Introduction
Gastric cancer is one of the most common human
digestive tract malignancies, affected by various factors,
including dietary habits, environmental factors and the prevalence
of Helicobacter pylori infection (1). A total of 951,600 new gastric cancer
cases and 723,100 mortalities are estimated to have occurred in
2012, making it the third leading cause of cancer-associated
mortality globally (1). Almost 66% of
gastric cancer cases and mortalities occur in less-developed
regions, particularly in Eastern Asia (1). China is one of the countries with a high
incidence of gastric cancer, and accounts for >40% of all new
gastric cancer cases worldwide (1,2). Previous
studies indicate a declining trend in gastric cancer-associated
mortalities has been reported due to improvements in
quality-of-life and treatment techniques (3,4). At
present, pre-operative chemotherapy is widely used as a preliminary
treatment for locally-advanced gastric cancer to aid total
resection and improve survival (5).
It is assumed that neoadjuvant chemotherapy has a relatively
short-term benefit, based on the tumor regression grade (6). Several studies have reported a close
association of the tumor regression grade with clinic opathological
characteristics and patient survival (6–9). However,
the tumor response to preoperative chemotherapy is not uniform
among patients, and there are currently no effective methods to
predict the outcome of treatment. Molecular markers have been
suggested to have potential for early detection of disease, and for
predicting response to therapy (10,11).
Therefore, identifying specific and sensitive novel markers that
may predict the efficacy of neoadjuvant chemotherapy may be useful
for making decisions in the management of patients with gastric
cancer.
Aldo-ketoreductase family 1 member B10 (AKR1B10) is
a member of the aldo-ketoreductasesuperfamily, a cytosolic
nicotinamide adenine dinucleotide phosphate-dependent
oxidoreductase enzyme that metabolizes carbohydrates, steroids,
prostaglandins and exogenous carbonyl compounds (12). Although the functions of AKR1B10
intumorigenes is remain unclear, it is hypothesized that AKR1B10
may serve functions in cancer development and progression via
multiple molecular mechanisms including the detoxification of
cytotoxic carbonyls, modulation of retinoic acid levels, and
regulation of cellular fatty acid synthesis and lipid metabolism
(13–15). AKR1B10 is predominantly expressed in
the gastrointestinal tract, particularly in the small intestine and
colon, and in other organs, including the liver, pancreas, thymus
and adrenal gland (16). However,
overexpression of AKR1B10 has been reported in numerous solid
tumors, including hepatocellular carcinoma, non-small cell lung
cancer, breast cancer and pancreatic cancer (17–20), while
reduced expression has been reported in colon, stomach, and head
and neck cancer (21). Only a small
number of studies have explored the association between AKR1B10
expression and clinic opathological parameters of gastric cancer.
To our knowledge, the present study is the first to have
investigated the immunohistochemical expression of AKR1B10 and
identified the clinic opathological parameters that predicted the
response of gastric tumors to neoadjuvant chemotherapy in 53
patients with gastric carcinoma.
Materials and methods
Reagents and cell lines
Unless otherwise stated, all chemicals were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Immobilon-P (cat. no. IPVH00010) was purchased from Merck Millipore
(Merck KGaA, Darmstadt, Germany). ECL kit (cat. no. WBKLS0500) was
from Millipore (Billerica, MA, USA). An antibody against rabbit
AKR1B10 used in the present study was synthesized and used as
previously described (22,23). The Lamin B1 antibodies (cat. no.
sc-20682) was supplied by Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). α-tubulin (cat. no. T6199) was purchased from Merck KGaA
(Darmstadt, Germany). Horseradish peroxidase (HRP) conjugated goat
anti-rabbit antibody (cat. no. HA1001) was obtained from Hua-an
Biotechnology (Hangzhou, Zhejiang, China). The human gastric cancer
cell lines AGS and BGC-823 were obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells were maintained in
RPMI-1640 growth medium supplemented with 10% fetal bovine serum
and penicillin-streptomycin. All cell lines were cultured as
described previously (24).
Western blot analysis for detection of
AKR1B10 expression in gastric cancer cell lines
AGS and BGC-823 cells were grown in Dulbecco's
modified Eagles' medium supplemented with 10% fetal calf serum and
100 units/ml penicillin and streptomycin (Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany). Cells were washed three times with
Hepes-buffered, modified Krebs-Henseleit buffer (118 mMNaCl, 4.69
mMKCl, 1.18 mMMgSO4, 1.29 mMCaCl2, 1.18
mMKH2PO4, 11.67 mMglucose, 25 mMHepes, pH 7.4
at 37°C) and gently re-suspended cells in 500 µl Hypotonic Buffer
(20 mMTris-HCl, pH 7.4, 10 mMNaCl, 3 mM MgCl2) by
pipetting up and down several times. Following a 15 min incubation
on ice, 25 µl detergent was added (10% NP40) and vortexed for 10
sec at the highest setting. The homogenate were centrifuged for 10
min at 1,000 × g at 4°C. The nuclear pellet was resuspended in 50
µl complete Cell Extraction Buffer (10 mMTris, pH 7.4, 2 mM
Na3VO4, 100 mMNaCl, 1% Triton X-100, 1 mM
EDTA, 10% glycerol, 1 mM EGTA, 0.1% SDS, 1 mMNaF, 0.5%
deoxycholate, 20 mM Na4P2O7, 1 mM PMSF) for
30 min on ice with vortexing at 10 min intervals. The extract was
centrifuged for 30 min at 14,000 × g at 4°C. Quantification of
protein concentration was performed using Bradford assay. Protein
samples (100 µg) were separated by 10% SDS-PAGE as described
previously (24,25). The membrane carrying the transferred
proteins were blocked with 5% non-fat milk powder in PBS, 0.3%
Tween 20 for 2 h at room temperature. The membranes were incubated
with AKR1B10 primary antibody (dilution, 1:5,000) for 1 h at room
temperature, washed with blocking buffer 3–4 times (5 min per wash
at room temperature), the membranes were then incubated with the
HRP-conjugated goat anti-rabbit antibody (dilution, 1:5,000)
secondary antibody. Protein bands were detected by developing blot
using the ECL kit (Merck Millipore) according to the manufacture's
protocol. Subsequently to confirm nuclear and cytoplasmic fractions
immunoblotting was carried out with nuclear marker anti-Lamin B1
antibodies (dilution, 1:500) and anti-tubulin antibodies (dilution,
1:2,000). The relative levels of the protein of interest were
calculated by quantification of band intensity with an Odyssey
infrared imaging system from LI-COR® Biosciences
(Lincoln, Nebraska, USA).
