Introduction
Differences exist between the incidence of gastric
cancer in a number of countries due to several factors including
race and dietary habits. Gastric cancer incidence in eastern Asia,
especially in China, where there were ~400,000 new cases in 2010,
accounting for 42% of the global total (1). At present, gastric cancer is one of the
most common diseases resulting in the mortality of patients with
cancer (2,3). Gastric cancer is difficult to be
detected early and the majority of patients with gastric cancer are
diagnosed at Stage II or III (4).
Studies demonstrate that the five-year survival rates of Chinese
patients with gastric cancer at Stage II and III are ~70 and 40%,
respectively (4,5). Clinical data demonstrated that there are
a number of difficulties in treating patients with gastric cancer
due to high recurrence rates and metastasis risks (6). T-cells may serve an important role in
suppressing tumor progression. A previous study demonstrated that
the immune system of gastric cancer patients and the antitumor
function of T-cells are suppressed in gastric cancer, and
therefore, resulting in tumor escape from the immune surveillance
and disease progression (7).
Programmed death-1 (PD-1) and T-cell immunoglobulin
mucin domain-3 (Tim-3) (8–11) are known to be associated with the
inhibition of T-cell function. The expression and function of the
T-cell surface PD-1 molecules in gastric cancer patients have been
reported in several studies (8–10). The
results demonstrate that the average expression levels of PD-1 on
the surface of T-cells in the peripheral blood and cancer tissues
of patients with gastric cancer increases significantly, and the
ability of T-cells that express PD-1 to secrete interferon (IFN)-γ
decreases significantly (8–10). However, to the best of our knowledge,
no research regarding the expression of Tim-3 on the surface of the
T-cells of gastric cancer patients, and the association with the
development of gastric cancer has been reported. Therefore, a study
of the expression of Tim-3 on the surface of T-cells in
paracancerous and cancerous tissues of patients with gastric cancer
and the effect on the function of T-cells may reveal the mechanism
of immune inhibition in gastric cancer patients. Interfering with
the immune inhibitory state of T-cells may be a novel therapeutic
target for the treatment of gastric cancer.
In the present study, the expression of Tim-3 on the
surface of T-cells in the peripheral blood, paracancerous and
cancerous gastric tissues was analyzed using flow cytometry, and
the association between Tim-3 expression and T staging of gastric
cancer was analyzed. Recombinant galectin-9 was used to activate
the Tim-3 signaling pathway. Flow cytometry following intracellular
staining was used to detect the ability of T-cells to secrete IFN-γ
and tumor necrosis factor (TNF)-α. In addition, the effects of
Tim-3 on T-cells inducing the inhibition of tumor growth were
evaluated in a human gastric cancer xenograft model. The expression
of Tim-3 in T-cells in paracancerous and cancerous gastric tissues,
and the effect of Tim-3 activation on T-cell function were assessed
to provide a scientific basis for novel therapeutic targets for
gastric cancer.
Materials and methods
Reagents
The key instruments and reagents used in the present
study included: The 37°C constant temperature incubator with 5%
CO2 purchased from Thermo Fisher Scientific, Inc.,
(Waltham, MA, USA); the flow cytometer was purchased from BD
Biosciences (Franklin Lakes, NJ, USA); cluster of differentiation
(CD)3, Tim-3, IFN-γ/TNF-α antibody purchased from Biolegend Inc.,
San Diego, CA, USA; galectin-9 purchased from Cloud-Clone Corp.,
(Katy, TX, USA); the RPMI-1640 medium and fetal bovine serum were
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA); Pan T Cell Isolation Kit was purchased from Miltenyi Biotec
GmbH (Bergisch Gladbach, Germany); collagenase and DNase were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany);
70-µm cell strainer was purchased from BD Biosciences; and a total
of 24 female BALB/c nude mice (6–7 weeks old; mean weight, 20 g)
were purchased from Beijing Vital River Experimental Animals Co.,
Ltd., (Beijing, China). Mice were maintained in a regulated
environment (23±1°C, 50–60% humidity) under a 12 h light/dark cycle
and provided food and water ad libitum.
Clinical data
A total of 22 patients (male:female, 12:10) with
gastric cancer had been hospitalized in Weihai Municipal Hospital
(Jinan, China) and Provincial Hospital Affiliated to Shandong
University (Weihai, China), and underwent surgery between January
30, 2015 and December 30, 2015. None of the patients in the group
received chemotherapy, radiotherapy or immunotherapy prior to the
operation. Paracancerous and cancerous tissue specimens were
obtained from patients with gastric cancer during the operation.
The tissue specimens were immediately placed in physiological
saline solution and tumor-infiltrating T-cells (TILs) were
extracted within 30 min for the experiment. Histopathological
diagnosis was performed using the specific diagnostic criteria
described in the literature (4). The
average age of the 22 patients was 55.06±16.83 years. The present
study was approved by the Medical Ethics committee of Provincial
Hospital Affiliated to Shandong University and Weihai Municipal
Hospital. Written informed consent was received from all patients
involved.
