Introduction
Neuroendocrine tumors (NETs, also named carcinoids)
are rare neoplasms that most commonly originate in the
gastrointestinal tract (1), with an
incidence of 2.5–5 cases per 100,000 population (2). Approximately 20–30% of these patients
present with carcinoid syndrome, which can occur regardless of the
presence of metastatic disease (3).
Carcinoid heart disease (CHD) is frequently associated with
carcinoid syndrome and is responsible for substantial morbidity and
mortality (4,5), with a 2-year survival of ~10% following
the onset of advanced heart failure symptoms (6). CHD is characterized by plaque-like,
fibrous endocardial thickening of the heart valves, mainly in the
right heart; mitral and aortic valves are very rarely involved.
There is considerable variation regarding the screening and
management of CHD (7). A recent
expert consensus (2) mentions
transthoracic echocardiogram (TTE) as the key modality in the
evaluation of CHD, but cardiac magnetic resonance (CMR) imaging can
be a valuable adjunct (8). Cardiac
valve replacement is the only intervention proven to improve
survival in CHD (6). We present a
series of imaging findings in CHD patients approved by the MD
Anderson Catheterization Laboratory Registry at The University of
Texas MD Anderson Cancer Center (Houston, TX, USA) with surgical
replacement of 1 to 4 heart valves.
Case series
Case 1
A 57-year-old male with a history of heart failure
due to severe tricuspid regurgitation caused by CHD presented with
chest pain, dyspnea, and bilateral lower extremity edema. Nuclear
perfusion stress test was negative for ischemia (Fig. 1A). CMR imaging 6 months prior to the
current presentation revealed a severely dilated right atrium and
right ventricle (Fig. 1B), with a
small and fixed medial tricuspid valve leaflet and severe tricuspid
regurgitation (Fig. 1C). Mild
mid-myocardial delayed enhancement in the proximal inferolateral
wall suggestive of non-ischemic cardiomyopathy was also noted.
Surgical tricuspid valve replacement was considered. On
preoperative TTE, the right atrium and right ventricle were
severely dilated, with severe tricuspid regurgitation (Fig. 1D and 1E). An interatrial shunt was
