Introduction
Ovarian cancer is one of the most common types of
tumor in women worldwide, and exhibits increasing morbidity and
mortality rates (1,2). Despite medical advances in surgical
techniques, radiation, chemotherapy and gene target therapy, the
five-year survival rate of patients with ovarian cancer remains
poor (3,4). Resistance to anti-ovarian cancer
therapies is a major issue in patients with recurrent ovarian
cancer (5,6). Currently, apoptotic resistance is an
important factor in chemotherapy-managed metastatic ovarian cancer
in patients (7,8). The biological relevance of Chinese
medicinal therapy in the progression of breast cancer is evidenced
by the increasing immunosuppression, which has demonstrated their
capacities to induce apoptosis and inhibit tumor aggressiveness
(9,10). Therefore, the development of Chinese
medicine monomers that overcome apoptotic resistance has been the
focus of investigations in the treatment of cancer.
Tanshinone IIA (Tan-IIA), a traditional Chinese
medicine extracted from Danshen, has been clinically used for the
treatment of human cancer (11–13). In
previous years, experimental investigation of the anticancer
mechanism of Tan-IIA against human breast cancer has identified the
downregulation of multiple genes, including P53 and B-cell lymphoma
2 (bcl-2), which are involved in cell cycle regulation, cell
proliferation, apoptosis and DNA synthesis (14). The pharmacological and therapeutic
properties of Tan-IIA in inducing the apoptosis of human cancer
cells have attracted increased interest through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential (15,16). A previous study indicated that Tan-IIA
can inhibit the growth of lung cancer H146 cells by upregulating
pro-apoptotic gene expression and decreasing mitochondrial membrane
potential (15). Of note, a previous
study indicated that Tan-IIA induced apoptosis via p38
mitogen-activated protein kinase (MAPK), and the downregulation of
excision repair cross-complementing 1 and lung-resistance protein
in cisplatin-resistant ovarian cancer cells (17). Therefore, Tan-IIA is widely used for
the treatment of ovarian cancer.
On these premises, the present study developed a
series of experimental strategies to identify the role of Tan-IIA
in ovarian cancer growth and examine the possible mechanism of its
activity. It was hypothesized that Tan-IIA induces the apoptosis of
ovarian cancer cells through attenuation of the phosphoinositide
3-kinase (PI3K)/AKT/c-Jun N-terminal kinase (JNK) signaling
pathways.
Materials and methods
Cell culture
The A2780 ovarian cancer cells were purchased from
American Type Culture Collection (Rockville, MD, USA). All cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in a 37°C humidified atmosphere of 5%
CO2.
MTT cytotoxicity assays
The A2780 cells (1×103) were incubated
with Tan-IIA (50, 100, 150, 200 and 250 µM) from Herbasin Co., Ltd.
Shenyang, China (purity >99.2%) in 96-well plates for 24 h, in
triplicate for each condition, with phosphate buffer solution (PBS)
added instead of the Tan-IIA as a control. Subsequently, 20 µl of
MTT (5 mg/ml) in PBS solution was added to each well, and the plate
was incubated for another 4 h. The majority of the medium was
removed and 100 µl of dimethyl sulfoxide was added to the wells to
solubilize the crystals. The optical density (OD) was measured
using a Bio-Rad ELISA reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at a wavelength of 490 nm.
Overexpression of PI3K
The A2780 cells (1×105) were cultured in
a six-well plate until 85% confluence, following which the medium
was removed from the culture plate followed by three PBS washes.
The A2780 cells were transfected with pLentivirus-PI3K (pPI3K)
using Lipofectamine 2000 (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). The PI3K-overexpressing A2780 cells were then
treated with Tan-IIA (150 µM) and/or PI3K inhibitor (50 mg/ml;
LY294002; Qiagen, Inc., Gaithersburg, MD, USA) for 12 h at 37°C to
analyze the protein expression levels, determined by western blot
analysis.
Cell invasion and migration
assays
The A2780 cells (1×105) were transfected
with pPI3K, PI3K inhibitor and/or Tan-IIA (150 µM). For the
migration assay, the A2780 cells were seeded onto a Matrigel
Invasion Chamber (BD Biosciences, San Jose, CA, USA) for 24 h at
37°C. For the invasion assay, the A2780 cells were suspended as a
density of 1×105 in 500 µl serum-free DMEM. The cells
were then added to the top of the BD BioCoat Matrigel Invasion
Chamber (BD Biosciences) for 24 h according to the manufacturer's
protocol. The cells invaded through the Matrigel were fixed in
methanol (25%) at 24-h post-treatment. The cells were then stained
with 0.1% crystal violet in 25% methanol, followed by washing in
cold 1X PBS. The tumor cell invasion and migration were counted in
at least three randomly selected stained fields of every membrane
under a microscope (IX71; Olympus Corporation, Tokyo, Japan) at 10X
magnification.