Peripheral blood mononuclear cells
(PBMCs)
Peripheral blood was obtained from patients with
gastric cancer and then diluted using sterile RPMI-1640 to the
ratio of 1:1. Following the dilution, the blood was added into a
centrifuge tube, which contained the lymphocyte separation medium
(the ratio of diluted blood to lymphocyte separation medium was
2:1) and then centrifuged for 20 min at room temperature, at a rate
of 250 × g. White lymphocytes were extracted using a glass pipette
and then the cells were added into a centrifuge tube containing 10
ml sterile RPMI-1640 and centrifuged at room temperature for 15 min
at the rate of 250 × g. RPMI-1640 was then discarded and the cells
were precipitated using 10 ml sterile RPMI-1640 and finally
centrifuged at room temperature for 8 min at the rate of 200 ×
g.
Isolation of TILs
The gastric cancer tissue was placed into sterile
PBS, then cut into pieces using surgical scissors and mechanical
grind using the grinding rod. Subsequently, tissues were treated
with 1 µg/ml collagenase, 25 µg/ml DNase, and 2% fetal bovine serum
in PBS at 37°C for 1–1.5 h. The tissue homogenates were filtered
with a 70-µm cell strainer, and then centrifuged at room
temperature for 15 min at the rate of 250 × g (12).
T-cell isolation
All types of T-cells (>90%) were purified by
negative selection using a Pan T Cell Isolation kit (cat. no.
130-096-535; Miltenyi Biotec GmbH), according to manufacturer's
protocol. In brief, non-T-cells were indirectly magnetically
labeled by using a cocktail of biotin-conjugated antibodies and
anti-Biotin MicroBeads. Highly pure untouched T-cells are obtained
by depletion of the magnetically labeled cells. The cells were
confirmed to be CD3+ T-cells by flow cytometry.
Flow cytometry and intracellular
staining method
A total of 1×105 negative selection
CD3+ T-cells were washed using 1 ml PBS containing 1%
bovine serum albumin (BSA) (Sigma-Aldrich; Merck KGaA) twice, each
time for 5 min. After the supernatant was discarded, 0.1 µl PBS
containing 1% BSA was added. Then, 5 µl fluorescein isothiocyanate
(FITC) anti-Tim-3 (cat. no. 345021; Biolegend, Inc.) antibody was
added for the incubation for 30 min at 4°C. The solution was washed
using 1 ml PBS containing 1% BSA twice, each for 5 mins. After the
supernatant was discarded, 0.1 ml PBS containing 1% BSA was added
for flow cytometry detection. Cytometric data were acquired by
using a BD Accuri C6 flow cytometer (BD Biosciences). For
intracellular staining, Galectin-9 was added into 1×105
purified T-cells to a final concentration 5 µg/ml to activate the
Tim-3 signaling pathway (11). After
72 h, cells were stimulated with 2 µl of the cell activation
cocktail (cat. no. 423303; Biolegend, Inc.) at 37°C for 6 h. The
cells were washed using 1 ml PBS containing 1% BSA twice, and fixed
cells using 0.5 µl fixation buffer (cat. no. 420801; Biolegend,
Inc.) per tube and incubated in the dark for 20 min at room
temperature. Subsequently, cells were washed using permeabilization
buffer (cat. no. 421002; Biolegend, Inc.) twice and then 5 µl
antigen presenting cell anti-TNF-α antibody (cat. no. 502913;
Biolegend, Inc.) and 5 µl FITC-anti-IFN-γ antibody (cat. no.
502505; Biolegend, Inc.) were added for 30 min in the dark at 4°C.
The cells were washed using permeabilization buffer twice. Cells
were analyzed using a flow cytometer.
Cell culture
The human breast cancer cell line MGC803 was
purchased from the Cell Center of Institute of Basic Medical
Sciences Chinese Academy of Medical Sciences (Shanghai, China). The
cells were placed into RPMI-1640 medium containing 10% fetal bovine
serum and incubated in the 37°C constant temperature incubator
containing 5% CO2. Subsequent experiments were then
conducted when the cells were at the logarithmic growth stage.
Gastric cancer xenograft model and
therapeutic method
MGC803 cells were digested with 0.25% trypsin (cat.
no. 59427C; Sigma-Aldrich; Merck KGaA), then washed using sterile
PBS for three times, and injected into nude mice subcutaneously in
the right flank of nude mice (1×106/100 µl). Day 0 was
marked when subcutaneous tumor nodules grew up to ~0.1
mm3 (~10 days). The nude mice were randomly divided into
three experimental groups, with each group containing 8 mice: The
untreated T-cells treatment group (PBS-treated CD3+
T-cells), the treatment group using galectin-9-stimulated T-cells
(CD3+ T-cells that were cultured for 72 h with 5 µg/ml
final concentration of galectin-9) and the PBS control group. Then,
the intratumor injection treatment was performed. All experiments
were approved by the Institutional Animal Care and Utilization
Committee of Medical Ethics committee of Provincial Hospital
Affiliated to Shandong University and Weihai Municipal Hospital.
