPs) and proteasome subunits
(PSs) in breast cancer tissue observed by the methods of RFDD-PCR
and proteomics. Breast Cancer Res Treat. 104:21–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grunda JM, Nabors LB, Palmer CA, Chhieng
DC, Steg A, Mikkelsen T, Diasio RB, Zhang K, Allison D, Grizzle WE,
et al: Increased expression of thymidylate synthetase (TS),
ubiquitin specific protease 10 (USP10) and survivin is associated
with poor survival in glioblastoma multiforme (GBM). J Neurooncol.
80:261–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitamura H, Kameda Y, Ito T and Hayashi H:
Atypical adenomatous hyperplasia of the lung. Implications for the
pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol.
111:610–622. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mori M, Kaji M, Tezuka F and Takahashi T:
Comparative ultrastructural study of atypical adenomatous
hyperplasia and adenocarcinoma of the human lung. Ultrastruct
Pathol. 22:459–466. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osanai M, Igarashi T and Yoshida Y: Unique
cellular features in atypical adenomatous hyperplasia of the lung:
Ultrastructural evidence of its cytodifferentiation. Ultrastruct
Pathol. 25:367–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beasley MB, Brambilla E and Travis WD: The
2004 World Health Organization classification of lung tumors. Semin
Roentgenol. 40:90–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu
J and Gao FH: MicroRNA-191 promotes pancreatic cancer progression
by targeting USP10. Tumour Biol. 35:12157–12163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai B, Ma W, Bi C, Yang F, Zhang L, Han Z,
Huang Q, Ding F, Li Y, Yan G, et al: Long noncoding RNA H19
mediates melatonin inhibition of premature senescence of c-kit(+)
cardiac progenitor cells by promoting miR-675. J Pineal Res.
61:82–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guturi KK, Bohgaki M, Bohgaki T, Srikumar
T, Ng D, Kumareswaran R, El Ghamrasni S, Jeon J, Patel P, Eldin MS,
et al: RNF168 and USP10 regulate topoisomerase IIα function via
opposing effects on its ubiquitylation. Nat Commun. 7:126382016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li D, Zeng Z, Yu T, Qin J, Wu J, Song JC,
Zhou ZY and Yuan JP: Expression and clinical implication of S100A12
in gastric carcinoma. Tumour Biol. 37:6551–6559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie KJ, He HE, Sun AJ, Liu XB, Sun LP and
Dong XJ: Expression of ERCC1, MSH2 and PARP1 in non-small cell lung
cancer and prognostic value in patients treated with platinum-based
chemotherapy. Asian Pac J Cancer Prev. 15:2591–2596. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levallet G, Dubois F, Fouret P, Antoine M,
Brosseau S, Bergot E, Beau-Faller M, Gounant V, Brambilla E,
Debieuvre D, et al: MSH2/BRCA1 expression as a DNA-repair signature
predicting survival in early-stage lung cancer patients from the
IFCT-0002 phase 3 trial. Oncotarget. 8:4313–4329. 2017. View Article : Google Scholar : PubMed/NCBI
|