Flow cytometric analysis
The A2780 cells (1×105) were cultured in
a six-well plate until 90% confluence was reached. Apoptosis was
assessed following incubation of the A2780 cells with Tan-IIA (150
µM) and/or PI3K inhibitor (50 mg/ml) for 48 h. The A2780 cells were
trypsinized and collected following incubation. The cells were then
washed in cold PBS, adjusted to 1×106 cells/ml with PBS,
labeled with Annexin V-FITC and PI using an Annexin V-FITC kit (BD
Biosciences), and analyzed with a FACScan flow cytometer (BD
Biosciences).
Western blot analysis
The A2780 cells (1×108) were homogenized
in lysate buffer containing protease-inhibitor and were centrifuged
at 6,000 × g at 4°C for 10 min. Protein concentration was measured
by a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Protein extracts (10 µg) were electrophoresed on 8–12% denaturing
gel and transferred to a polyvinylidene fluoride membrane (GE
Healthcare, Chicago, IL, USA) for western blotting analysis. as
previously described (18). For
western blot analysis, the following rabbit anti-rat primary
antibodies were used: JNK (1:1,000, cat. no. ab124956, Abcam
Cambridge, MA, USA), PI3K (1:500, cat. no. ab86714, Abcam), AKT
(1:500, cat. no. ab64148, Abcam), phosphorylated (p)JNK (1:1,000,
cat. no. ab47337, Abcam), pAKT (1:500, cat. no. ab8932, Abcam),
caspase-3 (1:1,000, cat. no. ab2171, Abcam), caspase-8 (1:1,000,
cat. no. ab25901, Abcam), caspase-9 (1:1,000, cat. no. ab52298,
Abcam), Bcl-2-like protein 2 (Bcl-w; 1:1,000, cat. no. ab38629,
Abcam), myeloid cell leukemia-1 long (Mcl-1L; 1:1,000, cat. no.
ab32087, Abcam) and β-actin (1:2,000, cat. no. ab8226, Abcam) for
12 h at 4°C. The antibodies were added following blocking (5%
skimmed milk) for 1 h 37°C and were subsequently incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
monoclonal antibody (cat. no. PV-6001, ZSGB-BIO, Beijing, China)
for 12 h at 4°C. A Ventana Benchmark automated staining system was
used for visualizing protein expression in tumor tissues (Olympus
BX51; Olympus Corporation).
Animal experiments
A total of 100 female Sprague-Dawley rats (8-week
old, 300–320 g body weight) were purchased from Slack Co., Ltd.
(Shanghai, China). All animals were housed in a
temperature-controlled facility at 23±1°C and relative humidity of
50±5% with a 12-h light/dark cycle. All rats had ad libitum
access to food and water. The rats were subcutaneously implanted
with A2780 tumor cells (1×107). The rats received either
Tan-IIA treatment (10 mg/kg) via gavage or treatment with the same
volume of PBS control (n=10 in each group, once a day for 1 week)
on day 5. The tumor volume was calculated as follows: Volume=(D ×
d2)/2, where D represents the maximal diameter and d
represents the minimal diameter. The rats were sacrificed on day 30
following a total of seven treatments.
Immunohistochemistry
Tumors from the xenograph rats were fixed using
formaldehyde (10%), followed by embedding in paraffin. The tumor
tissues were fabricated into 4-µm-thick tumor sections. Antigen
retrieval was performed on the tumor sections and the sections were
incubated for 12 h at 37°C with the following primary antibodies:
JNK (1:1,000, cat. no. ab124956, Abcam), PI3K (1:500, cat. no.
ab86714, Abcam), AKT (1:500, cat. no. ab64148, Abcam) The sections
were washed three times with PBS (3 min/wash), and then incubated
with HRP-conjugated anti-rabbit IgG (Bio-Rad Laboratories, Inc.) at
a 1:10,000 dilution for 1 h at 37°C. A Ventana Benchmark automated
staining system was used for examining the protein expression in
tumor tissues.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) analysis
For the analysis of apoptosis of tumor cells in
tumor tissues, a TUNEL assay (Biotool, Houston, TX, USA) were used
to detect TUNEL-positive cells. The tumor sections were fixed with
4% paraformaldehyde solution for 60 min at 4°C. The cells were then
washed with PBS three times and permeabilized by immersing cell
slides in 0.2% TritonX-100 solution in PBS for 30 min at 4°C.
Subsequently, the cells were incubated with equilibration buffer
for 30 min at 4°C. The myocardial cells were then incubated with 50
µl of the reaction mixture at 37°C for 60 min, and washed three
times with PBS. The cell nuclei were stained with
4′,6-diamidino-2-phenylindole for 60 min at 4°C. Finally, images of
the myocardial cells were captured with a ZEISS LSM 510 confocal
microscope (Carl Zeiss AG, Oberkochen, Germany) at 488 nm.