The treatments were performed on day 5 after MGC803 cells
injection, and there were 4 treatments, with the injection being
repeated every 3 days. The untreated CD3+ T-cells
(1×106/50 µl per mice; total of 8 mice) were injected
into mice in the conventional T-cells treatment group;
Tim-3-stimulated CD3+ T-cells (1×106/50 µl, 8
mice) were injected into the mice in the treatment group of the
Tim-3-stimulated CD3+ T-cells; the same volume of PBS
was injected into the mice in the PBS control group (8 mice)
instead of the cell suspension. At the same time, 5,000 U/kg
interleukin-2 (Biolegend, Inc.) was injected into abdominal cavity
or each mouse. The general state of the mice and the volume of the
tumor nodules were observed. The duration of the experiments was 1
month, and following that all mice were anesthetized/euthanized
using cervical dislocation. The computational formula for tumor
volume used was: Tumor volume (mm3)=axb2x0.5,
where ‘a’ denotes the long diameter of tumor (mm) and ‘b’ denotes
the short diameter of tumor (mm).
Statistical method
The results are expressed as the mean ± standard
deviation. The experiments were at repeated ≥3 times. Data analysis
was conducted using Graph Pad software 5 (GraphPad Software, Inc.,
La Jolla, CA, USA). Data between groups were analyzed using a
Student's t-test or one-way analysis of variance followed by a
Bonferroni-Dunn multiple comparison post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
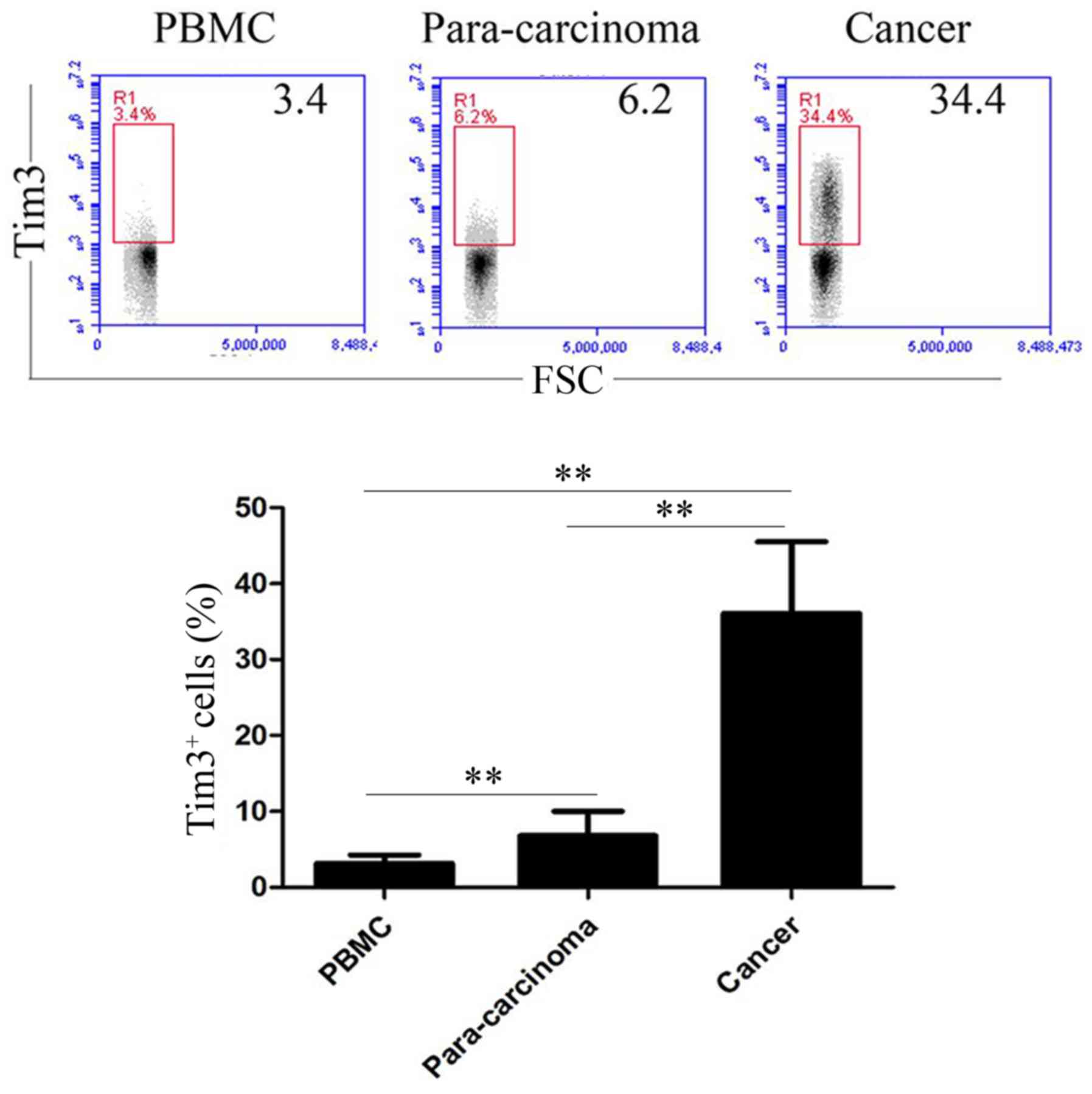
Expression of Tim-3 by T-cells
The expression of Tim-3 by T-cells in the peripheral
blood, paracancerous and cancerous tissues was assessed by flow
cytometry (Fig. 1). The percentages
of Tim-3+ cells within T-cells in the peripheral blood
the paracancerous gastric tissues were 3.14±1.13 and 6.83±3.18%,
respectively. The percentage of Tim-3+ cells within
T-cells in cancerous gastric tissues was 36.11±9.42% (Fig. 1). Compared with the expression levels
of Tim-3 on the surface of T-cells in the peripheral blood, the
expression levels of Tim-3 on the surface of para-carcinoma tissue
and cancer tissue of the patients with gastric cancer increased
significantly (P<0.01; Fig. 1).