Patients and samples
The present study recruited 53 patients with gastric
carcinoma who received different chemotherapy regimens, including
epirubicin + cisplatin + capecitabine (ECX), epirubicin + oxaplatin
+ tegafur (EOS), epirubicin + oxaplatin + capecitabine (EOX),
oxaplatin + leucovorin +5-FU (FOLFOX), and (S1) tegafur and
oxaplatin + tegafur (SOX) prior to surgery between January 2006 and
December 2015 at the Department of Surgical Oncology, Sir Run Run
Shaw Hospital, Zhejiang University School of Medicine (Zhejiang,
China). All specimens were biopsy materials taken subsequent to
preoperative chemotherapy. The patient's response to chemotherapy
was evaluated following two courses of chemotherapy according to
response evaluation criteria in solid tumors (RECIST) criteria
(26). Surgery was performed within
1–2 weeks of completion of neoadjuvant chemotherapy. Depending on
the location and macroscopic type of gastric cancer, patients
underwent total, distal, or proximal subtotal gastrectomy. The
patients age ranged between 33 and 77 years (median 58 years; mean
58.15). The study population comprised 40 males and 13 females.
Clinic opathological factors recorded were age, sex, tumor size,
tumor location, tumor staging, tumor depth, lymph node metastasis,
histological differentiation and response to neoadjuvant
chemotherapy. A receiver operating characteristics curve (Table I) was used to define the cut-off point
for various variables relative to AKR1B10 expression. The staging
of gastric cancer was according to the rules of the 6th edition of
American Joint Commission on Cancer system (AJCC) manual (27). Patients were recommended for
neoadjuvant chemotherapy based on the criteria of
histologically-proven gastric cancer, Eastern Cooperative Oncology
Group performance status (28),
clinical stage ≥T2 or lymph node metastasis, satisfactory organ
function, and no active associated malignancy. Patients were
monitored by upper gastrointestinal endoscopy and/or ultrasound
endoscopy and abdominal computed tomography scanning. For tumor
differentiation, well- and moderately differentiated
adenocarcinoma, papillary adenocarcinoma, and well-differentiated
mucinous adenocarcinoma were considered to be differentiated,
whereas poorly-differentiated adenocarcinoma, signet ring cell
carcinoma, and poorly-differentiated mucinous adenocarcinoma were
designated undifferentiated. Surgeons, oncologists and radiologists
individually evaluated all cases at the Department of Surgical
Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of
Medicine. The discrepancies were resolved by consensus review. The
present study protocol was based on the Sir Run Run Shaw Hospital's
policies and comprised chemotherapy and radio-chemotherapy,
dependent upon tumor location. Multiple chemotherapeutic regimens
were used, as aforementioned, and the tumor response to neoadjuvant
treatment was reviewed using the tumor regression grade (TRG) scale
introduced by Mandard et al (29). Tumor regression was graded as TRG 4,
complete regression; TRG 3, isolated cell nests; TRG 2, increased
number of residual cancer cells with predominant fibrosis; TRG 1,
residual cancer outgrowing fibrosis; and TRG 0, no regressive
changes. The patients were followed up until the last follow-up
date or until they succumbed to mortality. The median follow-up
period was 19 months (range, 1–67 months). Of the 53 patients, 17
(32.1%) succumbed during the follow-up period. The Human Ethics
Review Committee of Sir Run Run Shaw Hospital, Zhejiang University
School of Medicine approved the present study and informed written
consent was obtained from patients regarding the use of biopsy
materials for the present study.
 | Table I.Summary of patient's general
information and management involved in this study. Total number of
patients: 53. |
Table I.
Summary of patient's general
information and management involved in this study. Total number of
patients: 53.
| Clinical
parameters | Number of cases
(n) | (%) |
|---|
| Age, years |
|
|
|
<58 | 26 | 49.0 |
|
≥58 | 27 | 51.0 |
| Sex |
|
|
|
Male | 40 | 75.5 |
|
Female | 13 | 24.5 |
| Tumor size, cm |
|
|
|
<5 | 34 | 64.2 |
| ≥5 | 19 | 35.8 |
| Tumor location |
|
|
| Cardia,
fundus, body | 28 | 52.8 |
|
Antral | 25 | 47.2 |
| TNM stage |
|
|
|
I–II | 26 | 49.0 |
|
III–IV | 27 | 51.0 |
| Tumor depth |
|
|
|
T1-T2 | 11 | 20.8 |
|
T3-T4 | 42 | 79.2 |
| Lymph node
metastasis |
|
|
|
Yes | 36 | 68.0 |
| No | 17 | 32.0 |
| Histological
differentiation |
|
|
|
Differentiated | 24 | 45.3 |
|
Undifferentiated | 29 | 54.7 |
| Chemotherapy
regimen |
|
|
|
FOLFOX | 22 | 41.5 |
|
EOX | 16 | 30.2 |
| Others
(SOX, ECX, EOX, S1) | 13 | 24.5 |
|
Chemoradiotherapy | 2 | 3.8 |
| Chemotherapy
cycle |
|
|
| 2 | 8 | 15.1 |
| 3 | 17 | 32.1 |
| ≥4 | 28 | 52.8 |
| Tumor regression
grade |
|
|
|
0–2 | 33 | 62.3 |
|
3–4 | 20 | 37.7 |
| Surgical types |
|
|
|
Complete resection | 25 | 47.2 |
| Distal
resection | 25 | 47.2 |
|
Proximal resection | 1 | 1.9 |
| Partial
resection | 2 | 3.7 |
Immunohistochemistry (IHC)
AKR1B10 expression in paraffin-embedded tumor
samples (4-µm thick sections) were evaluated by IHC staining using
standard procedures (24,25). In brief, tissue slides were
deparaffinized in xylene and then rehydrated through graded
ethanols. The slides were incubated with the AKR1B10 antibody
(1:3,000) overnight at 4°C. The reacted antibody was visualized
with the Vector Laboratories ImmPRESS Detection kit, according to
the manufacturers protocols, included a horseradish
peroxidase-conjugated secondary antibody and a
diaminobenzidine-based stain. Finally, sections were counterstained
with Mayer's hematoxylin, dehydrated and mounted (23).
