Introduction
Colorectal cancer (CRC) is the second-leading cause
of cancer-associated mortality worldwide (1). Patients are frequently initially
diagnosed at an advanced stage of disease, when distant metastasis
is likely to be present. The mechanisms of metastasis in CRC remain
incompletely characterized.
High-fat diets (HFDs) are a risk factor for numerous
conditions, including obesity (2),
insulin resistance (3,4), non-alcoholic fatty liver disease
(5), hypertension and atherosclerosis
(6). HFD, particularly when including
animal fat, is also a risk factor for CRC (7–9). An
abnormal lipid profile contributes to the development of CRC.
Notarnicola et al (10)
observed 84 patients with CRC and reported that the levels of total
cholesterol (TC), low-density lipoprotein (LDL)-C and
LDL-C/high-density lipoprotein-C were all increased in patients
with metastasis compared with patients without metastasis. However,
the mechanism of abnormal lipid metabolism in affecting CRC
progress remains unclear.
Angiogenesis is an important contributing factor to
tumor metastasis. CD34 is the major marker for tumor angiogenesis,
invasiveness and metastasis. Tumor angiogenesis consists of
numerous steps, during which vascular endothelial growth factor
(VEGF) and angiotensin (ANG)2 are particularly important (11,12). VEGF
promotes the growth and migration of vascular endothelial cells,
induces tumor angiogenesis and promotes the permeability of
microvessels and venules (13,14). ANG2
can facilitate angiogenesis by disrupting endothelial cell-pericyte
interactions in primary tumors and enhancing the permeability of
vessels (13). ANG2 promotes the
VEGF-induced migration and proliferation of ECs, and facilitates
the growth of new blood vessels in the presence of growth factors
(15). Angiogenesis can be induced by
chronic inflammation (16), which may
result from HFD (17). Thus, HFD may
contribute to the progress of CRC by promoting angiogenesis.
The aim of the present study was to observe the
effect of HFD on the metastasis and angiogenesis of colon
orthotopic transplantation tumors in BABL/c mice, and to identify
the mechanism of the effect of HFD on metastasis. It was
demonstrated that HFD increased the level of cytokines, and induced
tumor angiogenesis, to promote colon orthotopic transplantation
tumor metastasis in BALB/c mice.
Materials and methods
Materials
ELISA kits for interleukin-6 (IL-6; cat. no.
550950), tumor necrosis factor (TNF)-α (cat. no. 560478) and mouse
lipoprotein were purchased from BD Biosciences (Franklin Lakes, NJ,
USA). Anti-vascular endothelial growth factor (VEGF) antibody
(EP1176Y), anti-CD34 antibody (EP373Y) and anti-angiotensin 2
(ANG2) antibody [EPR2891(2)] (all from Abcam, Cambridge, UK; all
rabbit monoclonal), and kits for the determination of triglyceride
(TG; cat. no. 006304) and total cholesterol (TC; cat. no. 006301)
were purchased from Beijing BHKT Clinical Reagent Co., Ltd.
(Beijing, China).
Animals
A total of 41 six-week-old BALB/c mice (specific
pathogen-free grade; half male, half female; body weights ~20±2 g)
were purchased from and housed at the Shanghai Laboratory Animals
Center (SLAC) of the Chinese Academy of Sciences (Shanghai, China).
The housing conditions were as follows: 24±2°C, 50±10% relative
humidity, and a 12-h light/dark cycle. The animals had ad
libitum access to food and water. The housing facility and all
animal experiments were conducted in accordance with the guidelines
established by the Beijing Administration Office of Laboratory
Animals; the study protocol was approved by the Institutional
Animal Care and Use Committee of SLAC.
Cell line
CT26 mouse colon cancer cells were provided as a
gift by SLAC. The cells were incubated at 37°C. They were
maintained in Dulbecco's modified Eagle's medium containing 100
ml/l fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 100,000 U/l of penicillin and 100 mg/l of
streptomycin in a humidified atmosphere of 5% CO2.
Colon cancer orthograft model
CT26 cells (5×106 cells suspended in 0.2
ml Matrigel) were injected into the right axillary subcutaneous
region of a BALB/c mouse. When the tumor grew to ~1 cm3,
the mouse was sacrificed and the tumor was excised. The tumor lump
was sliced into 1 mm3 pieces to make a colon cancer
orthograft model.
The remaining BALB/c mice were divided into the
following four groups: Tumor, tumor-HFD, HFD and control, with 10
mice in each group. The mice in the tumor-HFD and HFD groups were
fed with HFD as previously described (18) whereas the other two groups were fed
with a control diet. The HFD contained 92 g/kg protein, 203 g/kg
fat and 128 g/kg carbohydrate, while the control diet contained 78
g/kg protein, 22 g/kg fat and 320 g/kg carbohydrate.