observed on TEE (Fig. 1F, arrow),
documented with contrast injection (Fig.
1G) and Doppler color (Fig. 1H,
arrow). The patient received a 31-mm Epic bioprosthetic tricuspid
valve (St. Jude Medical, Inc., St. Paul, MN, USA) and had an
uneventful postoperative course, with significantly improved heart
failure symptoms. Good functional status was maintained at the last
follow-up, 8 months after the surgery.
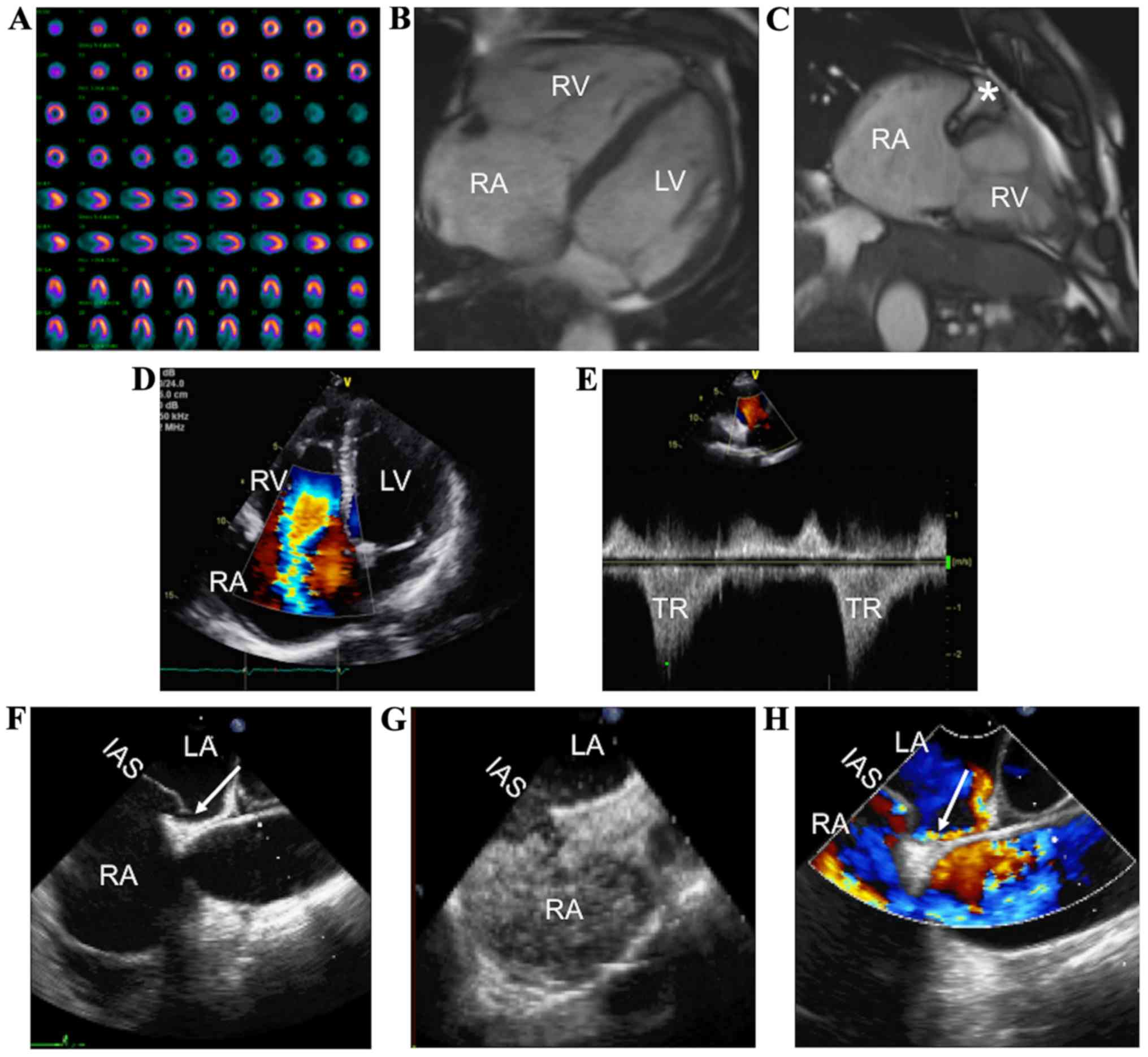 | Figure 1.CHD in a patient who underwent
tricuspid valve replacement. (A) Normal nuclear perfusion stress
test. (B and C) Cardiac magnetic resonance imaging with evidence of
severe RA and RV dilation, and TV thickening (*) with severe TR. (D
and E) Preoperative transthoracic echocardiography showing severe
TR. Preoperative transesophageal echocardiography revealing
persistent foramen ovale (F, arrow) with contrast (G) and Doppler
studies (H, arrow). CHD, carcinoid heart disease; RA, right atrium;
RV, right ventricle; TV, tricuspid valve; TR, tricuspid
regurgitation; IAS, interatrial septum; LV, left ventricle. |
Case 2
A 40-year-old male with a 3-year history of CHD
presented for increasing dyspnea, abdominal distention, and lower
extremity edema. CMR two years before the current presentation
showed severe right atrium and right ventricle dilation (Fig. 2A), dilated tricuspid annulus, immobile
tricuspid leaflets with no coaptation, severe tricuspid
regurgitation (Fig. 2B), mild
pulmonic regurgitation, and a pulmonic stenotic jet (Fig. 2C, arrow). Surgical replacement of the
tricuspid and pulmonic valves was considered. Preoperative TTE
showed severely dilated right atrium and right ventricle (Fig. 2D), with severe tricuspid regurgitation
(Fig. 2E) and pulmonic regurgitation
(Fig. 2F). The patient underwent
surgical tricuspid and pulmonic valves replacement with Epic
bioprosthetic valves (29 and 23 mm, respectively; St. Jude Medical,
Inc.), without perioperative complications and with symptoms
resolution. On postoperative TTE, the right ventricular size
normalized (Fig. 2G) and the
bioprosthetic tricuspid and pulmonic valves were functioning
normally (Fig. 2H and I). The patient