Immunohistochemical evaluation and
scoring
Protein expression was quantified by two independent
pathologists of Sir Run Run Shaw Hospital (Zhejiang University
School of Medicine) who were blinded to the clinical data.
Representative images were captured under a light microscope
(Olympus BX61, Shanghai, China; magnification, ×600). AKR1B10
expression was detected in the cytoplasm of tumor cells in gastric
carcinoma and assessed based on the staining intensity and
proportion of positive cells. The staining intensity was scored
from 0 to 3+ as follows: 0, no staining; 1+, weak staining; 2+,
moderate staining; and 3+, strong staining. AKR1B10-positive cells
were expressed as a percentage and divided into four grades: Grade
0, <5% positive; grade 1, 5–25% positive; grade 2, 26–50%
positive; and grade 3, >50% positive cells. The total score was
obtained by multiplying these two results (range 0–9) and samples
were divided into two groups: Negative expression (≤4) and positive
expression (>4). The threshold value of 4 was selected since the
median score (30) of AKR1B10
expression in gastric cancer samples was 4.5 in the present
study.
Statistical analysis
Statistical analysis was carried out using SSPS
software for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA).
The significant associations between AKR1B10 expression and various
clinic opathological parameters were determined by the
χ2 or Fisher's exact tests. The Kaplan-Meier test was
used to evaluate patient survival and the log-rank test for data
analysis. Prognostic factors were assessed by univariate and
multivariate analyses (Cox proportional hazards regression model).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Among the 53 patients, 40 were men and
13 women
Their age ranged from 33 to 77 years (median, 58
years). The tumor size in the majority of patients was <5 cm
(64.2%). In addition, lymph node metastasis was present in 68% of
patients and the depth of tumor invasion was T3 or T4 in 79.2%.
Following neoadjuvant chemotherapy 20 patients (37.7%) exhibited
tumor regression (TRG 3 or 4) as defined here, and complete
regression occurred in 5 patients. The patient data is summarized
in Table I.
Expression of AKR1B10 in gastric
cancer cell lines
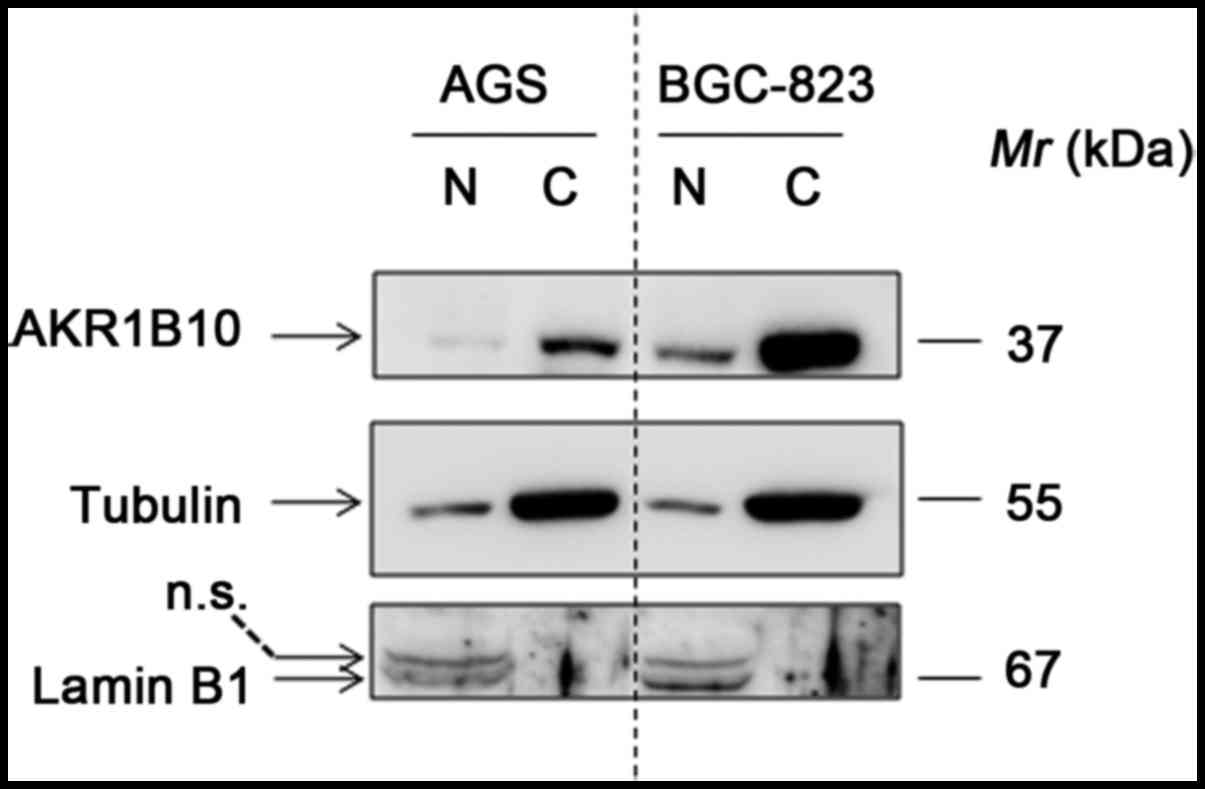
Western blotting was carried out to detect the
AKR1B10 expression in nuclear and cytoplasmic fractions of gastric
cancer cell lines. The results identified that AKR1B10 can be
readily detected, and was predominantly expressed in cytoplasm in
both AGS and BGC-823 cells. Notably, the expression of AKR1B10 is
approximately 5-fold higher in BGC-823 cells than that in AGS cells
(Fig. 1).