At 4 weeks, the mice were fasted for 12 h prior to
surgery. The mice were anesthetized by injection with 4.5% chloral
hydrate into the abdominal cavity. Subsequent to disinfection, they
were cut along the right midline to expose the colon, and the
muscular layer of the colon was opened with 1 ml sterile needles. A
piece of tumor was adhered to the cecum incision with medical OB
biological glue in mice from the tumor-HFD and tumor groups,
whereas mice in the HFD and control groups were treated with OB
glue alone. The abdomen was closed. From the next day, the mice
were fed with their previous diet continuously for 3 weeks.
Measurement of body and tumor
weights
The body weights of mice were measured once a week
with an electronic weighting scale. The tumor weights were measured
by an electronic microbalance, following the sacrifice of mice and
the excision of the tumors.
Quantification of metastasis
Metastasis was scored at five levels: 1, no
metastasis; 2, one incidence of visceral metastasis; 3, 2
incidences of visceral metastases; 4, 3 incidences of visceral
metastases and 5, >4 incidences of visceral metastases.
Tissue preparation
All mice were sacrificed by the cervical dislocation
method. Blood was sampled from the retro-orbital plexus. Blood
serum was extracted by centrifugation (15,970 × g at 4°C for 5 min)
and stored at −20°C until analysis. The lungs, liver, spleen,
pancreas, greater omentum and distal rectum were stored in
Eppendorf tubes with formalin at room temperature. Colon tumor
tissues were stored at −80°C.
Immunohistochemistry
Paraffin-embedded tissues were sectioned,
deparaffinized, and rehydrated, and then washed in PBS (3×3 min).
Antigen retrieval was performed in the microwave for 20 min in
citrate buffer (2102-100; pH 6.0, 0.01 ml/l; BioVision, Inc.,
Milpitas, CA, USA); sections were left at room temperature to cool,
washed in PBS 3 times for 3 min, treated with 0.3%
H2O2 at room temperature for endogenous
peroxidase ablation (20 min) and washed in PBS 3 times for 3 min
again. The sections were incubated with antibodies against CD34,
VEGF and ANG2 (1:100) for 2 h at 37°C. Subsequent to washing, the
sections were incubated with goat anti-rabbit IgG, which was
horseradish peroxidase-conjugated (1:300; A0208; Beyotime Institute
of Biotechnology, Shanghai, China) at 37°C for 30 min. Subsequent
to further washing, the sections were reacted with
3,3-diaminobenzidine, left at room temperature without light for 10
min, and then counterstained with hematoxylin, dehydrated, cleared
and mounted with neutral gums.
The positive proportion of immunohistochemical
staining and the optical density (OD) were measured by an IMS Cell
Image Analysis system (Shanghai ShenTeng Information Technology
Co., Ltd., Shanghai, China). Each section was analyzed at least in
three fields of view. The immunohistochemistry index was calculated
as the positive proportion × OD.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Tumor tissues were treated with TRIzol (Thermo
Fisher Scientific, Inc.), chloroform and isopropyl alcohol to
extract RNA. Following the reverse transcription of RNA to cDNA
(Moloney Murine Leukemia Virus; 200 U/ml; Invitrogen; Thermo Fisher
Scientific, Inc.), primers were added for amplification with 40
cycles of 95°C for 30 sec and 60°C for 30 sec. The primers were
synthesized by Shanghai Yingjun Biological Technology Co., Ltd.
(Shanghai, China; Table I). The
Fluorescence quantitative PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China) was used according to the manufacturer's protocols.
The products were subjected to a biological electrophoresis image
analysis system for software-based PCR densitometry (FR-2000;
Shanghai Furi Science & Technology Co., Ltd., Shanghai, China).
GAPDH was used as a reference gene.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequence
(5′-3′) | Product length,
bp |
|---|
| Mouse GAPDH |
| 123 |
|
Forward |
AGGTCGGTGTGAACGGATTTG |
|
|
Reverse |
TGTAGACCATGTAGTTGAGGTCA |
|
| Mouse angiotensin
2 |
| 146 |
|
Forward |
CCTCGACTACGACGACTCAGT |
|
|
Reverse |
TCTGCACCACATTCTGTTGGA |
|
| Mouse vascular
endothelial growth factor |
| 131 |
|
Forward |
AGCTACTGCCGTCCAATT |
|
|
Reverse |
TCCAGGGCTTCATCGTTA |
|
ELISA assays
Serum IL-6 (cat. no. 550950), TNF-α (cat. no.