was free of heart failure symptoms at 13 months post-surgery.
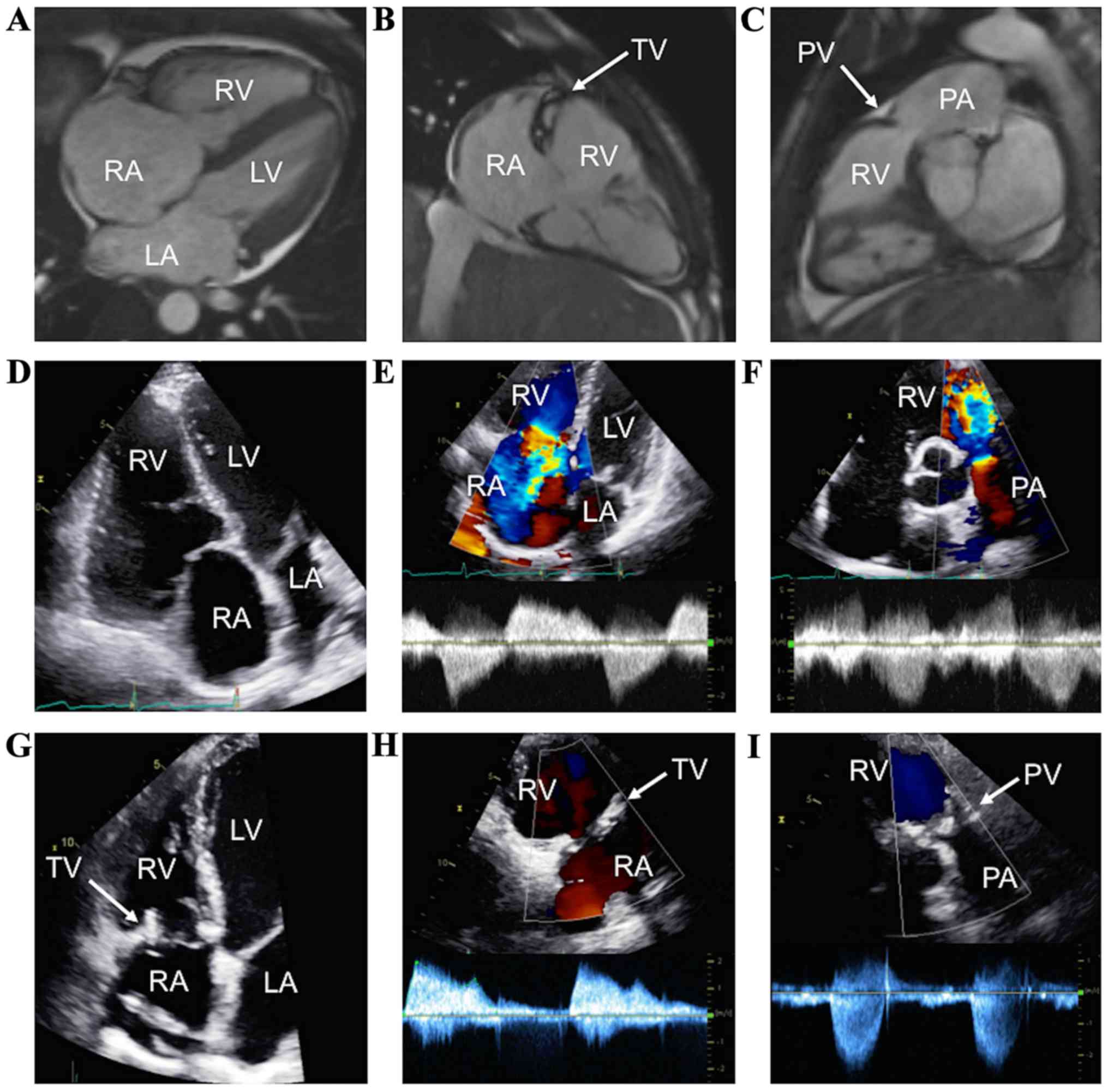 | Figure 2.CHD in a patient who underwent
simultaneous tricuspid and pulmonary valve replacement. (A and B,
arrow) Cardiac magnetic resonance imaging revealing severely
diseased TV, and (C, arrow) mild carcinoid involvement of the PV.
(D and E) Preoperative TTE with severe tricuspid and (F) pulmonary
regurgitation. (G-I) Postoperative TTE showing bioprosthetic TV and
PV, with normal function. CHD, carcinoid heart disease; TV,
tricuspid valve; LA, left atrium; PV, pulmonary valve; LV, left
ventricle; PA, pulmonary artery; TTE, transthoracic
echocardiography; RA, right atrium; RV, right ventricle. |
Case 3
A 78-year-old man with CHD and severe tricuspid
regurgitation diagnosed 2 years prior to the current admission
presented with mild dyspnea. TTE revealed enlarged right atrium and
right ventricle (Fig. 3A), severe
tricuspid regurgitation (Fig. 3B),
moderate pulmonic regurgitation, and moderate aortic regurgitation.
CMR showed severe right atrium and right ventricle enlargement
(Fig. 3C and D), immobile and
thickened tricuspid valve leaflets with severe tricuspid
regurgitation, and thickened aortic valve leaflets, with moderate
aortic regurgitation. In the presence of cardiac symptoms, the
decision was made to replace the tricuspid, pulmonic, and aortic
valves in one surgery. A 23-mm Labcor TBLP Supra bioprosthetic
aortic valve (Labcor Laboratõrios, Belo Horizonte, Brazil), a 29-mm
Epic pulmonary bioprosthetic valve, and a 29-mm Epic tricuspid
bioprosthetic valve (both from St. Jude Medical, Inc.) were
implanted. After a successful surgery with no perioperative
complications and with symptomatic relief, TTE showed normal right
atrial and right ventricular sizes (Fig.
3E), without residual tricuspid regurgitation (Fig. 3F). CMR performed postoperatively
revealed normal right heart sizes (Fig.
3G), with visible bioprosthetic tricuspid valve (Fig. 3G and H arrows), aortic valve (Fig. 3I and J, thin arrows), and pulmonic
valve (Fig. 3J, thick arrow). Good
functional status was reported 11 months following the
intervention.