Expression of AKR1B10 in gastric
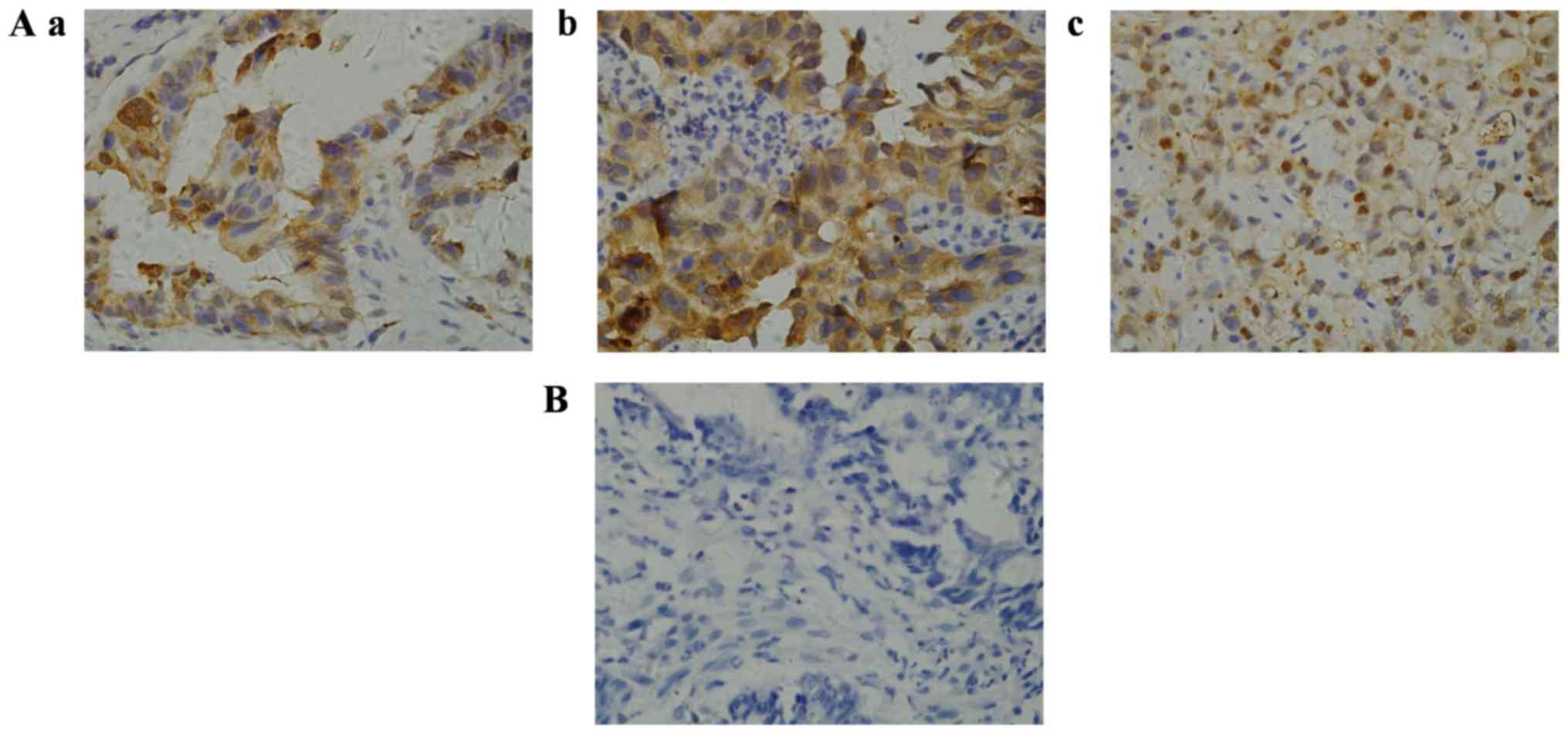
cancer and its association with clinic opathological features
The expression and subcellular localization of
AKR1B10 were determined in paraffin-embedded biopsy specimens of
gastric cancer. Immunohistochemistry revealed that AKR1B10 protein
was primarily expressed in the cytoplasm of gastric cancer cells
(Fig. 2), and demonstrated that the
antibody used had a strong potency as well as consistency with a
previous study (31). AKR1B10
immunoreactivity was detected in 31 gastric carcinoma samples. The
positive rate of AKR1B10 protein expression was 58.5% with an
overall score >4, whereas 22 (41.5%) samples exhibited negative
expression with a score ≤4. To evaluate the role of AKR1B10 in
gastric cancer the correlation between AKR1B10 expression and the
patients' clinic opathological features was assessed (Table II). No correlation was been observed
between the expression level of AKR1B10 protein and patient age,
sex, tumor size, tumor location, tumor staging, tumor depth or and
histological differentiation. However, the frequency of
AKR1B10-positive reactivity was higher in gastric cancer with lymph
node metastasis than in that without metastasis (69.4%, 25/36 vs.
35.3%, 6/17; P=0.020). The proportion of AKR1B10-positive samples
was higher in patients with decreased tumor regression following
neoadjuvant chemotherapy than in those with tumor regression grades
3 and 4 (69.7%, 23/33 vs. 40%, 8/20; P=0.033).
 | Table II.Correlation of Aldo-ketoreductase
family 1 member B10 expression with clinic opathological parameters
in gastric cancer patient with neoadjuvant chemotherapy. |
Table II.