560478), TG (cat. no. 006304), TC (cat. no. 006301) and lipoprotein
a (cat. no. 006340) were examined by using Quantikine ELISA kits
(BD Biosciences Company, USA) according to the manufacturer's
protocol. Optical density was measured in an ELISA reader at 450 nm
following a 15-min incubation at room temperature.
Data analysis
The measurement data are expressed as the mean ±
standard deviation. Statistical analysis was performed using SPSS
21.0 (IBM Corp., Armonk, NY, USA). Comparisons between groups were
tested by one-way analysis of variance followed by a least
significant difference test. Differences in metastasis were
analyzed by a Wilcoxon Rank-Sum test. Correlation analysis was
performed by Pearson correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
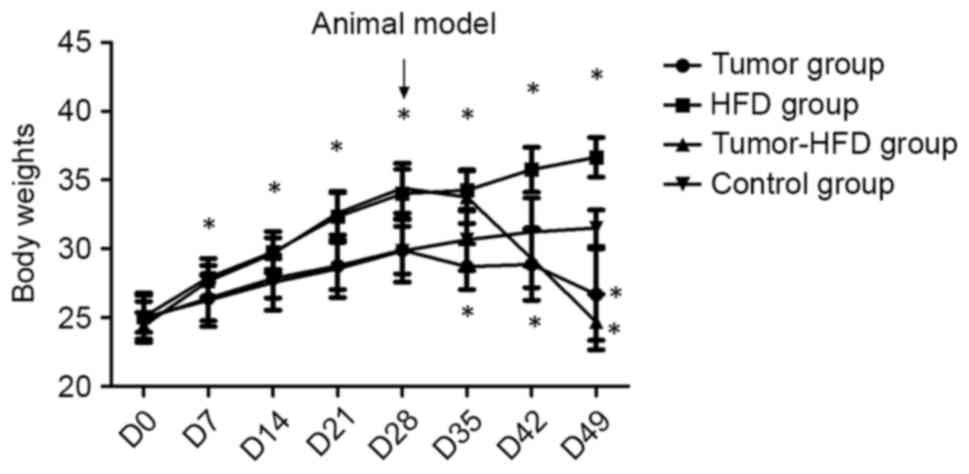
Normal mouse body weight, but not
orthotopic tumor-transplanted mouse body weight, increases
following HFD
The body weights of the mice were measured twice a
week. There was no difference between the mean body weights of the
four groups before the experiment (P>0.05). One week later, the
body weights of mice in the HFD group had significantly increased
compared with the control group (P<0.05), whereas the body
weights of mice decreased following colon orthotopic
transplantation in the tumor-HFD and tumor groups. At the end of
the experiment, the mean weight of the mice was higher in the HFD
group than in the tumor-HFD group or control group (P<0.05), and
was lower in the tumor and tumor-HFD groups than in the control
group (P<0.05). The body weights of the mice in the tumor-HFD
group were the lowest among the four groups (Fig. 1).
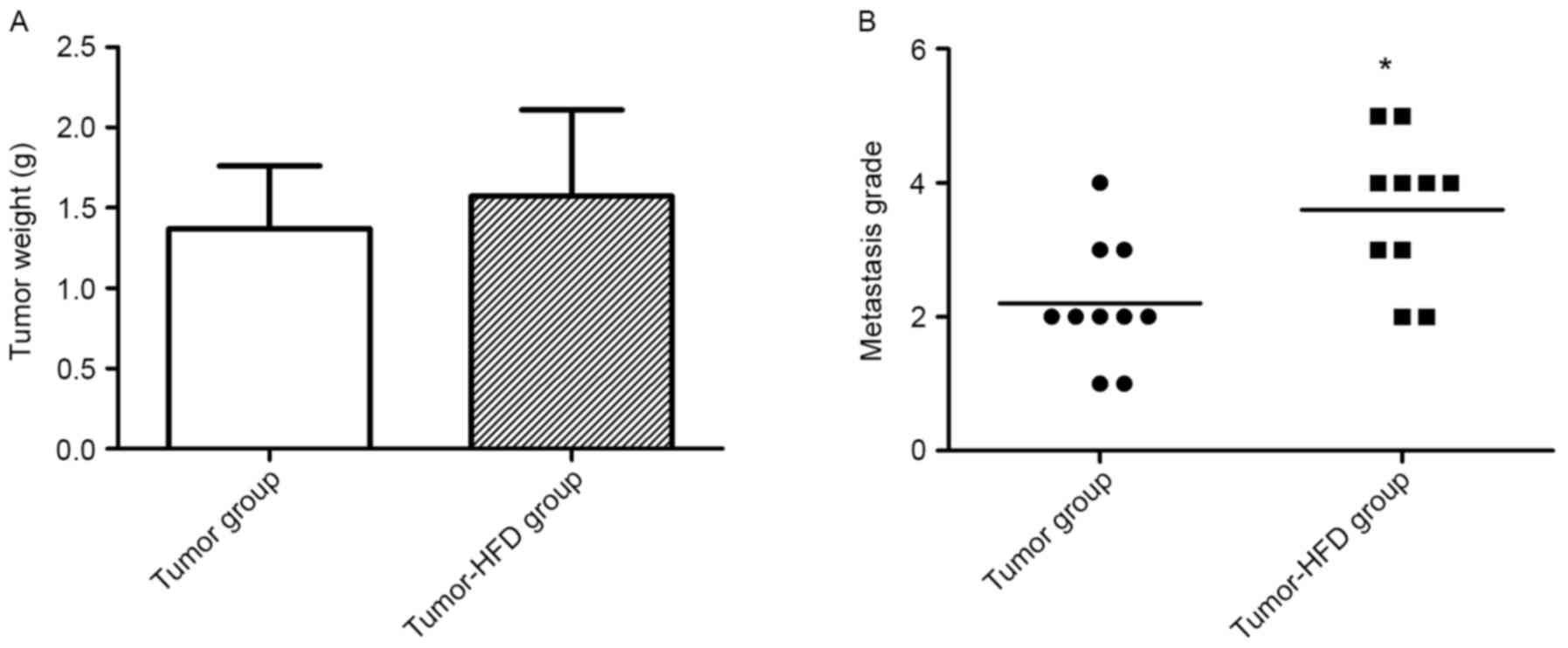