Additionally, the expression of Tim-3 within T-cells was
significantly increased in cancerous gastric tissues compared with
that in paracancerous gastric tissues (P<0.01; Fig. 1).
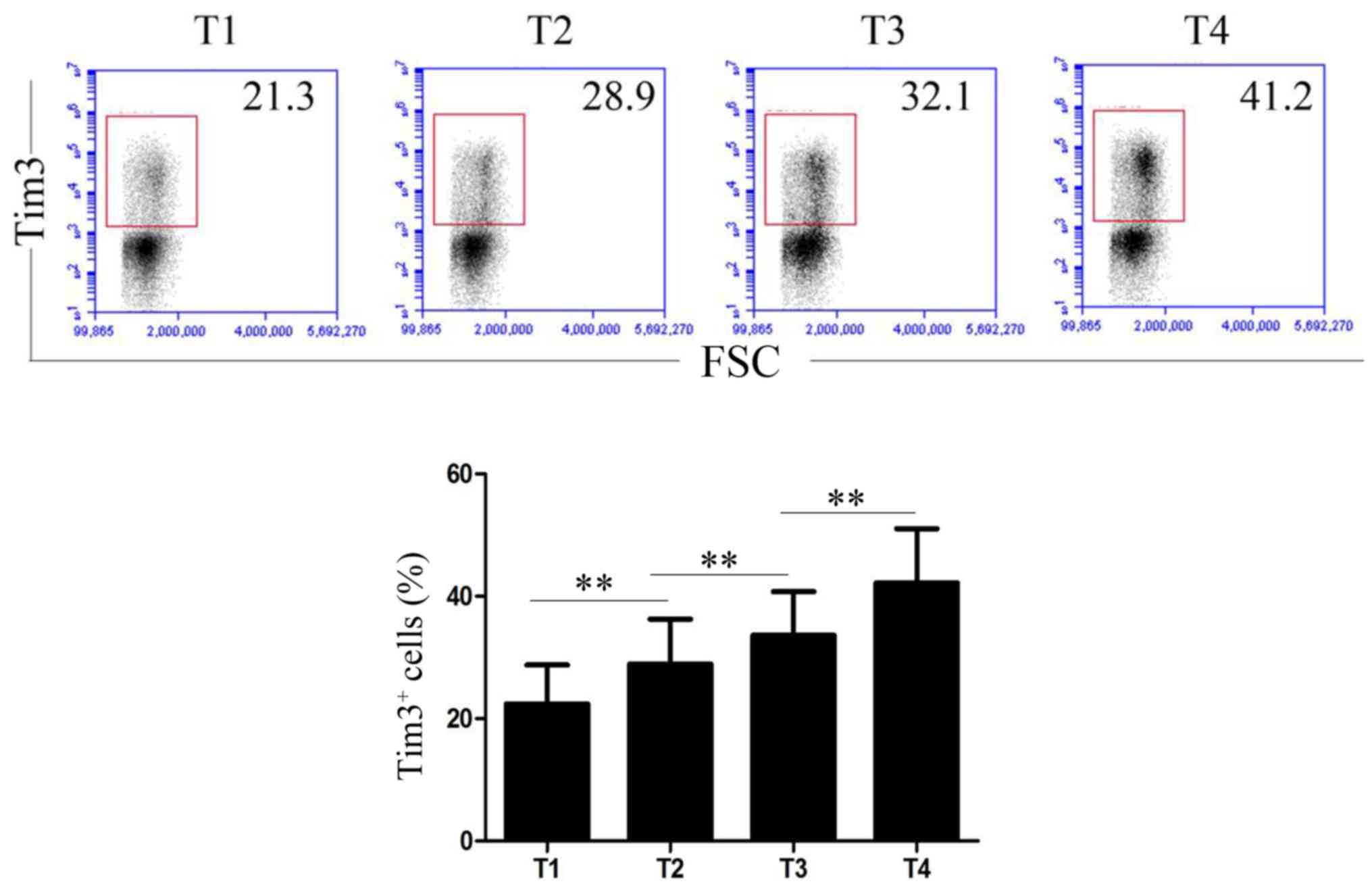
Tim-3 expression is increased at
advanced stages of gastric cancer
The association between Tim-3 expression level and T
staging of gastric cancer is illustrated in Fig. 2. The percentage of Tim-3+
T-cells in gastric cancer tissues at stages T1, T2, T3 and T4 were
22.41±6.35, 28.94±7.35, 33.62±7.16 and 42.16±8.93%, respectively.