Statistical analysis
All data are expressed as the mean ± standard
deviation of triplicate independent experiments and were analyzed
using Student's t-test or one-way analysis of variance with a
Tukey's HSD test. All data were analyzed using SPSS 19.0 (IBM SPSS,
Armonk, NY, USA) and GraphPad Prism version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA0 with the assistance of Microsoft Excel
(Microsoft Corporation, Redmond, WI, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Tan-IIA significantly inhibits ovarian
cancer cell growth and aggressiveness through the inhibition of
target PI3K
The anti-ovarian cancer efficacy of Tan-IIA was
analyzed in vitro in the A2780 human ovarian cancer cell
line. Following treatment with different concentrations of Tan-IIA
(50, 100, 150, 200 and 250 µM) for 24 h, A2780 cell growth was
inhibited by the treatment of Tan-IIA (Fig. 1A). The 50% inhibitory concentration of
Tan-IIA for A2780 cells was identified as 150 µM. As shown in
Fig. 1B and C, 150 µM of Tan-IIA
markedly inhibited the migration and invasion of A2780 cells,
compared with that in the PBS-treated group (blank control). It was
demonstrated that Tan-IIA (150 µM) downregulated the expression of
PI3K in A2780 cells, compared with that in the control (Fig. 1D). It was also observed that the PI3K
inhibitor (LY29400/PI3KIR) enhanced the Tan-IIA-inhibited
(Tan-IIA+PI3KIR) growth, migration and invasion of A2780 cells
(Fig. 1E-G). However, the