Metastasis severity is increased by
HFD in mice transplanted with orthotopic tumors
The mean tumor weight was detected when the mice
were sacrificed at 3 weeks after the colon cancer orthotopic
transplantation surgery. The tumor weight was greater in the
tumor-HFD group (1.57±0.54 g) than in the tumor group (1.37±0.40
g), but there was no significant difference between these two
groups (P=0.368; Fig. 2A).
A total of 2 out of 10 mice died in the tumor-HFD
group due to intestinal obstruction diagnosed by autopsy, while
there was no mortality in the tumor group. The metastases in the
lung, liver, spleen, pancreas, rectum and greater omentum were
observed by immunohistochemistry. The metastases in the tumor group
included two cases at level 1, five cases at level 2, two cases at
level 3 and 1 case at level 4, whereas there were two cases at
level 2, two cases at level 3, four cases at level 4 and 2 cases at
level 5 in the tumor-HFD group. The severity of metastasis was
greater in the tumor-HFD group than in the tumor group (P<0.05;
Fig. 2B).
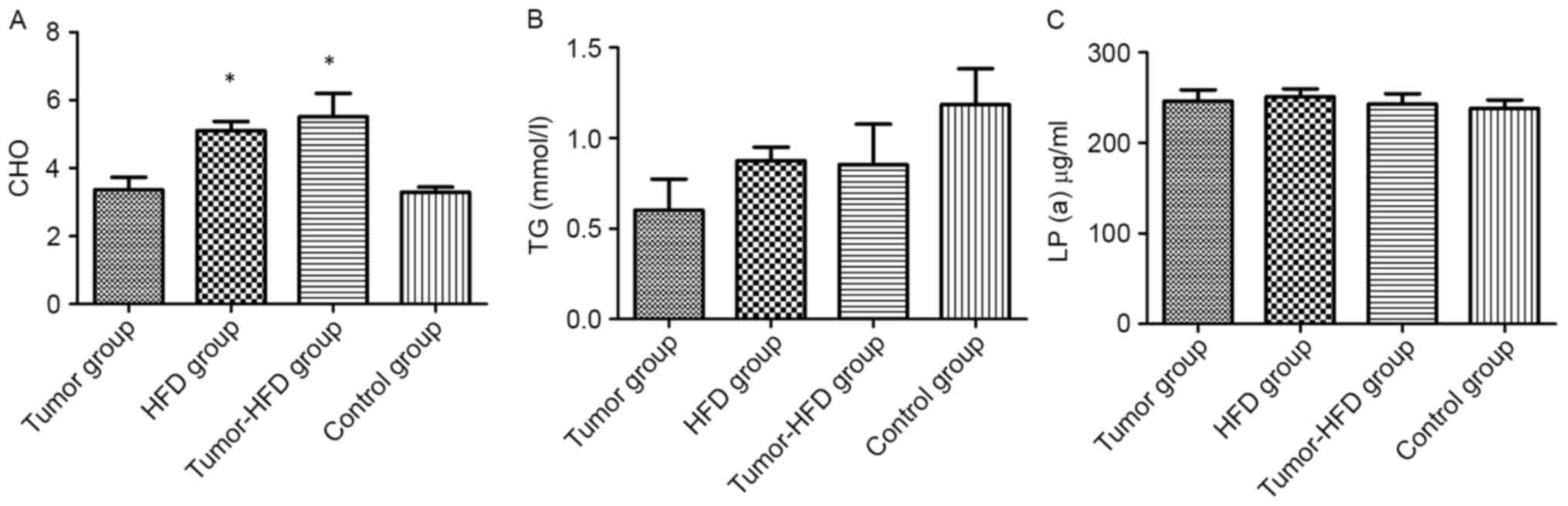
Serum cholesterol level is increased
in mice transplanted with orthotopic tumors following HFD
ELISA was applied to determine whether HFD increased
the serum cholesterol level. The serum cholesterol levels in the
HFD and tumor-HFD groups were 5.10±0.84 and 5.51±1.93 mmol/l,
respectively, which were higher than in the control group
(3.29±0.48 mmol/l; P<0.05). No difference was observed in serum
cholesterol levels between the tumor group (3.36±1.17 mmol/l) and
the control group (P>0.05). No difference was observed in serum
TG or lipoprotein levels among the four groups (P>0.05; Fig. 3).