With the increase of gastric cancer stages, the expression level of
Tim-3 on T-cell was significantly increased (P<0.01; Fig. 2).
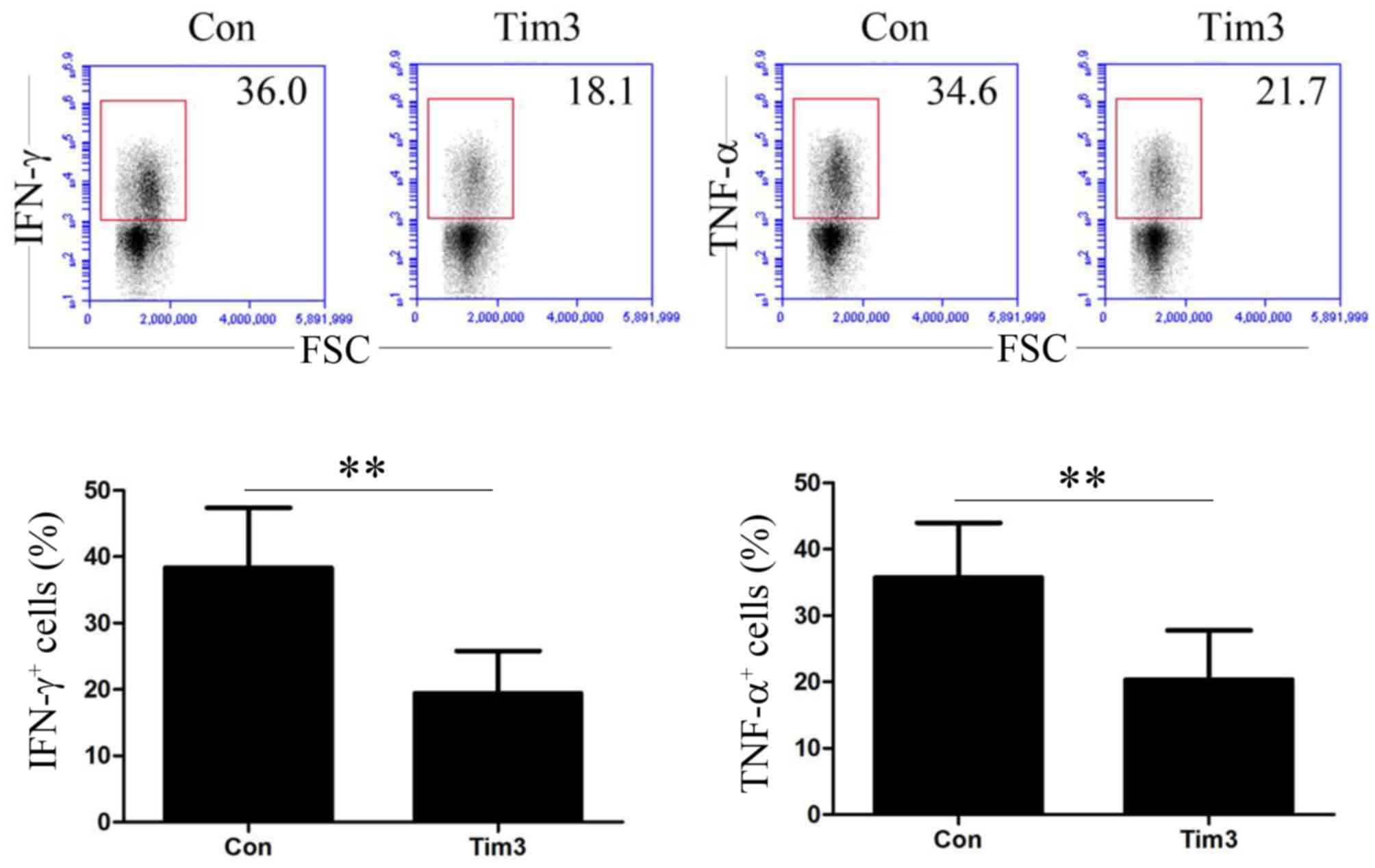
Effects of galectin-9-mediated Tim-3
activation on cytokine secretion of T-cells
T-cells were treated with control or galectin-9
antibody and IFN-γ and TNF-α expression was assessed by flow
cytometry The effects of Tim-3 activation on cytokine secretion of
T-cells is demonstrated in Fig. 3.
The percentages of IFN-γ+ and TNF-α+ T-cells
in the control groups were 38.34±9.04 and 35.73±8.26%,
respectively. Following the activation of Tim-3 signaling pathway,
the percentages of IFN-γ+ and TNF-α+ T-cells
were 19.45±6.36 and 20.35±7.42%, respectively. Therefore,
galectin-9-mediated Tim-3 activation may significantly inhibit the
capability of T-cells to secrete IFN-γ and TNF-α (P<0.01;
Fig. 3).