Correlation of Aldo-ketoreductase
family 1 member B10 expression with clinic opathological parameters
in gastric cancer patient with neoadjuvant chemotherapy.
|
| AKR1B10
expression |
|---|
|
|
|
|---|
| Clinical
factors | Positive (%) | Negative (%) | P-value |
|---|
| Age, years |
|
| 0.565 |
|
<58 | 15 (57.7) | 11 (42.3) |
|
|
≥58 | 16 (59.3) | 11 (40.7) |
|
| Sex |
|
| 0.108 |
|
Male | 21 (52.5) | 19 (47.5) |
|
|
Female | 10 (76.9) | 3 (23.1) |
|
| Tumor size, cm |
|
| 0.211 |
|
<5 | 18 (52.9) | 16 (47.1) |
|
| ≥5 | 13 (68.4) | 6 (31.6) |
|
| Tumor location |
|
| 0.236 |
| Cardia,
fundus, body | 14 (51.9) | 13 (48.1) |
|
|
Antral | 17 (65.4) | 9 (34.6) |
|
| Tumor staging |
|
| 0.171 |
|
I–II | 13 (50.0) | 13 (50.0) |
|
|
III–IV | 18 (66.7) | 9 (33.3) |
|
| Tumor depth |
|
| 0.259 |
|
T1-T2 | 5 (45.5) | 6 (54.5) |
|
|
T3-T4 | 26 (61.9) | 16 (38.1) |
|
| Lymph node
metastasis |
|
| 0.020a |
|
Yes | 25 (69.4) | 11 (30.6) |
|
| No | 6 (35.3) | 11 (64.7) |
|
| Histological
differentiation |
|
| 0.399 |
|
Differentiated | 15 (62.5) | 9 (37.5) |
|
|
Undifferentiated | 16 (55.2) | 13 (44.8) |
|
| Tumor regression
grade |
|
| 0.033a |
|
0–2 | 23 (69.7) | 10 (30.3) |
|
|
3–4 | 8 (40.0) | 12 (60.0) |
|
| Chemotherapy
regimen |
|
| 0.583 |
|
FOLFOX | 13 (59.1) | 9 (40.9) |
|
|
EOX | 8 (50.0) | 8 (50.0) |
|
| Others
(SOX, ECX, EOS, S1) | 8 (61.5) | 5 (38.5) |
|
|
Chemoradiotherapy | 2 (100.0) | 0 (0.0) |
|
| Chemotherapy
cycle |
|
| 0.768 |
| 2 | 4 (50.0) | 4 (50.0) |
|
| 3 | 11 (64.7) | 6 (35.3) |
|
| ≥4 | 16 (57.1) | 12 (42.9) |
|
Survival analysis
Overall survival of between 1 and 67 months occurred
in the 53 gastric cancer cases, and the median overall survival was
19 months. Follow up results indicated that the mean survival time
of patients with gastric cancer with neoadjuvant chemotherapy with
AKR1B10-negative samples was 25.0 months, while in those with
AKR1B10-positive samples the mean overall survival was 19.5 months.
Patients were classified as alive (67.9%; n=36/53) or dead (32.1%;
n=17/53) according to the final follow-up on Dec 31, 2015.
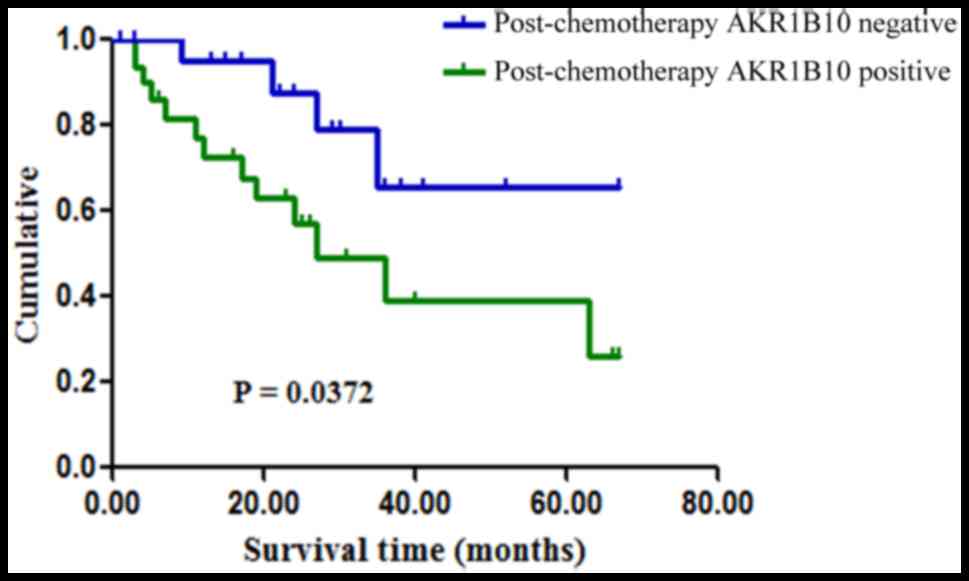
Kaplan-Meier survival analysis indicated that patients with
positive AKR1B10 expression had poorer survival rates than those
with negative AKR1B10 expression (P=0.0372; Fig. 3). Univariate analysis for age, sex,
tumor size, tumor location, tumor stage, tumor depth, lymph node
metastasis, histological type, and tumor response to neoadjuvant
chemotherapy revealed significant associations of tumor location
(P=0.0237), tumor stage (P=0.0066), lymph node metastasis
(P=0.0006) and tumor regression (P=0.0496) with overall survival,
while the other factors exhibited no such association (Table III).
 | Table III.Univariate and multivariate survival
analyses of various clinicopathological parameters in gastric
cancer patient with neoadjuvant chemotherapy. |
Table III.