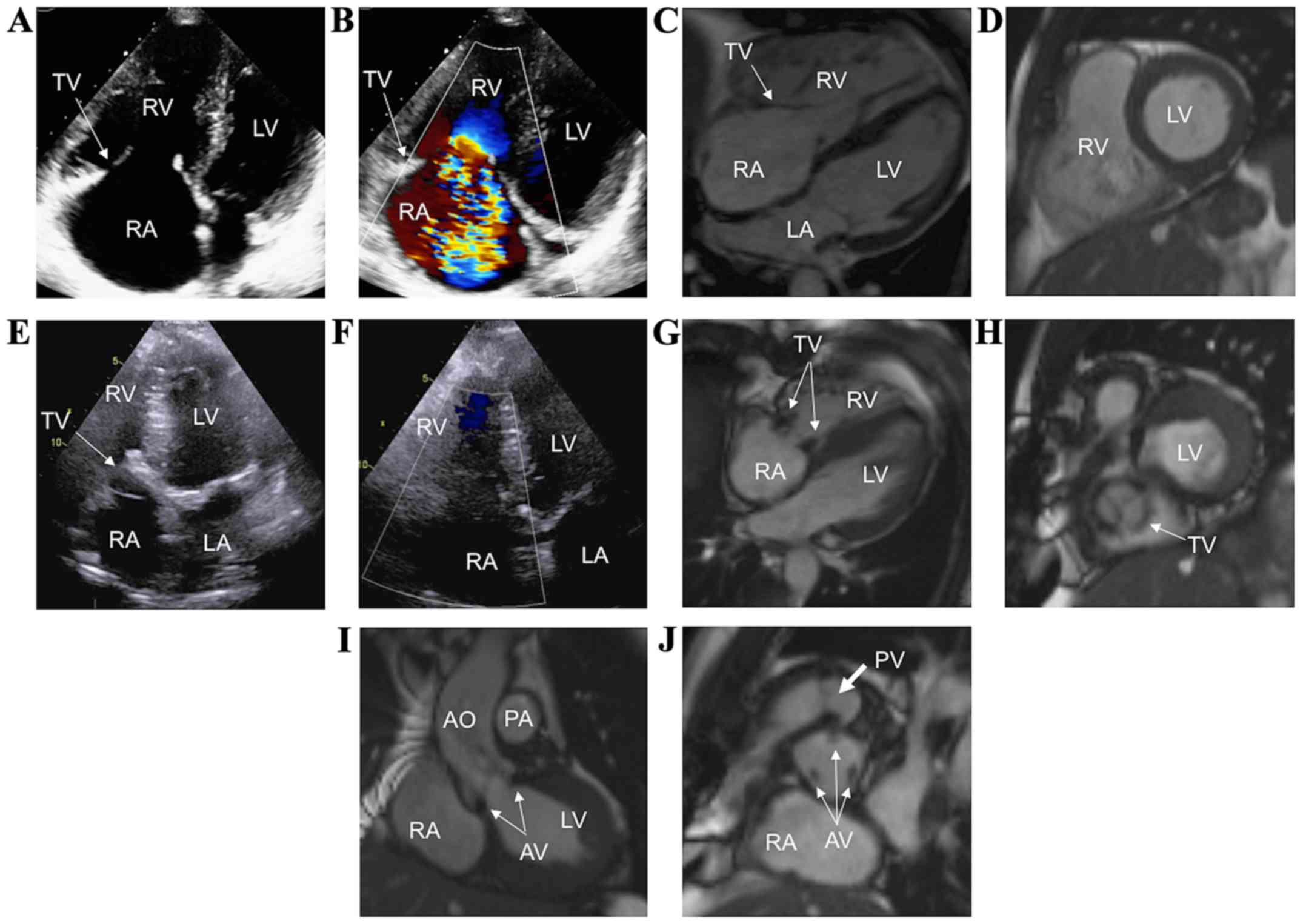 | Figure 3.CHD in a patient who underwent
simultaneous tricuspid, pulmonic, and aortic valve replacement. (A
and B) TTE showing severe TR. (C and D) Preoperative CMR imaging
illustrating dilated right heart chambers and severe TR. (E and F)
Postoperative TTE showing the bioprosthetic TV, without residual
TR. Postoperative CMR imaging showing the bioprosthetic TV (G and
H, arrows), aortic valve (I and J, thin arrows), and pulmonary
valve (J, thick arrow) in different views. CHD, carcinoid heart
disease; TTE, transthoracic echocardiography; TR, tricuspid
regurgitation; CMR, cardiac magnetic resonance; TV, tricuspid
valve; AO, ascending aorta; LA, left atrium; LV, left ventricle;
PA, pulmonary artery; RA, right atrium; RV, right ventricle. |
Case 4
A 54-year-old female with a history of
neuroendocrine tumor and subsequent concern for CHD presented with
orthopnea, paroxysmal nocturnal dyspnea, and lower extremity edema.
While CMR 9 months prior showed normal cardiac chamber sizes
(Fig. 4A) and mild-to-moderate aortic
regurgitation and mitral regurgitation (Fig. 4B), current TTE confirmed rapid
progression of her valvular heart disease, with right ventricular
volume overload, thickened mitral and aortic valves (Fig. 4C, zoom on the mitral valve), severe
tricuspid regurgitation (Fig. 4D),
severe pulmonic regurgitation (Fig.