overexpression of PI3K eliminated the Tan-IIA-induced inhibited
growth and aggressiveness of A2780 cells (Fig. 1H-J). These results indicated that
Tan-IIA inhibited ovarian cancer cell growth and aggressiveness
through the inhibition of target PI3K.
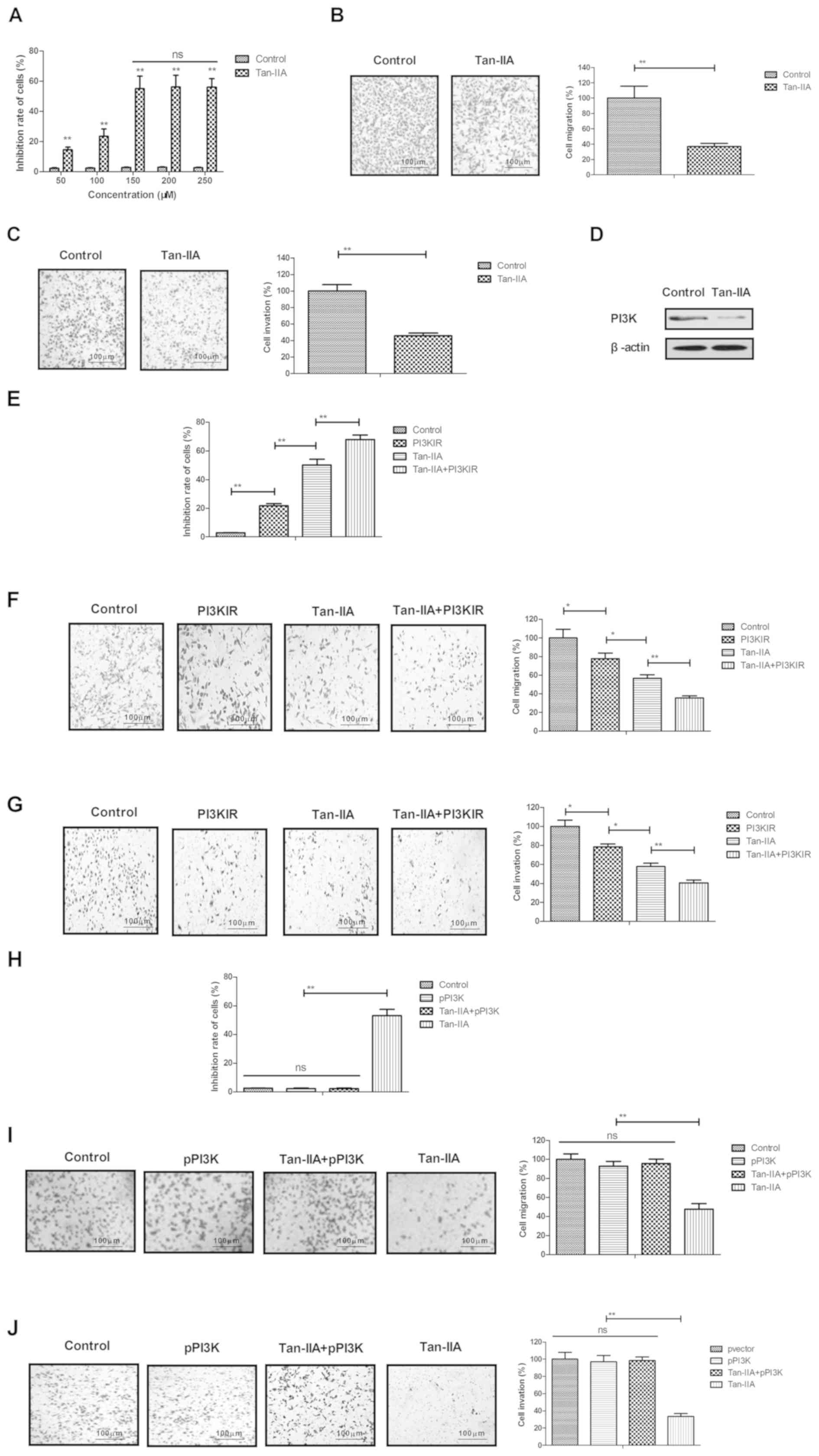 | Figure 1.Tan-IIA significantly inhibits ovarian
cancer cell growth and aggressiveness through inhibition of target
PI3K. (A) Tan-IIA (50, 100, 150, 200 and 250 µM) inhibited A2780
cell growth. Tan-IIA (150 µM) inhibited the (B) migration and (C)
invasion of A2780 cells, compared with PBS-treated group. (D)
Tan-IIA (150 µM) downregulated the expression of PI3K in A2780
cells, compared with the control. PI3K inhibitor (LY294002; PI3KIR)
promoted Tan-IIA-inhibited (Tan-IIA+PI3KIR) A2780 cell (E) growth,
(F) migration and (G) invasion. Overexpression of PI3K inhibited
Tan-IIA-inhibited (H) growth, (I) migration and (J) invasion (J) of
A2780 cells. Tan-IIA, tanshinone IIA; PI3K, phosphoinositide
3-kinase; pPI3K, pLentivirus-PI3K; NS, not significant. *P<0.05
and **P<0.01 vs. control. |
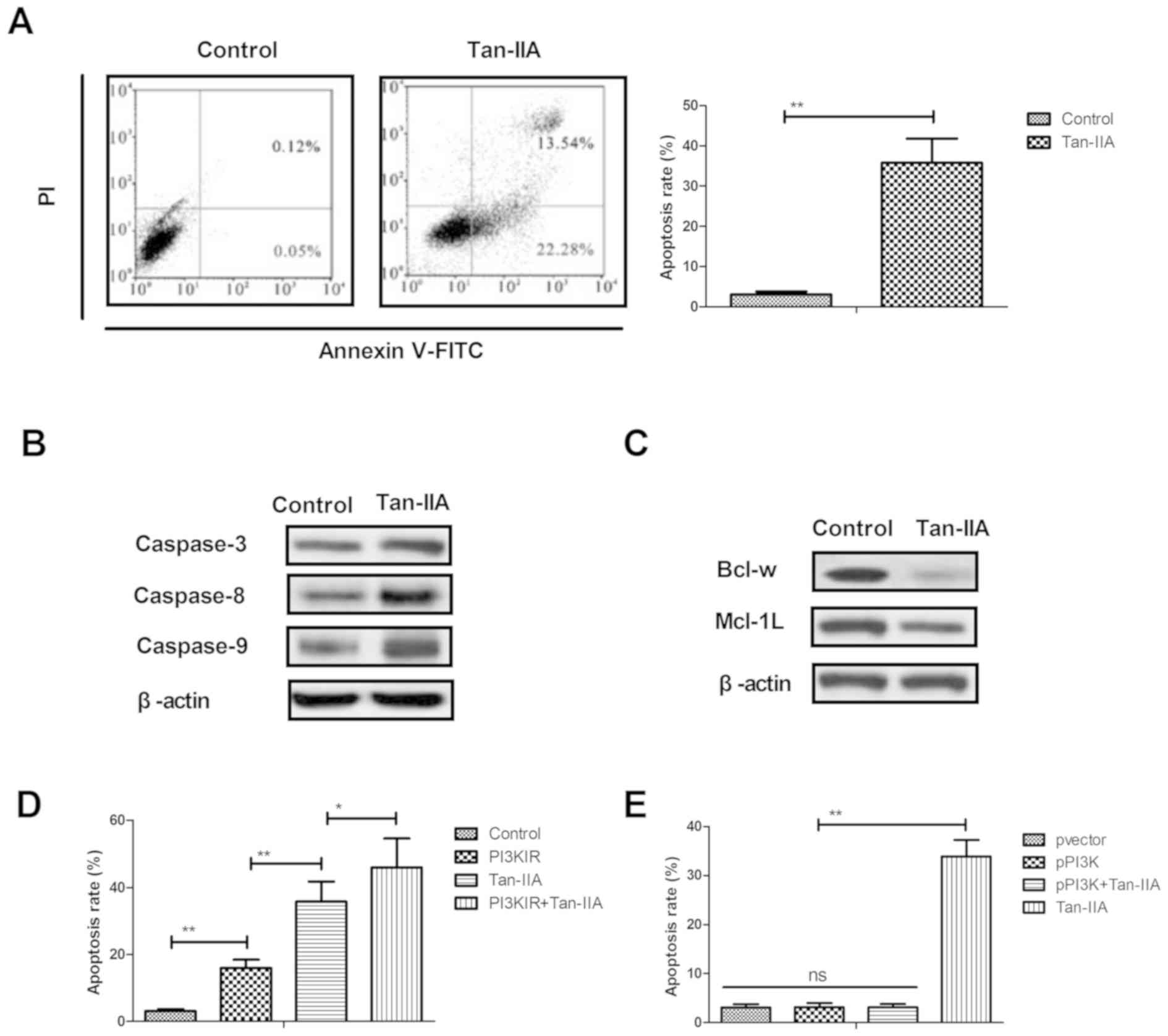
Tan-IIA significantly induces the
apoptosis of ovarian cancer cells through the inhibition of target
PI3K
The present study further examined the efficacy of
Tan-IIA (150 µM) on the apoptosis of ovarian cancer cells. Tan-IIA
significantly increased the apoptosis of human A2780 ovarian cancer
cells, compared with the control (Fig.