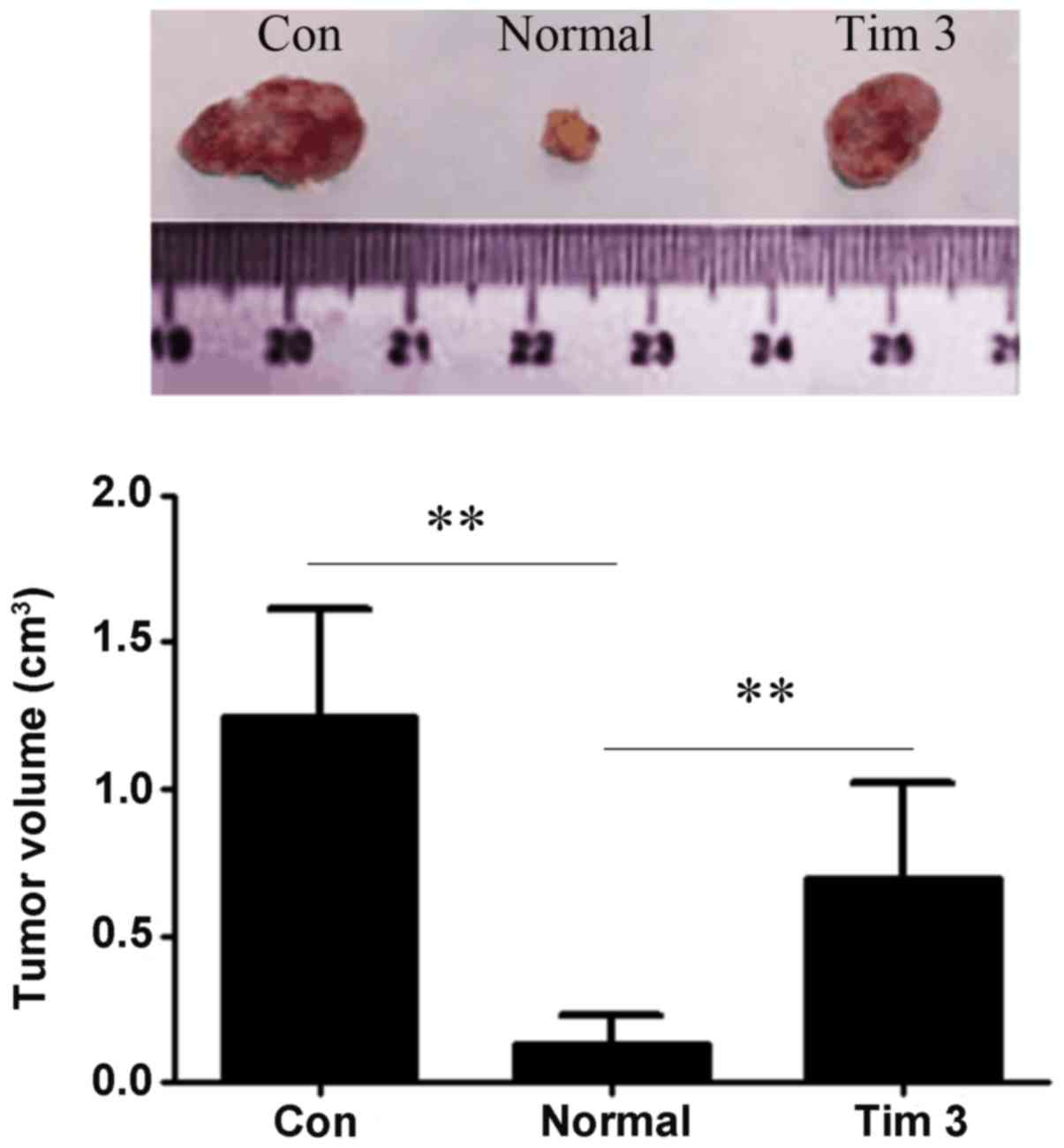
Galectin-9-mediated Tim-3 activation
affects tumor growth in vivo
A tumor xenograft model was used, and the mice were
treated with 3 types of cells and tumor growth was assessed after
30 days Experimental results of nude mice tumor-bearing test are
illustrated in Fig. 4. The tumor
volumes of tumor-bearin g mice in control, untreated T-cells and
Tim-3-stimulated T-cell groups were 1.27±0.38, 0.12±0.10 and
0.68±0.33 cm3, respectively. Tim-3 stimulation
significantly weakened the ability of T-cells to inhibit tumor
growth (P<0.01; Fig. 4).
Discussion
The Tim family is a transmembrane glycoprotein
encoded by the Tim gene. Its basic structure includes a signal
peptide, immunoglobulin V area, mucin area, transmembrane region
and intracellular tail region containing phosphate sites (13). Tim family members include Tim-1, Tim-3
and Tim-4 (14). Tim-3 is one of the
newly-discovered family members (15–17).