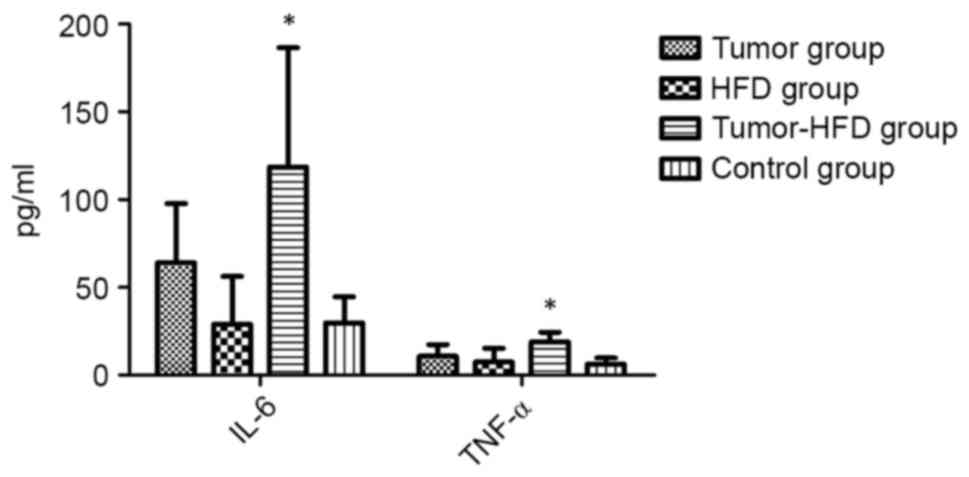
Serum levels of IL-6 and TNF-α
increase in mice transplanted with orthotopic tumors following
HFD
The results of ELISA revealed that the serum levels
of IL-6 and TNF-α, two cytokines associated with inflammation,
increased markedly (118.57±68.07 and 18.94±5.39 pg/ml,
respectively) in the tumor-HFD group compared with the other groups
(P<0.05; Fig. 4). Although the
serum levels of IL-6 and TNF-α in the tumor group (64.06±33.56,
10.69±6.74 pg/ml) were higher than those in the control group
(29.79±14.78, 6.19±3.51 pg/ml), the differences were not
statistically significant (P>0.05). The serum levels of IL-6 and
TNF-α in the HFD group were similar to those in the control group
(P>0.05).
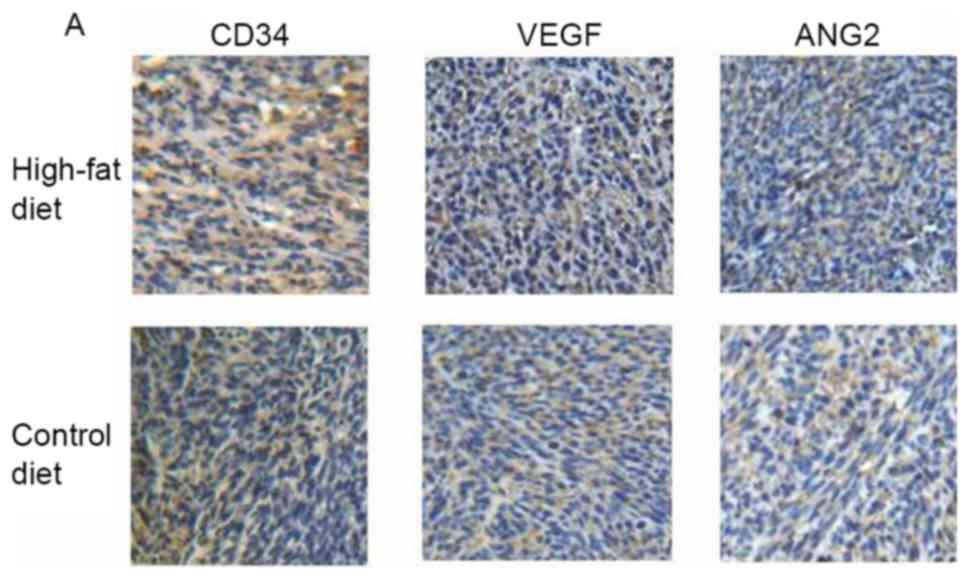
Expression of CD34 protein, and VEGF
and ANG2 protein and mRNA levels in colon cancer tissue, are
increased following HFD
The results of IHC staining for CD34, a glycoprotein
expressed on endothelial cells and platelets, revealed that the
expression of CD34 was higher in the tumor-HFD group than in the
tumor group (Fig. 5A and B).