Univariate and multivariate survival
analyses of various clinicopathological parameters in gastric
cancer patient with neoadjuvant chemotherapy.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Clinical
factors | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age | 0.9304 | 1.040 | 0.3953–2.759 | 0.0643 | 7.958 | 0.962–3.985 |
| Sex | 0.4931 | 1.481 | 0.4819–4.549 | 0.2361 | 7.595 | 0.737–3.452 |
| Tumor size | 0.9655 | 0.9783 | 0.3619–2.644 | 0.1360 | 0.205 | 0.025–1.648 |
| Tumor location | 0.0237 | 0.3081 | 0.1111–0.8545 | 0.6691 | 1.347 | 0.343–5.298 |
| Tumor staging | 0.0066 | 0.2626 | 0.1000–0.6897 | 0.9064 | 1.806 | 0.385–2.936 |
| Tumor depth | 0.2384 | 0.4545 | 0.1225–1.686 | 0.0992 | 0.476 | 0.197–1.150 |
| Lymph node
metastasis | 0.0006 | 5.562 | 2.087–14.82 | 0.0021 | 4.750 | 1.744–12.934 |
| Histological
differentiation | 0.5747 | 0.7574 | 0.2869–1.999 | 0.0504 | 0.465 | 0.216–1.000 |
| Tumor regression
grade | 0.0496 | 2.635 | 1.002–6.931 | 0.0365 | 0.515 | 0.277–0.957 |
| AKR1B10 | 0.0372 | 2.797 | 1.063–7.359 | 0.0344 | 5.230 | 1.133–24.131 |
Furthermore, multivariate analysis was performed on
the factors. Analysis identified lymph node metastasis, tumor
regression grade and AKR1B10 expression as independent prognostic
predictors for overall survival, presented in Table III.
Discussion
Gastric cancer is a heterogeneous disease with
distinct biological behaviors. Previously, neoadjuvant chemotherapy
has been considered the primary choice of treatment in locally
advanced gastric cancer due to the survival advantage of combined
chemotherapy and surgery over surgery alone (5,32).
Multiple studies have demonstrated the efficacy of chemotherapeutic
agents by assessing the clinical and pathological responses of
tumors (6). A World Health
Organisation-based evaluation of the clinical response in gastric
cancer using conventional staging modalities, including endoscopic
ultrasonography and CT contrast, was inaccurate (33,34), while
assessment of post-chemotherapy histological change, another
technique for assessing the tumor response, was considered highly
useful (35). Mandard's tumor
regression grade (29) predicts the
pathological response to cytotoxic agents. The clinical usefulness
of these studies remains unclear, therefore, it is important to
identify precise molecular markers which predict the effectiveness
of neoadjuvant chemotherapy for improved management of patients
with gastric cancer. In the present study, it was identified that
AKR1B10 protein expression in gastric cancer was significantly
associated with patient survival. In this context, to the best of
our knowledge, this is the first study to demonstrate the role of
AKR1B10 in gastric cancer with neoadjuvant chemotherapy.
Multiple studies have identified that AKR1B10 is
highly expressed in many solid tumors outside the digestive tract,
including non-small cell lung cancer, breast cancer, pancreatic
cancer and hepatic cancer (17–20).
Clinicopathological studies in liver tumors have reported that
AKR1B10 may be a useful biomarker of tumor proliferation and
differentiation as well as being responsible for the initial phases
of hepatocarcinogenesis (17,35). Notably, downregulated AKR1B10
expression has been reported in gastrointestinal cancers without
pre-operative treatment and its expression correlates with
increased overall survival (30,36). In
the present study, the expression of AKR1B10 protein was
investigated using immunohistochemical staining in 53 gastric
cancer biopsies taken from patients who received preoperative
chemotherapy. Immunoreactivity was predominantly detected in the
cytoplasm. This result was consistent with the expression of
AKR1B10 in primary resected gastric tumor reported by Yao et
al (31). Among the 53 samples,
AKR1B10 positive expression was observed in 31/53 gastric carcinoma
samples and tended to be increased in patients with lymph node
metastasis compared with patients without metastasis. Furthermore,
the rate of AKR1B10-positivity was significantly increased in
samples from patients with less tumor regression compared with
patients exhibiting complete or nearly-complete regression.
Although AKR1B10-negative protein expression was observed in tumor
regression grades 3 and 4, the associations between AKR1B10
expression and other clinical factors were not significant.
Furthermore, Kaplan-Meier survival curves were used to investigate
the association between AKR1B10 expression and survival. The
results revealed that patients with AKR1B10-positive samples
experienced significantly poorer survival than those with
AKR1B10-negative samples. A previous study by Yao et al
(36) reported that positive AKR1B10
staining was also markedly associated with lymph node and distant
metastasis, tumor size, and Tumor-Node-Metastasis stage. The
present results differ from previous studies, possibly due to the
fact that the samples were biopsy materials taken following
preoperative chemotherapy. Concurrently, survival time and other
clinic opathological factors were investigated by univariate
analysis to identify any associations. Notably, it was identified
that tumor stage, tumor location, lymph node metastasis and tumor
regression were significantly associated with survival. Wang et
al (6) reported similar results
regarding tumor regression. Additionally, lymph node metastasis,
tumor regression grade and AKR1B10 expression were selected as
independent prognostic predictors via multivariate analysis. Based
on the above findings, it may be concluded that AKR1B10 expression
is associated with lymph node metastasis and a worse prognosis in
patients with a poor response to neoadjuvant chemotherapy.