Previous studies have demonstrated that Tim-3 is an immune negative
regulating molecule and expressed on the surface of differentiated
and mature Th1 cells (11,18). Previous studies demonstrated that
Tim-3, cytotoxic T lymphocyte associated antigen-4 (CTLA−4)
and PD-1 are classified as the immune system's inhibitory receptors
(11,19). A number of studies demonstrated that
the expression of Tim-3 in a variety of tumor tissues is associated
with tumor development (20,21).
In addition to the close association with the
occurrence and development of tumors, Tim-3 is also associated with
T-cell depletion (22). Previous
studies demonstrated that the expression level of Tim-3 increases
on the surface of T-cells infiltrating various tumor tissues
(11,23). There are a number of studies regarding
Tim-3 expression on the T-cell surface of gastric cancer patients
(11,22,23). The
present study assessed the expression of Tim-3 on the surface of
T-cells in patients with gastric cancer. According to the results
of the present study, the expression levels of Tim-3 on the surface
of T-cells was significantly increased in para-carcinoma and cancer
gastric tissues compared with that in peripheral blood.
Furthermore, the expression level of Tim-3 on the surface of
T-cells is significantly increased in cancer tissues compared with
that in para-carcinoma tissues. At the same time, with the increase
in the T staging of gastric cancer, the expression level of Tim-3
on the surface of T-cells gradually increases. The results of the
present study suggest that Tim-3 may participate in the decrease of
T-cell function during tumor development due to it inhibiting the
secretion of TNF-α and IFN-γ by T-cells, and therefore promote the
occurrence and development of the tumor.
To further clarify the association between Tim-3
expression on the surface of T-cells in gastric cancer patients and
T-cell function, the present study used recombinant ligand
galectin-9 to activate Tim-3 on the surface of T-cells. Galectin-9
is the most commonly used ligand to activate the Tim-3 pathway in
the study of all types of diseases (11,22–24). The
present study used a Galectin-9 antibody, a ligand to Tim-3 to
activate the Tim-3 signaling pathway in T-cells. Results
demonstrated that, following the activation of Tim-3 signaling, the
ability of T-cells to secrete IFN-γ and TNF-α decreased.
Furthermore, a human gastric cancer xenograft model was employed.
The mice were treated with T-cells that were activated or
non-activated by Tim-3 stimulation and tumor growth was evaluated.
The results demonstrated that the effect of Tim-3-stimulated
T-cells on inhibiting tumor growth decreased. The results of the
present study suggest that Tim-3 may inhibit T-cell function by
inhibiting IFN-γ and TNF-α secretion, therefore participating in
gastric cancer initiation and progression. In conclusion, the
increase of expression level of Tim-3 on T-cell surface in gastric
cancer tissues may be associated with the development gastric
cancer. The present data provide a new understanding of the effect
of Tim-3 on T-cells, which may have implications for T-based cancer
immunotherapy by optimizing T-cell effector function, possibly by
neutralization of the effect of Tim-3 on T-cells.
Acknowledgements
The authors would like to thank Dr Li Ruilin
(Department of Oncology, Weihai Municipal Hospital, Weihai, China)
for advice on the experimental ideas.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL, JY and HZ made substantial contributions to
conception and design. HZ, SBS and SWS were responsible for the
analysis and interpretation of data. LL, JY and SBS were involved
in drafting the manuscript or revising it critically for important
intellectual content. LL agreed to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Weihai Municipal Hospital (Weihai, China). Written
informed consent was received from all patients involved.
Patient consent for publication
All of the study participants provided consent for
the publication of data.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Cheng G, Li M, Wu J, Ji M, Fang C, Shi H,
Zhu D, Chen L, Zhao J, Shi L, et al: Expression of Tim-3 in gastric
cancer tissue and its relationship with prognosis. Int J Clin Exp
Pathol. 8:9452–9457. 2015.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore MA, Eser S, Igisinov N, Igisinov S,
Mohagheghi MA, Mousavi-Jarrahi A, Ozentürk G, Soipova M, Tuncer M
and Sobue T: Cancer epidemiology and control in North-Western and
Central Asia-past, present and future. Asian Pac J Cancer Prev. 11
Suppl 2:S17–S32. 2010.
|
|
4
|
Qiu MZ, Wang ZQ, Zhang DS, Liu Q, Luo HY,
Zhou ZW, Li YH, Jiang WQ and Xu RH: Comparison of 6th and 7th AJCC
TNM staging classification for carcinoma of the stomach in China.
Ann Surg Oncol. 18:1869–1876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JM, Kim JH, Park SS, Kim SJ, Mok YJ
and Kim CS: Prognostic factors and availability of D2 lymph node
dissection for the patients with stage II gastric cancer:
Comparative analysis of subgroups in stage II. World J Surg.