Similarly, the protein expression of VEGF, a key cytokine in tumor
angiogenesis, was higher in the tumor-HFD group than in the tumor
group (1.77±0.83 and 10.67±3.00); the differences were all
statistically significant (P<0.05). The results of IHC staining
for ANG2, another cytokine implicated in tumor angiogenesis, were
13.33±4.30 and 12.33±1.75 in the two groups, respectively, with no
difference between them (P>0.05).
The results of RT-PCR demonstrated that the VEGF
mRNA level was significantly higher in the tumor-HFD group than in
the tumor group (1.24±0.69 vs. 0.74±0.23; P<0.05), whereas there
the ANG2 mRNA level did not differ between the groups (Fig. 5C).
Correlation analysis between IL-6,
TNF-α, CD34 and VEGF, and metastasis
Correlation analysis results demonstrated that the
frequency of metastasis was associated with the IL-6 serum level
(r=0.61), and the expression of CD34 (r=0.941) and VEGF (0.679)
protein in tumor tissue (all P<0.05). There was no evident
correlation between metastasis and TNF-α (P>0.05).
Discussion
The purpose of the present study was to determine
whether HFD increases colon tumor metastasis in BALB/c mice. It was
observed that the metastasis frequency was more severe, with a
higher level of serum cholesterol, in the tumor-HFD group compared
with the tumor group. This indicated that high cholesterol induced
by HFD may have served an important role in the metastasis of colon
cancer. The link between dysregulated lipid levels, including high
levels of cholesterol, LDL-C and TG, and colon cancer progression
has previously been reported (19).
Another previous study indicated that a high serum cholesterol
level was associated with the distant metastasis of colon cancer
(10). Cholesterol is a crucial
component of cell membranes, and contributes to the organization of
lipid rafts, which contain a large number of cancer-associated
signaling and adhesion molecules (20). Due to the increase in the cholesterol
level of tumor cells, increased lipid synthesis is also recognized
as a common feature of metabolic reprogramming in cancer cells
(21). Dysregulated cholesterol
synthesis and sterol-dependent proliferation have been identified
in various cancer cell types and may lead to cancer progression
(21).
In the present study, it was observed that HFD
increased the serum IL-6 and TNF-α levels of mice with colon
cancer. Previous studies have indicated that the metabolic syndrome
induced by HFD can cause inflammation (22), particularly through the oxidation of
cholesterol (23,24). However, HFD did not induce an
inflammatory effect in normal mice, which may be due to the limited
time that HFD was received. In the colon cancer model mice fed a
normal diet, IL-6 and TNF-α increased to a non-significant extent
compared with the control mice. A correlation analysis identified
that metastasis may have been associated with the serum level of
IL-6. This indicated that the inflammation induced by HFD may
contribute to colon cancer metastasis.
Clinical and epidemiological research has
demonstrated an association between cancer and inflammatory
metabolic diseases; common molecular characteristics between the
two have been demonstrated. Hirsch et al (21) hypothesized that there was a common
core signaling pathway network controlling normal cell growth and
behavior. Once the core gene network is affected by genetics or the
environment, a range of diseases develop, which may account for the
association between cancer and metabolic disease. Regarding how a
common transcriptional program contributed to this diverse range of
human diseases, Hirsch et al suggested that the interplay
between cell-type-specific factors and a common transcriptional
program leads to cell-type-specific transcription profiles and
phenotypes associated with these specific disease states. In this
view, the combination of cell-type-specific factors with a common
disease program can lead to inappropriate cell proliferation as
associated with either cancer or metabolic diseases (21). Inflammation serves an important role
in both colon cancer and metabolic diseases (25,26), which
may be the main mechanism for the dysregulation of cholesterol, as
induced by HFD, promoting colon cancer metastasis.