4E), severe eccentric mitral regurgitation (Fig. 4F, arrow), severe mitral stenosis (mean
gradient of 13.1 mmHg), and severe aortic regurgitation (Fig. 4G). She successfully underwent surgical
replacement of the aortic, mitral, tricuspid, and pulmonic valves
with Epic bioprosthetic valves (aortic valve, 21 mm; pulmonary
valve, 21 mm; mitral valve, 29 mm; and tricuspid valve, 29 mm; St.
Jude Medical, Inc.) during the same procedure, with an excellent
postoperative course. TTE on postoperative follow-up showed normal
biventricular size and function, with normally functioning
bioprostheses (Fig. 4H and I, zoom on
the mitral valve). No heart failure symptoms were present at 1-year
follow-up.
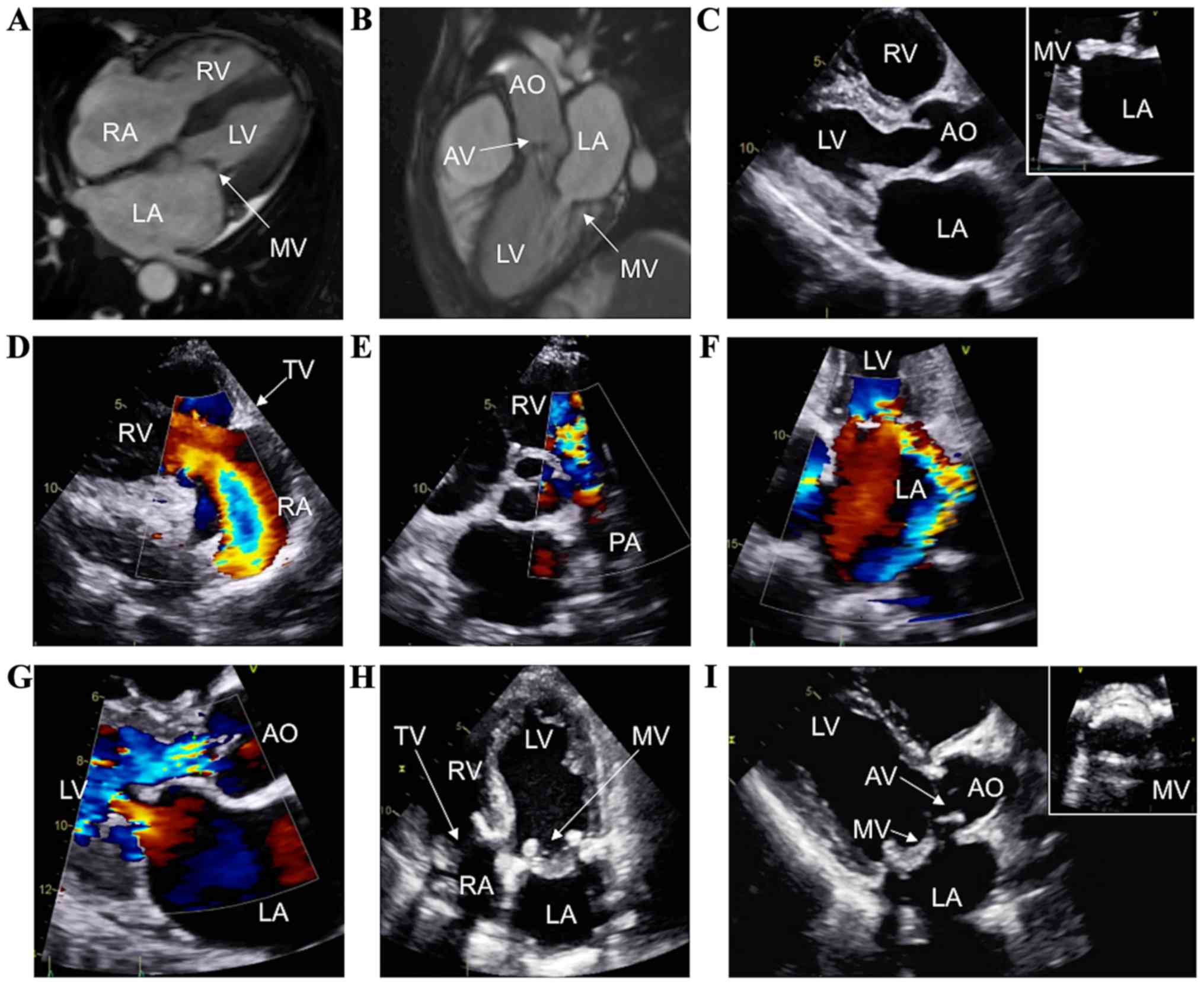 | Figure 4.CHD in a patient who underwent
simultaneous tricuspid, pulmonic, mitral, and aortic valve
replacement. (A and B) Cardiac magnetic resonance imaging showing a
normal right heart and moderate carcinoid involvement of the MV and
AV. Preoperative TTE assessment showing thickening of the AV and MV
(C, zoom on the MV), severe tricuspid (D) and pulmonary
regurgitation (E), and moderate mitral (F) and aortic regurgitation
(G). Postoperative TTE revealing the implanted bioprostheses of the
tricuspid valve, MV, and AV (H-I, zoom on the MV). CHD, carcinoid
heart disease; MV, mitral valve; AV, aortic valve; AO, ascending
aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery;
RA, right atrium; RV, right ventricle. |
Diagnosing carcinoid heart disease
Despite progress in the medical and surgical
management of patients with carcinoid disease, CHD remains a major
cause of morbidity and mortality (4).