2A). The expression levels of caspases-3, caspase-8 and
caspases-9 were markedly upregulated by Tan-IIA in the A2780 cells
(Fig. 2B). Tan-IIA treatment
decreased the expression levels of mitochondria protective Bcl-w
and Mcl-1L in the human ovarian cancer cells (Fig. 2C). As shown in Fig. 2D and E, the results showed that PI3K
inhibitor (LY294002) enhanced the Tan-IIA-induced apoptosis of
A2780 cells, whereas the overexpression of PI3K had the reverse
effect. These results suggested that Tan-IIA induced the apoptosis
of ovarian cancer cells through the inhibition of target PI3K.
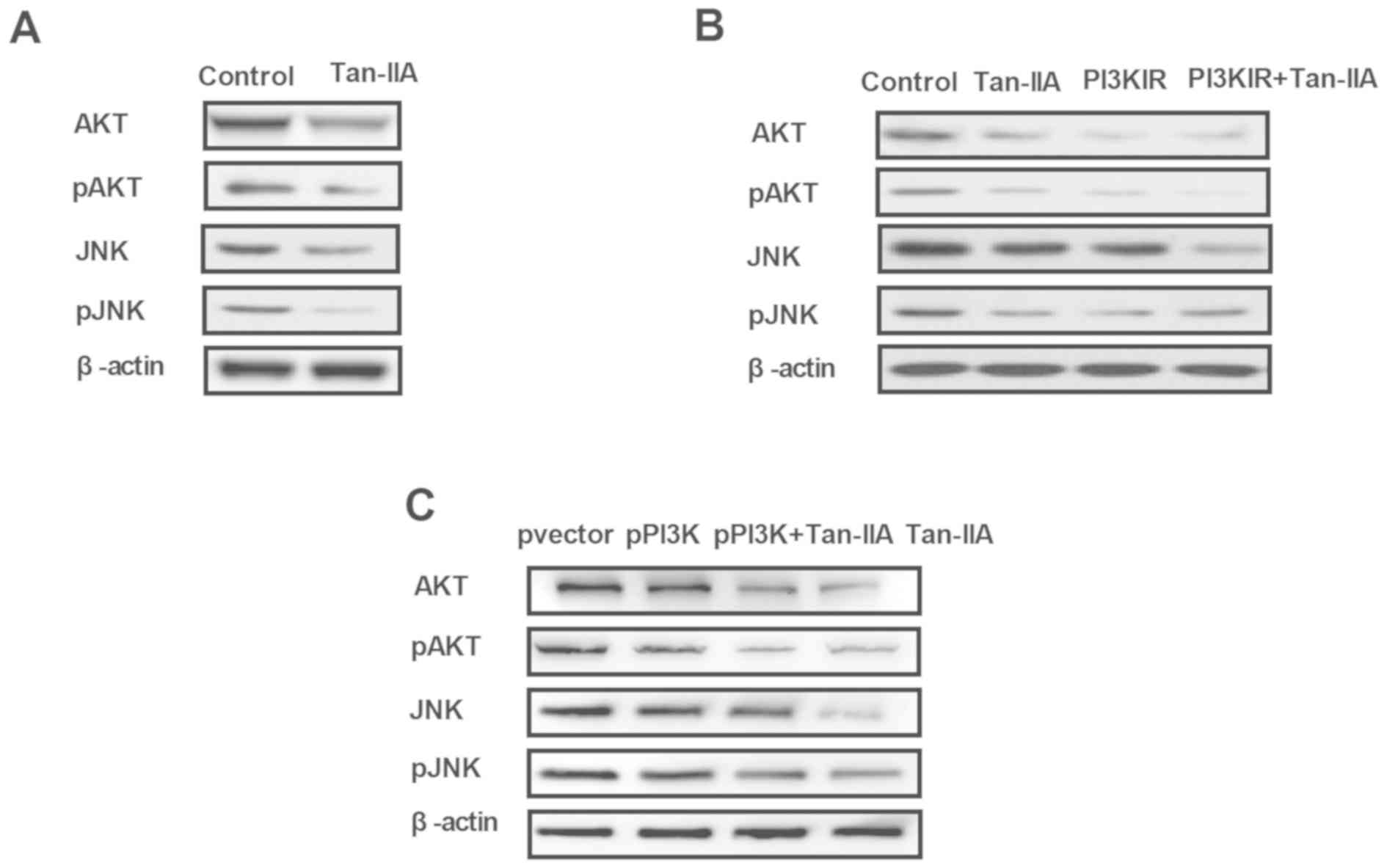
Tan-IIA regulates the expression of
key protein molecules of the PI3K/AKT/JNK signaling pathways in
ovarian cancer cells
To understand the inhibitory effects of Tan-IIA on
the apoptosis of A2780 cells, the present study investigated the
potential signaling pathway in vitro. Tan-IIA decreased the
expression and phosphorylation of AKT and JNK in the A2780 cells
(Fig. 3A). In the Tan-IIA-treated
group, PI3K inhibitor (LY294002) enhanced the Tan-IIA-induced
inhibited expression and phosphorylation of AKT and JNK in the
A2780 cells (Fig. 3B). The results
indicated that the overexpression of PI3K eliminated the
Tan-IIA-inhibited expression and phosphorylation of AKT and JNK
(Fig. 3C). These results suggested
that Tan-IIA regulated the expression of key protein molecules of
the PI3K/AKT/JNK signaling pathways in ovarian cancer cells.