32:1037–1044. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Y, Qiu MZ, Wang DS, Zhang DS, Ren C,
Bai L, Luo HY, Wang ZQ, Wang FH, Li YH, et al: Adjuvant
chemotherapy for elderly patients with gastric cancer after D2
gastrectomy. PLoS One. 8:e531492013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takano S, Saito H and Ikeguchi M: An
increased number of PD-1+ and Tim-3+
CD8+ T-cells is involved in immune evasion in gastric
cancer. Surg Today. 46:1341–1347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hassan SS, Akram M, King EC, Dockrell HM
and Cliff JM: PD-1, PD-L1 and PD-L2 gene expression on t-cells and
natural killer cells declines in conjunction with a reduction in
PD-1 protein during the intensive phase of tuberculosis treatment.
PLoS One. 10:e01376462015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee KA, Shin KS, Kim GY, Song YC, Bae EA,
Kim IK, Koh CH and Kang CY: Characterization of age-associated
exhausted CD8+ T-cells defined by increased expression
of Tim-3 and PD-1. Aging Cell. 15:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HJ, Park JS, Jeong YH, Son J, Ban YH,
Lee BH, Chen L, Chang J, Chung DH, Choi I, et al: Correction: PD-1
upregulated on regulatory T-cells during chronic virus infection
enhances the suppression of CD8+ T-cell immune response
via the interaction with PD-L1 expressed on CD8+
T-cells. J Immunol. 195:5841–5842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ngiow SF, von Scheidt B, Akiba H, Yagita
H, Teng MW and Smyth MJ: Anti-TIM3 antibody promotes T cell
IFN-γ-mediated antitumor immunity and suppresses established
tumors. Cancer Res. 71:3540–3551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan L, Xu B, Yuan P, Zhou J, Qin P, Han
L, Chen G, Wang Z, Run Z, Zhao P, et al: Tumor-infiltrating
CD4+ T-cells in patients with gastric cancer. Cancer
Cell Int. 17:1142017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McIntire JJ, Umetsu SE, Akbari O, Potter
M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT and DeKruyff RH:
Identification of Tapr (an airway hyperreactivity regulatory locus)
and the linked Tim gene family. Nat Immunol. 2:1109–1116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shakhov AN, Rybtsov S, Tumanov AV,
Shulenin S, Dean M, Kuprash DV and Nedospasov SA: SMUCKLER/TIM4 is
a distinct member of TIM family expressed by stromal cells of
secondary lymphoid tissues and associated with lymphotoxin
signaling. Eur J Immunol. 34:494–503. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markwick LJ, Riva A, Ryan JM, Cooksley H,
Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A,
et al: Blockade of PD1 and TIM3 restores innate and adaptive
immunity in patients with acute alcoholic hepatitis.
Gastroenterology. 148:590–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao JL, Li LJ, Fu R, Wang HQ, Jiang HJ,
Yue LZ, Zhang W, Liu H, Ruan EB, Qu W, et al: Elevated
TIM3+ hematopoietic stem cells in untreated
myelodysplastic syndrome displayed aberrant differentiation,
overproliferation and decreased apoptosis. Leuk Res. 38:714–721.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roth CG, Garner K, Eyck ST, Boyiadzis M,
Kane LP and Craig FE: TIM3 expression by leukemic and non-leukemic
myeloblasts. Cytometry B Clin Cytom. 84:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge RT, Zeng L, Mo LH, Xu LZ, Zhang HP, Yu
HQ, Zhang M, Liu ZG, Liu ZJ and Yang PC: Interaction of TIM4 and
TIM3 induces T helper 1 cell apoptosis. Immunol Res. 64:470–475.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Liu X, Zhu J, Li W, Guo L, Han X,
Song B, Cheng S and Jie L: Polymorphisms of co-inhibitory molecules
(CTLA-4/PD-1/PD-L1) and the risk of non-small cell lung cancer in a
Chinese population. Int J Clin Exp Med. 8:16585–16591.
2015.PubMed/NCBI
|
|
20
|
Li Z, Li N, Zhu Q, Zhang G, Han Q, Zhang
P, Xun M, Wang Y, Zeng X, Yang C, et al: Genetic variations of PD1
and TIM3 are differentially and interactively associated with the
development of cirrhosis and HCC in patients with chronic HBV
infection. Infect Genet Evol. 14:240–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ngiow SF, Teng MW and Smyth MJ: Prospects
for TIM3-targeted antitumor immunotherapy. Cancer Res.
71:6567–6571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jayaraman P, Sada-Ovalle I, Beladi S,
Anderson AC, Dardalhon V, Hotta C, Kuchroo VK and Behar SM: Tim3
binding to galectin-9 stimulates antimicrobial immunity. J Exp Med.
207:2343–2354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bu M, Shen Y, Seeger WL, An S, Qi R,
Sanderson JA and Cai Y: Ovarian carcinoma-infiltrating regulatory
T-cells were more potent suppressors of CD8(+) T-cell inflammation
than their peripheral counterparts, a function dependent on TIM3
expression. Tumour Biol. 37:3949–3956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oomizu S, Arikawa T, Niki T, Kadowaki T,
Ueno M, Nishi N, Yamauchi A and Hirashima M: Galectin-9 suppresses
Th17 cell development in an IL-2-dependent but Tim-3-independent
manner. Clin Immunol. 143:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|