It is believed that CHD most commonly involves the right side of
the heart because of the inactivation of humoral substances by the
lung (9). Left-sided involvement can
occur in <10% of patients with CHD (9), usually because of a concomitant
right-to-left shunt such as a patent foramen ovale (7), or more rarely because of lung metastasis
or high disease burden with unusually increased levels of
circulating serotonin (10). The most
common forms of valvular dysfunction noted in patients with CHD
include tricuspid or pulmonic regurgitations, but concomitant
pulmonic stenosis is also often found (9). Irreversible ventricular dysfunction can
develop with valvular regurgitation before the onset of clinical
symptoms, hence the need for careful serial observations and
periodic serial cardiac imaging (11).
TTE is the main diagnostic tool used for the
diagnosis and evaluation of CHD. However, if TTE is suboptimal, TEE
can significantly enhance assessment of the mitral, aortic, and
tricuspid valves. Advances in three-dimensional echocardiography
and CMR can further delineate the pathophysiology and severity of
valvular dysfunction. Assessment of myocardial strain is not well
established in CHD, but may add value for the evaluation of right
ventricular function (12,13). CMR and CT may be particularly helpful
in assessing the right heart, describing features of carcinoid
valve disease, valve pathology, and right ventricular function
(14). CMR also provides additional
diagnostic accuracy in patients with multivalvular disease
(11), who pose difficulties in
determining disease severity, and may influence long-term
management (15). Positron emission
tomography may also add benefit by identifying cardiac metastases
of the carcinoid tumor (15). Little
information is available on the predictability of disease
progression with imaging. Current data suggest that disease
progression is mainly correlated with biochemical variables (e.g.,
serum 5-HIAA), rather than imaging or clinical findings (16). Post-valve replacement imaging
assessment is not currently well documented and is generally
performed based on clinical judgment.
Treatment, surgical outcomes and disease
progression
The main objective of medical therapy in CHD is
symptomatic improvement. In the presence of heart failure,
treatment with diuretics and angiotensin-converting enzyme
inhibitors (17,18) is useful for symptomatic relief from
volume overload. Somatostatin analogs work by reducing serum levels
of serotonin, but have not been shown to prevent the development of
CHD (19). Novel agents, such as
everolimus (20) and telotristat
(21), have shown benefit for
carcinoid syndrome, but their role in the prevention of CHD has not
yet been established (19).
Early diagnosis of CHD and regular follow-up is
essential in order to identify patients likely to benefit from
surgery, the only effective treatment for CHD (6). Standard indications for valve surgery
apply, but mainly for patients whose metastatic carcinoid disease
and syndrome are well controlled. There is no standard preoperative
evaluation in these patients. The main driver of mortality in CHD
patients is not carcinomatosis, but severe tricuspid regurgitation
(22). Postoperative mortality is
mainly driven by the disease, rather than procedure-related
complications (23,24). Because cardiac surgery for CHD had
such high mortality rates, it was reserved for severely symptomatic
patients with advanced cardiac disease. Late surgical referral has
been proposed as a potential factor contributing to high
postoperative rates (25). However,
recent data suggest that there have been significant improvements
in the postoperative mortality rates and median survival (13). Nowadays, there is a trend of earlier
intervention, due to increased perioperative mortality in patients
with severe heart failure and improvements in cardiac surgery
(26). Cardiac surgery in CHD still
has higher mortality rates than the general population, but
survival and symptomatic relief are better than with medical
treatment alone (4,6,27). The
feasibility of multiple valve replacement has been reported,
leading to significant functional improvement (28,29), as
was the case with our patients.
Evidence suggests similar outcomes following either
mechanical or bioprosthetic valve implantation (24). Bioprosthetic valves are increasingly
used due to the decreased need for anticoagulation, despite concern
for premature bioprosthetic degeneration as a consequence of the
carcinoid tumor (15,30,31). In
cases of early prosthetic degeneration, reintervention with
implantation of a new bioprosthetic valve is indicated (13). Of note, in a case series of 3
patients, stentless bioprostheses in the pulmonic position have
been associated with premature restenosis requiring early
reintervention (32). Transcatheter
valve replacements have been described for the pulmonary (33) and tricuspid valves (34) in both native and bioprosthetic valves,
but safety and outcome data are limited.
In conclusion, CHD has a wide spectrum of severity.