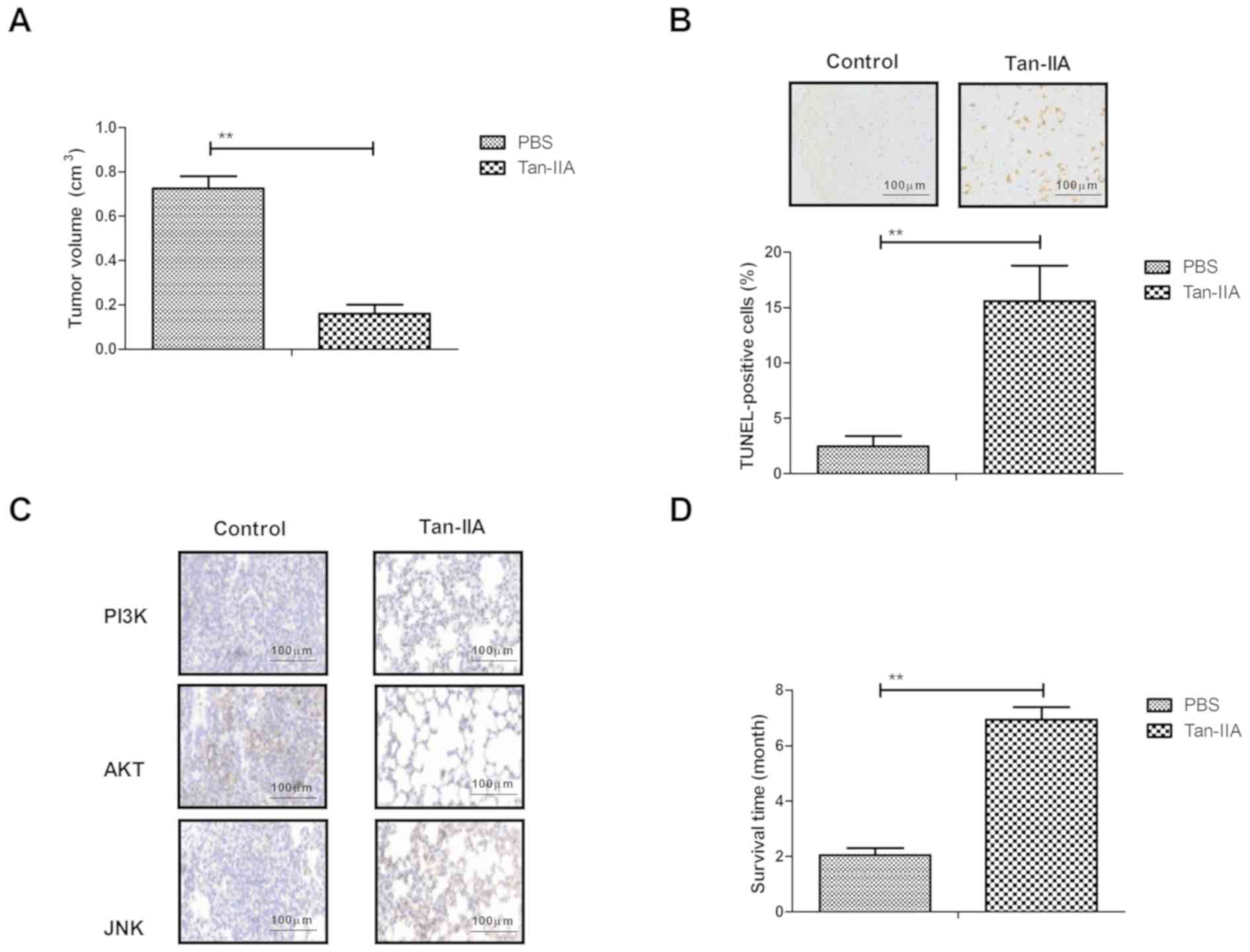
Tan-IIA exerts therapeutic effects on
ovarian cancer lesion model rats
The present study further investigated the in
vivo therapeutic effects of Tan-IIA on an ovarian cancer lesion
model in rats. As shown in Fig. 4A,
Tan-IIA treatment significantly inhibited ovarian cancer growth,
compared with the PBS-treated rats. The Tan-IIA-treated ovarian
cancer lesion model rats showed increased apoptotic bodies in tumor
tissues, compared with the PBS-treated rats (Fig. 4B). The immunohistochemical assay
demonstrated that the expression levels of PI3K, AKT and JNK were
decreased by Tan-IIA treatment in the tumor tissues, compared with
those in the control group (Fig. 4C).