In the tumor microenvironment, inflammatory factors
affect tumor angiogenesis (27). In
the present study, HFD increased the CD34 in tumor tissue, in
addition to the serum levels of IL-6 and TNF-α in colon cancer
model mice. IL-6 and TNF-α have been demonstrated to serve
important roles in tumor angiogenesis (28,29). VEGF
is the most critical cytokine in angiogenesis; Eldesoky et
al (30) reported that the serum
levels of IL-6 and VEGF from 35 patients with colon cancer prior to
surgery were higher than in a control group, and the patients that
experienced higher cancer invasiveness and metastasis had higher
IL-6 and VEGF levels. TNF-α derived from the tumor can promote
angiogenesis by promoting the secretion of VEGF by fibroblasts
(31). Likewise, tumors derived from
cells with TNF-α knockdown exhibit a phenotype with reduced
vascularization and invasiveness (32).
The increased CD34 and VEGF protein and mRNA in
tumor tissue indicated that tumor angiogenesis likely increased in
colon cancer model mice that received HFD. As ANG2 protein and mRNA
were not elevated in tumor tissues by HFD, VEGF induced by HFD may
have promoted tumor angiogenesis. A correlation analysis
demonstrated that metastasis was associated with the expression of
CD34 and VEGF protein in tumor tissue. This indicated that the
metastasis promotion by HFD may be associated with the induced
inflammatory effect and angiogenesis. However, the specific
mechanism remains to be determined through further study.
In conclusion, HFD promoted the distant metastasis
of colon cancer model mice. HFD also elevated the serum level of
cholesterol and the pro-inflammatory cytokines IL-6 and TNF-α, and
potentially promoted angiogenesis in colon cancer model mice.
Acknowledgments
Not applicable.
Funding
The study was supported by SLAC and grants from the
Shanghai Municipal Health Bureau of China (grant no.
ZYSNXD-CC-MZY054, to PW).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX performed the animal experiment and was a major
contributor in writing the manuscript. ZY performed the
histological examination. YZ performed the animal experiment. XL
performed the serum examination and RT-PCR. JJ analyzed the data.
MY performed the animal experiment. DS collected the specimens from
animals. PW designed the whole examination. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Animal Care and Use Committee of SLAC.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao Z, Zhang L and Ji G: Efficacy of
polyphenolic ingredients of Chinese herbs in treating dyslipidemia
of metabolic syndromes. J Integr Med. 12:135–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng X, Scott A, Wang Y, Wang L, Zhao Y,
Doerner S, Satake M, Croniger CM and Wang Z: PTPRT regulates
high-fat diet-induced obesity and insulin resistance. PLoS One.
9:e1007832014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song HY, Zhang L, Pan JL, Yang LL and Ji
G: Bioactivity of five components of Chinese herbal formula
Jiangzhi granules against hepatocellular steatosis. J Integr Med.
11:262–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Zhang H, Zheng H and Jiang Y:
Hepatic inflammation scores correlate with common carotid
intima-media thickness in rats with NAFLD induced by a high-fat
diet. BMC Vet Res. 10:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khanna AK: Enhanced susceptibility of
cyclin kinase inhibitor p21 knockout mice to high fat diet induced
atherosclerosis. J Biomed Sci. 16:662009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Potter JD: Nutrition and colorectal
cancer. Cancer Causes Control. 7:127–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yiu HY, Whittemore AS and Shibata A:
Increasing colorectal cancer incidence rates in Japan. Int J
Cancer. 109:777–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CS, Hsu HS, Li CI, Jan CI, Li TC, Lin
WY, Lin T, Chen YC, Lee CC and Lin CC: Central obesity and
atherogenic dyslipidemia in metabolic syndrome are associated with
increased risk for colorectal adenoma in a Chinese population. BMC
Gastroenterol. 10:512010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Notarnicola M, Altomare DF, Correale M,
Ruggieri E, D'Attoma B, Mastrosimini A, Guerra V and Caruso MG:
Serum lipid profile in colorectal cancer patients with and without
synchronous distant metastases. Oncology. 68:371–374. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He T, Qi F, Jia L, Wang S, Song N, Guo L,
Fu Y and Luo Y: MicroRNA-542-3p inhibits tumour angiogenesis by
targeting angiopoietin-2. J Pathol. 232:499–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hosseini H, Rajabibazl M, Ebrahimizadeh W
and Dehbidi GR: Inhibiting angiogenesis with human single-chain
variable fragment antibody targeting VEGF. Microvasc Res. 97:13–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Song N, Ding Y, Yuan S, Li X, Cai
H, Shi H and Luo Y: Pulmonary vascular destabilization in the
premetastatic phase facilitates lung metastasis. Cancer Res.