A comprehensive imaging evaluation is necessary for an accurate
preoperative assessment. Surgical treatment can be successfully
attempted even in cases of multivalvular involvement, if
appropriately guided by a multimodality imaging assessment and a
multidisciplinary team. The management of patients with CHD is
complex and involves a multidisciplinary effort (6). An integrative approach, involving both
the Oncology and Cardiology teams, is recommended to achieve an
accurate evaluation of the severity of the lesions, their clinical
significance, and follow-up. Early recognition of CHD and prompt
surgical intervention, before advanced heart failure has occurred,
may improve the outcome of these patients. The accurate timing of
surgery is crucial, but there are no clear guidelines on this
matter, and published data suggest a trend of improved survival
with earlier intervention. This approach may shift the main driver
of mortality from the cardiac involvement to the primary malignancy
and lead to improved outcomes.
Acknowledgements
The authors would like to thank the patients,
physicians, and staff who assisted in this study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DVB, TD and SMB contributed to the conception and
design of study. JLM, SH, PK, AD, DH, JY, BK, IG and CI were major
contributors to the acquisition and analysis of the data. DVB, TD,
SMB, JLM, SH, PK, AD, DH, JY, BK, IG and CI were responsible for
drafting the study and revising it critically for important
intellectual content. All authors read and approved the final
version of study, and agree to be accountable for all aspects of
the study in ensuring that questions related to the accuracy or
integrity of any part of the study are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The University of Texas MD Anderson Cancer Center
Institutional Review Board (Houston, TX, USA) approved the study
and waived the need for written informed consent, given the
retrospective nature of the manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thanasupawat T, Hammje K, Adham I, Ghia
JE, Del Bigio MR, Krcek J, Hoang-Vu C, Klonisch T and
Hombach-Klonisch S: INSL5 is a novel marker for human
enteroendocrine cells of the large intestine and neuroendocrine
tumours. Oncol Rep. 29:149–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davar J, Connolly HM, Caplin ME, Pavel M,
Zacks J, Bhattacharyya S, Cuthbertson DJ, Dobson R,
Grozinsky-Glasberg S, Steeds RP, et al: Diagnosing and managing
carcinoid heart disease in patients with neuroendocrine tumors: An
expert statement. J Am Coll Cardiol. 69:1288–1304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zavras N, Schizas D, Machairas N, Damaskou
V, Economopoulos N and Machairas A: Carcinoid syndrome from a
carcinoid tumor of the pancreas without liver metastases: A case
report and literature review. Oncol Lett. 13:2373–2376. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhattacharyya S, Davar J, Dreyfus G and
Caplin ME: Carcinoid heart disease. Circulation. 116:2860–2865.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comaru-Schally AM and Schally AV: A
clinical overview of carcinoid tumors: Perspectives for improvement
in treatment using peptide analogs (Review). Int J Oncol.
26:301–309. 2005.PubMed/NCBI
|
|
6
|
Connolly HM, Schaff HV, Abel MD, Rubin J,
Askew JW, Li Z, Inda JJ, Luis SA, Nishimura RA and Pellikka PA:
Early and late outcomes of surgical treatment in carcinoid heart
disease. J Am Coll Cardiol. 66:2189–2196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhattacharyya S, Toumpanakis C, Burke M,
Taylor AM, Caplin ME and Davar J: Features of carcinoid heart
disease identified by 2- and 3-dimensional echocardiography and
cardiac MRI. Circ Cardiovasc Imaging. 3:103–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amano Y, Mandai M, Baba T, Hamanishi J,
Yoshioka Y, Matsumura N and Konishi I: Recurrence of a carcinoid
tumor of the ovary 13 years after the primary surgery: A case
report. Oncol Lett. 6:1241–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pellikka PA, Tajik AJ, Khandheria BK,
Seward JB, Callahan JA, Pitot HC and Kvols LK: Carcinoid heart
disease. Clinical and echocardiographic spectrum in 74 patients.
Circulation. 87:1188–1196. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mansencal N, Mitry E, Forissier JF, Martin
F, Redheuil A, Lepère C, Farcot JC, Joseph T, Lacombe P, Rougier P,
et al: Assessment of patent foramen ovale in carcinoid heart
disease. Am Heart J. 151:1129.e1–1129.e6. 2006. View Article : Google Scholar
|
|
11
|
Zoghbi WA, Adams D, Bonow RO,
Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J,
Lang RM, et al: Recommendations for noninvasive evaluation of
native valvular regurgitation. J Am Soc Echocardiogr. 30:303–371.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haugaa KH, Bergestuen DS, Sahakyan LG,
Skulstad H, Aakhus S, Thiis-Evensen E and Edvardsen T: Evaluation
of right ventricular dysfunction by myocardial strain
echocardiography in patients with intestinal carcinoid disease. J
Am Soc Echocardiogr. 24:644–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castillo JG, Silvay G and Solís J: Current
concepts in diagnosis and perioperative management of carcinoid
heart disease. Semin Cardiothorac Vasc Anesth. 17:212–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mollet NR, Dymarkowski S and Bogaert J:
MRI and CT revealing carcinoid heart disease. Eur Radiol.