AKR1B10 catalyzes the reduction of highly
electrophilic compounds, including cytotoxic α β-unsaturated
carbonyls as by-products of cell metabolism and lipid peroxidation
(34). Unsaturated carbonyls may
induce protein dysfunction, DNA damage and apoptosis (35). AKR1B10 is able to protect host cells
from carbonyl lesions by detoxifying cellular carbonyls and their
glutathione conjugates through the conversion of reactive carbonyl
groups into less active hydroxyls (37–39). A
study carried out by Luo et al (40) demonstrated that AKR1B10 protein is
secreted by a lysosome-mediated non-classical pathway and is
considered to be a tumor marker. It is hypothesized that AKR1B10
promotes cancer cell growth via a number of mechanisms. A study by
Matsunaga et al (41)
suggested functions for AKR1B10 in cancer cells, including
detoxifying carbonyl compounds, promoting fatty acid and lipid
synthesis, reducing farnesal and geranylgeraniol to their alcohols,
and reducing retinoic acid. The present study validated the
expression of AKR1B10 in AGS and BGC-823 gastric carcinoma cell
lines. It was identified that AKR1B10 is localized to the
cytoplasm, and is highly expressed in both cell lines. However,
much may be learned about the functions of AKR1B10 by either
knockout or over expression models in gastric carcinoma cell lines
in future studies. AKR1B10 inactivation is not a consequence of
promoter hypermethylation or chromosome rearrangement in colon
cancer, rather, it results from specific regulation by carcinogenic
transcription factors. Several studies have identified multiple
putative oncogenic and tumor-suppressor protein-binding sites
within the AKR1B10 promoter region, including the transcription
factors nuclear factor erythroid 2 like 2, activator protein 1,
tumor protein p53, and nuclear factor κ-light-chain-enhancer of
activated B cells, and antioxidant response elements (42,43).
Despite advances in research, the current understanding of the
underlying enzymatic mechanisms, pharmacological modulation, gene
regulation and physiological roles of AKR1B10 remain uncertain,
thus additional studies are required to resolve the function of
AKR1B10 in cellular growth and survival in gastric cancer.
To conclude, AKR1B10 is expressed in the cytoplasm
of tumor cells in gastric cancer. Positive expression of AKR1B10
protein is associated with lymph node metastasis and a decreased
tumor response to neoadjuvant chemotherapy. AKR1B10-positivity also
predicts poorer overall survival in gastric cancer, and may be a
useful therapeutic marker for determining appropriate treatment
management.
Acknowledgements
The authors would like to thank Ms HY Wang for
technical support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81750110546,
31571476 and 31370772), Science Technology Department of Zhejiang
Provence (grant no. 2017C33082 and 2017C37165).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XT and ZNJ conceived the study design; SMUA, ZNJ,
ZHZ, YL, XJW and XT analyzed the data; SMUA and XT drafted the
manuscript; all authors read and approved the final manuscript.
Ethics approval and patient consent for
publication
The present study was approved by the Human Ethics
Review Committee of Sir Run Run Shaw Hospital, Zhejiang University
School of Medicine.
Patient consent for publication
Patients provided written informed consent and were
informed that resected specimens were stored by the hospital, and
potentially used for scientific research and publication, and their
privacy would be maintained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasako M, Sano T, Yamamoto S, Kurokawa Y,
Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai
K, et al: D2 lymphadenectomy alone or with para-aortic nodal
dissection for gastric cancer. N Engl J Med. 359:453–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LB, Teng RY, Jiang ZN, Hu WX, Dong
MJ, Yuan XM, Chen WJ, Jin M and Shen JG: Clinicopathologic
variables predicting tumor response to neoadjuvant chemotherapy in
patients with locally advanced gastric cancer. J Surg Oncol.
105:293–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carlomagno C, Pepe S, D'Armiento FP,
D'Armiento M, Cannella L, De Stefano A, Crispo A, Giordano M and De
Placido S: Predictive factors of complete response to neoadjuvant
chemoradiotherapy in patients with rectal cancer. Oncology.
78:369–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langer R, Ott K, Feith M, Lordick F,
Siewert JR and Becker K: Prognostic significance of
histopathological tumor regression after neoadjuvant chemotherapy
in esophageal adenocarcinomas. Mod Pathol. 22:1555–1563. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suárez J, Vera R, Balén E, Gómez M, Arias
F, Lera JM, Herrera J and Zazpe C: Pathologic response assessed by
mandard grade is a better prognostic factor than down staging for
disease-free survival after preoperative radiochemotherapy for
advanced rectal cancer. Colorectal Dis. 10:563–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith FM, Reynolds JV, Kay EW, Crotty P,
Murphy JO, Hollywood D, Gaffney EF, Stephens RB and Kennedy MJ:
COX-2 overexpression in pretreatment biopsies predicts response of
rectal cancers to neoadjuvant radiochemotherapy. Int J Radiat Oncol
Biol Phys. 64:466–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Novell A, Morales S, Valls J, Panadés MJ,
Salud A, Iglesias E, Vilardell F, Matias-Guiu X and Llombart-Cussac
A: Novel biomarkers in primary breast core biopsies to predict poor
response to neoadjuvant chemotherapy and appearance of metastases.