Tan-IIA treatment significantly prolonged the survival rate of the
experimental rats in a100-day observation (Fig. 4D). These results suggested that
Tan-IIA exerted therapeutic effects on the rats of the ovarian
cancer lesion model.
Discussion
Ovarian cancer exhibits resistance mechanisms
towards chemotherapeutic drugs due to apoptotic resistance
(19). A previous study indicated
that the attenuation of PI3K and extracellular signal-regulated
kinase 1/2 contributes to a decrease in the apoptotic resistance of
nonadherent ovarian cancer cells (20). Evidence has suggested that Tan-IIA can
inhibit ovarian cancer cell growth by inducing apoptosis via the
downregulation of p38 MAPK (17). The
present study further analyzed the role of Tan-IIA in inducing the
apoptosis of ovarian cancer cells in vitro and in
vivo. It was found that Tan-IIA significantly inhibited the
growth and aggressiveness of ovarian cancer cells via attenuation
of the PI3K/AKT/JNK signaling pathways. The findings suggest that
Tan-IIA significantly induced the apoptosis of ovarian cancer cells
through the upregulation of caspases-3, caspase-8, caspases-9 and
the downregulation of Bcl-w and Mcl-1L in A2780 cells.
A previous study demonstrated that Tan-IIA inhibited
BxPC3-derived xenograft tumor growth by increasing the protein
expression levels of PKR-like endoplasmic reticulum kinase,
activating transcription factor 6, inositol-requiring enzyme 1α,
CCAAT-enhancer-binding protein homologous protein, caspase 3 and
caspase 12 (21). The results of the
present study showed that Tan-IIA inhibited ovarian tumor growth by
inducing apoptosis through the regulation of apoptosis-associated
protein expression. Yu et al showed that Tan-IIA suppressed
gastric cancer cell migration by decreasing expression levels of
Ki-67, proliferating cell nuclear antigen, matrix metalloproteinase
(MMP)-2, MMP-9 and forkhead box M1 (22). In the present study, it was shown that
Tan-IIA suppressed the migration and invasion of ovarian cancer
cells by inhibiting the target PI3K. Antitumor experiments have
indicated that Tan-IIA significantly inhibited growth of Lewis mice
with lung cancer by decreasing the expression of Bcl-2 and
endostatin (23). The present study
demonstrated that Tan-IIA treatment decreased the expression levels
of Bcl-w and Mcl-1L in ovarian cancer cells, which contributed to
the apoptosis of ovarian cancer cells.
Although the efficacy of Tan-IIA in ovarian cancer
has been investigated in a previous study, the molecular mechanism
mediated by Tan-IIA has not been clearly documented (17). A previous review revealed that the
PI3K/AKT/mammalian target of rapamycin pathways are potential
targeting members in the treatment of ovarian cancer (24). In the present study, the results
revealed that Tan-IIA induced the apoptosis of ovarian cancer cells
via the downregulation of PI3K/AKT/JNK signaling pathways.
Additionally, the in vivo assay showed that Tan-IIA
treatment inhibited the growth of ovarian cancer through increasing
the apoptosis of tumor cells. In particular, the overexpression of
PI3K eliminated the Tan-IIA-induced apoptosis of ovarian cancer
cells.