69:7529–7537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang H, Bhat A, Woodnutt G and Lappe R:
Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer.
10:575–585. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carvalho MI, Pires I, Prada J, Raposo TP,
Gregório H, Lobo L and Queiroga FL: High COX-2 expression is
associated with increased angiogenesis, proliferation and tumoural
inflammatory infiltrate in canine malignant mammary tumours: A
multivariate survival study. Vet Comp Oncol. 15:619–631. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jakobsdottir G, Xu J, Molin G, Ahrné S and
Nyman M: High-fat diet reduces the formation of butyrate, but
increases succinate, inflammation, liver fat and cholesterol in
rats, while dietary fibre counteracts these effects. PLoS One.
8:e804762013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perry B, Zhang J, Saleh T and Wang Y:
Liuwei Dihuang, a traditional Chinese herbal formula, suppresses
chronic inflammation and oxidative stress in obese rats. J Integr
Med. 12:447–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhao XW, Liu DB, Han CZ, Du LL,
Jing JX and Wang Y: Lipid levels in serum and cancerous tissues of
colorectal cancer patients. World J Gastroenterol. 20:8646–8652.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murai T: Cholesterol lowering: Role in
cancer prevention and treatment. Biol Chem. 396:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirsch HA, Iliopoulos D, Joshi A, Zhang Y,
Jaeger SA, Bulyk M, Tsichlis PN, Liu Shirley X and Struhl K: A
transcriptional signature and common gene networks link cancer with
lipid metabolism and diverse human diseases. Cancer Cell.
17:348–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barquilla Cano P, Pagano ES,
Jiménez-Ortega V, Fernández-Mateos P, Esquifino AI and Cardinali
DP: Melatonin normalizes clinical and biochemical parameters of
mild inflammation in diet-induced metabolic syndrome in rats. J
Pineal Res. 57:280–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jusakul A, Yongvanit P, Loilome W, Namwat
N and Kuver R: Mechanisms of oxysterol-induced carcinogenesis.
Lipids Health Dis. 10:442011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kampschulte M, Stöckl C, Langheinrich AC,
Althöhn U, Bohle RM, Krombach GA, Stieger P, Churin Y, Kremer S,
Dierkes C, et al: Western diet in ApoE-LDLR double-deficient mouse
model of atherosclerosis leads to hepatic steatosis, fibrosis, and
tumorigenesis. Lab Invest. 94:1273–1282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flores MBS, Rocha GZ, Damas-Souza DM,
Osório-Costa F, Dias MM, Ropelle ER, Camargo JA, de Carvalho RB,
Carvalho HF, Saad MJA and Carvalheira JBC: RETRACTED:
Obesity-induced increase in tumor necrosis factor-α leads to
development of colon cancer in mice. Gastroenterology.
143(741–753): e42012.PubMed/NCBI
|
|
26
|
Al Rifai M, Silverman MG, Nasir K, Budoff
MJ, Blankstein R, Szklo M, Katz R, Blumenthal RS and Blaha MJ: The
association of nonalcoholic fatty liver disease, obesity, and
metabolic syndrome, with systemic inflammation and subclinical
atherosclerosis: The multi-ethnic study of atherosclerosis (MESA).
Atherosclerosis. 239:629–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hefler LA, Grimm C, Ackermann S, Malur S,
Radjabi-Rahat AR, Leodolter S, Beckmann MW, Zeillinger R, Koelbl H
and Tempfer CB: An interleukin-6 gene promoter polymorphism
influences the biological phenotype of ovarian cancer. Cancer Res.
63:3066–3068. 2003.PubMed/NCBI
|
|
29
|
Middleton K, Jones J, Lwin Z and Coward
JI: Interleukin-6: An angiogenic target in solid tumours. Crit Rev
Oncol Hematol. 89:129–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eldesoky A, Shouma A, Mosaad Y and
Elhawary A: Clinical relevance of serum vascular endothelial growth
factor and interleukin-6 in patients with colorectal cancer. Saudi
J Gastroenterol. 17:170–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Danese S, Sans M, de la Motte C, Graziani
C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A
and Fiocchi C: Angiogenesis as a novel component of inflammatory
bowel disease pathogenesis. Gastroenterology. 130:2060–2073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kulbe H, Thompson R, Wilson JL, Robinson
S, Hagemann T, Fatah R, Gould D, Ayhan A and Balkwill F: The
inflammatory cytokine tumor necrosis factor-alpha generates an
autocrine tumor-promoting network in epithelial ovarian cancer
cells. Cancer Res. 67:585–592. 2007. View Article : Google Scholar : PubMed/NCBI
|