13:L14–L18. 2003. View Article : Google Scholar
|
|
15
|
Dobson R, Burgess MI, Pritchard DM and
Cuthbertson DJ: The clinical presentation and management of
carcinoid heart disease. Int J Cardiol. 173:29–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobson R, Burgess MI, Valle JW, Pritchard
DM, Vora J, Wong C, Chadwick C, Keevi B, Adaway J, Hofmann U, et
al: Serial surveillance of carcinoid heart disease: Factors
associated with echocardiographic progression and mortality. Br J
Cancer. 111:1703–1709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tantu M, Belu E, Bobescu E, Armean SM,
Armean P, Constantin MM and Domnariu CD: Role of angiotensin
converting enzyme (ACE) inhibitors in hypertension and
cardiovascular protection management. Farmacia. 62:443–451.
2014.
|
|
18
|
Edwards NC, Yuan M, Nolan O, Pawade TA,
Oelofse T, Singh H, Mehrzad H, Zia Z, Geh JI, Palmer DH, et al:
Effect of valvular surgery in carcinoid heart disease: An
observational cohort study. J Clin Endocrinol Metab. 101:183–190.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassan SA, Banchs J, Iliescu C, Dasari A,
Lopez-Mattei J and Yusuf SW: Carcinoid heart disease. Heart.
103:1488–1495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pavel ME, Hainsworth JD, Baudin E, Peeters
M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM,
et al RADIANT-2 Study Group, : Everolimus plus octreotide
long-acting repeatable for the treatment of advanced neuroendocrine
tumours associated with carcinoid syndrome (RADIANT-2): A
randomised, placebo-controlled, phase 3 study. Lancet.
378:2005–2012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kulke MH, O'Dorisio T, Phan A, Bergsland
E, Law L, Banks P, Freiman J, Frazier K, Jackson J, Yao JC, et al:
Telotristat etiprate, a novel serotonin synthesis inhibitor, in
patients with carcinoid syndrome and diarrhea not adequately
controlled by octreotide. Endocr Relat Cancer. 21:705–714. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Connolly HM, Nishimura RA, Smith HC,
Pellikka PA, Mullany CJ and Kvols LK: Outcome of cardiac surgery
for carcinoid heart disease. J Am Coll Cardiol. 25:410–416. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manoly I, McAnelly SL, Sriskandarajah S
and McLaughlin KE: Prognosis of patients with carcinoid heart
disease after valvular surgery. Interact Cardiovasc Thorac Surg.
19:302–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel C, Mathur M, Escarcega RO and Bove
AA: Carcinoid heart disease: Current understanding and future
directions. Am Heart J. 167:789–795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mortelmans P, Herregods MC, Rega F and
Timmermans P: The path to surgery in carcinoid heart disease: A
retrospective study and a multidisciplinary proposal of a new
algorithm. Acta Cardiol. Jun 18–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gustafsson BI, Hauso O, Drozdov I, Kidd M
and Modlin IM: Carcinoid heart disease. Int J Cardiol. 129:318–324.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luis SA and Pellikka PA: Carcinoid heart
disease: Diagnosis and management. Best Pract Res Clin Endocrinol
Metab. 30:149–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arghami A, Connolly HM, Abel MD and Schaff
HV: Quadruple valve replacement in patients with carcinoid heart
disease. J Thorac Cardiovasc Surg. 140:1432–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilhelmi M, Fritz MK, Fischer S, Haverich
A and Harringer W: Triple valve replacement in a patient with
severe carcinoid heart disease. Cardiovasc Surg. 10:287–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mokhles P, van Herwerden LA, de Jong PL,
de Herder WW, Siregar S, Constantinescu AA, van Domburg RT and
Roos-Hesselink JW: Carcinoid heart disease: Outcomes after surgical
valve replacement. Eur J Cardiothorac Surg. 41:1278–1283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ridker PM, Chertow GM, Karlson EW, Neish
AS and Schoen FJ: Bioprosthetic tricuspid valve stenosis associated
with extensive plaque deposition in carcinoid heart disease. Am
Heart J. 121:1835–1838. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaefer A, Sill B, Schoenebeck J,
Schneeberger Y, Reichenspurner H and Gulbins H: Failing stentless
bioprostheses in patients with carcinoid heart valve disease. J
Cardiothorac Surg. 10:412015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heidecker B, Moore P, Bergsland EK,
Merrick SH and Rao RK: Transcatheter pulmonic valve replacement in
carcinoid heart disease. Eur Heart J Cardiovasc Imaging.
16:10462015.PubMed/NCBI
|
|
34
|
Khan JN, Doshi SN, Rooney SJ, Bhabra MS
and Steeds RP: Transcatheter pulmonary and tricuspid valve-in-valve
replacement for bioprosthesis degeneration in carcinoid heart
disease. Eur Heart J Cardiovasc Imaging. 17:1142016.PubMed/NCBI
|


