Histol Histopathol. 32:909–915. 2017.PubMed/NCBI
|
|
12
|
Barski OA, Tipparaju SM and Bhatnagar A:
The aldo-keto reductase superfamily and its role in drug metabolism
and detoxification. Drug Metab Rev. 40:553–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan R, Zu X, Ma J, Liu Z, Adeyanju M and
Cao D: Aldo-keto reductase family 1 B10 gene silencing results in
growth inhibition of colorectal cancer cells: Implication for
cancer intervention. Int J Cancer. 121:2301–2306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crosas B, Hyndman DJ, Gallego O, Martras
S, Parés X, Flynn TG and Farrés J: Human aldose reductase and human
small intestine aldose reductase are efficient retinal reductases:
Consequences for retinoid metabolism. Biochem J. 373:973–979. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Yan R, Luo D, Watabe K, Liao DF
and Cao D: Aldo-keto reductase family 1 member B10 promotes cell
survival by regulating lipid synthesis and eliminating carbonyls. J
Biol Chem. 284:26742–26748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hyndman DJ and Flynn TG: Sequence and
expression levels in human tissues of a new member of the aldo-keto
reductase family. Biochim Biophys Acta. 1399:198–202. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heringlake S, Hofdmann M, Fiebeler A,
Manns MP, Schmiegel W and Tannapfel A: Identification and
expression analysis of the aldo-ketoreductase1-B10 gene in primary
malignant liver tumours. J Hepatol. 52:220–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukumoto S, Yamauchi N, Moriguchi H, Hippo
Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H,
Yamamoto S, et al: Overexpression of the aldo-keto reductase family
protein AKR1B10 is highly correlated with smokers' non-small cell
lung carcinomas. Clin Cancer Res. 11:1776–1785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma J, Luo DX, Huang C, Shen Y, Bu Y,
Markwell S, Gao J, Liu J, Zu X, Cao Z, et al: AKR1B10
overexpression in breast cancer: Association with tumor size, lymph
node metastasis and patient survival and its potential as a novel
serum marker. Int J Cancer. 131:E862–E871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung YT, Matkowskyj KA, Li H, Bai H,
Zhang W, Tsao MS, Liao J and Yang GY: Overexpression and oncogenic
function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic
carcinoma. Mod Pathol. 25:758–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laffin B and Petrash JM: Expression of the
aldo-ketoreductases AKR1B1 and AKR1B10 in human cancers. Front
Pharmacol. 3:1042012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo L, Chen Y, Wu D, Shou J, Wang S, Ye J,
Tang X and Wang Jun X: Differential expression patterns of Nqo1,
AKR1B8 and Ho-1 in the liver and small intestine of C57BL/6 mice
treated with sulforaphane. Data Brief. 5:416–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo L, Chen Y, Wu D, Shou J, Wang S, Ye J,
Tang X and Wang XJ: Butylated hydroxyanisole induces distinct
expression patterns of Nrf2 and detoxification enzymes in the liver
and small intestine of C57BL/6 mice. Toxicol Appl Pharmacol.
288:339–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed SM, Wu X, Jin X, Zhang X, Togo Y,
Suzuki T, Li Y, Kanematsu A, Nojima M, Yamamoto S, et al:
Synergistic induction of apoptosis by mapatumumab and
anthracyclines in human bladder cancer cells. Oncol Rep.
33:566–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang X, Wang H, Fan L, Wu X, Xin A, Ren H
and Wang XJ: Luteolin inhibits Nrf2 leading to negative regulation
of the Nrf2/ARE pathway and sensitization of human lung carcinoma
A549 cells to therapeutic drugs. Free Radic Biol Med. 50:1599–1609.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et
al: Staging system for breast cancer: Revisions for the 6th edition
of the ajcc cancer staging manual. Surg Clin North Am. 83:803–819.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P and Samama
G: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawasaki Y, Ishigami S, Arigami T,
Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H,
Nakajo A, et al: Clinicopathological significance of nuclear factor
(erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer.
BMC Cancer. 15:52015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao HB, Xu Y, Chen LG, Guan TP, Ma YY, He
XJ, Xia YJ, Tao HQ and Shao QS: AKR1B10, a good prognostic
indicator in gastric cancer. Eur J Surg Oncol. 40:318–324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LB, Shen JG, Xu CY, Chen WJ, Song XY
and Yuan XM: Neoadjuvant chemotherapy versus surgery alone for
locally advanced gastric cancer: A retrospective comparative study.
Hepatogastroenterology. 55:1895–1898. 2008.PubMed/NCBI
|
|
33
|
Mallery S, DeCamp M, Bueno R, Mentzer SJ,
Sugarbaker DJ, Swanson SJ and Van Dam J: Pretreatment staging by
endoscopic ultrasonography does not predict complete response to
neoadjuvant chemoradiation in patients with esophageal carcinoma.
Cancer. 86:764–769. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brown WA, Thomas J, Gotley D, Burmeister
BH, Lim KH, Martin I, Walpole ET, Thomson DB, Harvey JA and
Smithers BM: Use of oesophagogastroscopy to assess the response of
oesophageal carcinoma to neoadjuvant therapy. Br J Surg.
91:199–204. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biondo S, Navarro M, Marti-Rague J,
Arriola E, Pares D, Del Rio C, Cambray M and Novell V: Response to
neoadjuvant therapy for rectal cancer: Influence on long-term
results. Colorectal Dis. 7:472–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao HB, Xu Y, Chen LG, Guan TP, Ma YY, Tao
HQ and Shao QS: Expression of aldo-keto reductase family 1 member
B10 in gastric cancer tissues and its clinical significance.
Zhonghua Wei Chang Wai Ke Za Zhi. 16:183–187. 2013.(In Chinese).
PubMed/NCBI
|
|
37
|
Jacobs AT and Marnett LJ: Systems analysis
of protein modification and cellular responses induced by
electrophile stress. Acc Chem Res. 43:673–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
LoPachin RM, Gavin T, Petersen DR and
Barber DS: Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein
toxicity: Nucleophilic targets and adduct formation. Chem Res
Toxicol. 22:1499–1508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong L, Liu Z, Yan R, Johnson S, Zhao Y,
Fang X and Cao D: Aldo-keto reductase family 1 B10 protein
detoxifies dietary and lipid-derived alpha, beta-unsaturated
carbonyls at physiological levels. Biochem Biophys Res Commun.
387:245–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo DX, Huang MC, Ma J, Gao Z, Liao DF and
Cao D: Aldo-keto reductase family 1, member B10 is secreted through
a lysosome-mediated non-classical pathway. Biochem J. 438:71–80.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsunaga T, Wada Y, Endo S, Soda M,
El-Kabbani O and Hara A: Aldo-keto reductase 1B10 and its role in
proliferation capacity of drug-resistant cancers. Front Pharmacol.
3:52012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Zhong L, Krishack PA, Robbins S,
Cao JX, Zhao Y, Chung S and Cao D: Structure and promoter
characterization of aldo-keto reductase family 1 B10 gene. Gene.
437:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishinaka T, Miura T, Okumura M, Nakao F,
Nakamura H and Terada T: Regulation of aldo-keto reductase AKR1B10
gene expression: Involvement of transcription factor Nrf2. Chem
Biol Interact. 191:185–191. 2011. View Article : Google Scholar : PubMed/NCBI
|