In conclusion, the present study provided a novel
strategy of Tan-IIA use, which induced ovarian cancer apoptosis by
inducing changes in apoptosis-associated genes and causing growth
inhibition. The findings of the present study indicated that
Tan-IIA offers potential against ovarian cancer through the
downregulation of PI3K/AKT/JNK signaling pathways, which indicates
the anticancer activity of Tan-IIA for further translation into
clinics. Further investigations are required focusing on
identifying a novel potential mechanism for the treatment of
ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and YZ performed the experiments. YEG designed
the experiment.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Woman's Hospital, School of Medicine, Zhejiang University
(Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed-Lecheheb D and Joly F: Ovarian
cancer survivors' quality of life: A systematic review. J Cancer
Surviv. 10:789–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohammadi V, Dehghani S, Larijani B and
Azadbakht L: Ovarian cancer risk and nonisoflavone flavonoids
intake: A systematic review of epidemiological studies. J Res Med
Sci. 21:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khoja L, Nolan K, Mekki R, Milani A,
Mescallado N, Ashcroft L, Hasan J, Edmondson R, Winter-Roach B,
Kitchener HC, et al: Improved survival from ovarian cancer in
patients treated in phase III trial active cancer centres in the
UK. Clin Oncol (R Coll Radiol). 28:760–765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards HM, Noer MC, Sperling CD,
Nguyen-Nielsen M, Lundvall L, Christensen IJ and Høgdall C:
Survival of ovarian cancer patients in Denmark: Results from the
Danish gynaecological cancer group (DGCG) database, 1995–2012. Acta
Oncol. 55 Suppl 2:S36–S43. 2016. View Article : Google Scholar
|
|
5
|
Tomek S, Horak P, Pribill I, Haller G,
Rössler M, Zielinski CC, Pils D and Krainer M: Resistance to
TRAIL-induced apoptosis in ovarian cancer cell lines is overcome by
co-treatment with cytotoxic drugs. Gynecol Oncol. 94:107–114. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Zheng F, Xing H, Gao Q, Wei W, Lu
Y, Wang S, Zhou J, Hu W and Ma D: Resistance to
chemotherapy-induced apoptosis via decreased caspase-3 activity and
overexpression of antiapoptotic proteins in ovarian cancer. J
Cancer Res Clin Oncol. 130:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong YY, Chen HP, Tan BZ, Yu HH and Huang
XS: Triptolide avoids cisplatin resistance and induces apoptosis
via the reactive oxygen species/nuclear factor-κB pathway in
SKOV3PT platinum-resistant human ovarian cancer cells.
Oncol Lett. 6:1084–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lum E, Vigliotti M, Banerjee N, Cutter N,
Wrzeszczynski KO, Khan S, Kamalakaran S, Levine DA, Dimitrova N and
Lucito R: Loss of DOK2 induces carboplatin resistance in ovarian
cancer via suppression of apoptosis. Gynecol Oncol. 130:369–376.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang AL, Zhang TT, Zhou N, Wu CY, Lin MH
and Liu YJ: MiRNA-10b sponge: An anti-breast cancer study in vitro.
Oncol Rep. 35:1950–1958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhargava-Shah A, Foygel K, Devulapally R
and Paulmurugan R: Orlistat and antisense-miRNA-loaded PLGA-PEG
nanoparticles for enhanced triple negative breast cancer therapy.
Nanomedicine (Lond). 11:235–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su CC and Lin YH: Tanshinone IIA
down-regulates the protein expression of ErbB-2 and up-regulates
TNF-alpha in colon cancer cells in vitro and in vivo. Int J Mol
Med. 22:847–851. 2008.PubMed/NCBI
|
|
12
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
13
|
Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang
J and Wang W: Potential anticancer activity of tanshinone IIA
against human breast cancer. Int J Cancer. 116:799–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Q, Zhang P, Zhang X and Chen J:
Experimental study of the anti-cancer mechanism of tanshinone IIA
against human breast cancer. Int J Mol Med. 24:773–780. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Rep. 3:645–650. 2010.PubMed/NCBI
|
|
16
|
Won SH, Lee HJ, Jeong SJ, Lee HJ, Lee EO,
Jung DB, Shin JM, Kwon TR, Yun SM, Lee MH, et al: Tanshinone IIA
induces mitochondria dependent apoptosis in prostate cancer cells
in association with an inhibition of phosphoinositide 3-kinase/AKT
pathway. Biol Pharm Bull. 33:1828–1834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.PubMed/NCBI
|
|
18
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-Based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaffaroni N, Pennati M, Colella G, Perego
P, Supino R, Gatti L, Pilotti S, Zunino F and Daidone MG:
Expression of the anti-apoptotic gene survivin correlates with
taxol resistance in human ovarian cancer. Cell Mol Life Sci.
59:1406–1412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang MK, Zhou HY, Yam JW and Wong AS:
c-Met overexpression contributes to the acquired apoptotic
resistance of nonadherent ovarian cancer cells through a cross talk
mediated by phosphatidylinositol 3-kinase and extracellular
signal-regulated kinase 1/2. Neoplasia. 12:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiu TL and Su CC: Tanshinone IIA
increases protein expression levels of PERK, ATF6, IRE1α, CHOP,
caspase3 and caspase12 in pancreatic cancer BxPC3 cell-derived
xenograft tumors. Mol Med Rep. 15:3259–3263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Wang X, Li Y and Tang B: Tanshinone
IIA suppresses gastric cancer cell proliferation and migration by
downregulation of FOXM1. Oncol Rep. 37:1394–1400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Hu K, Tang S, Xu LF and Luo YC:
Anti-tumor activity of tanshinone IIA in combined with
cyclophosphamide against Lewis mice with lung cancer. Asian Pac J
Trop Med. 9:1084–1088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazzoletti M and Broggini M: PI3K/AKT/mTOR
inhibitors in ovarian cancer. Curr Med Chem. 17:4433–4447. 2010.
View Article : Google Scholar : PubMed/NCBI
|