Introduction
Cardia cancer is a malignant digestive system tumor
occurring at the cardia, which is also one of the major tumors in
middle-aged and elderly people, frequently occurring in people aged
>40 years and accounting for ~1/10 of digestive system tumors
(1,2).
Its mortality rate is decreasing year by year with the improvement
in diagnosis and treatment, but the therapeutic effect is not
ideal, and a considerable proportion of conditions still cannot be
controlled, leading to the deterioration of cardia cancer cells and
poor efficacy of radiochemotherapy, so that patients are not
treated and cured. Cardia cancer is deemed to be caused by many
factors, among which genetic factor is considered to be the chief
culprit in addition to unhealthy diet and environmental factor.
Changes in gene structure result in changes in protein levels
(3,4).
Gene polymorphism indicates that the base structure and quantity of
the same gene in different populations are different, which will
lead to changes in the level of translation, thereby affecting the
physical growth and metabolic function relating to such a gene
(5). Currently, mutations in K-rat
sarcoma (K-ras) gene are considered to be the major cause of
malignant tumors. K-ras, as a member of the ras gene family,
encodes the K-ras protein, which is correlated with tumor
formation, proliferation, migration, diffusion and angiogenesis.
Numerous studies have confirmed that K-ras may be involved in
regulating multiple signaling pathways, and uncontrolled cell
proliferation represents a tendency of tumor development (6). Fascin protein is highly expressed in
many tumor tissues and plays an important role in the
proliferation, invasion and metastasis of tumors, which is an
independent factor in the prognosis of many tumors (7,8). There are
rare studies on the correlation of cardia cancer with K-ras
mutations and fascin expression up to now. Therefore, this study
linked K-ras gene mutations and fascin expression with cardia
cancer and explored the relationship of cardia cancer with
pathogenic genes from a genetic perspective.
Materials and methods
General data
A total of 90 cardia cancer patients (average age
58.34±12.01 years and medially weighing 67.23±12.34 kg) treated in
Jining First People's Hospital (Jining, China) from March 2014 to
March 2017 were enrolled, including 56 males and 34 females. Based
on pathological staging, 24 patients had stage Tis
tumor, 43 patients had stage T1-T2 tumor, and 13 patients had stage
T3-T4 tumor. All the patients were informed and signed the informed
consent. This study was approved by the Ethics Committee of Jining
First People's Hospital.
Extraction of genomic deoxyribonucleic
acid (DNA)
Paraffin-embedded cardia specimens were collected
from all patients, from whom genomic DNA was extracted using a
genomic extraction reagent (Qiagen, Inc., Valencia, CA, USA). The
concentration and purity of DNA were determined using a NanoDrop,
and DNA was stored at −20°C.
Sequencing of K-ras gene
K-ras gene was subjected to quantitative polymerase
chain reaction (qPCR) amplification and purification and then
sequenced on an automated gene analyzer (ABI3130XL). The results
were analyzed using the sequencing analysis software Chromas.
Single-nucleotide polymorphism (SNP)
typing of K-ras gene and detection of relative expression of fascin
gene through qPCR
Primer sequences and their TaqMan probe sequences at
SNP site were designed by Oligo 6.0 (Table II). Primers were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). Reaction was performed using a qPCR
instrument under the following conditions: i) 1 cycle of 94°C for 3
min; and ⅱ) 42 cycles of 94°C for 15 sec and 60°C for 60 sec. After
each cycle, the fluorescence value was read. The experimental
results were generated by the built-in software of the instrument.
Each sample was tested in triplicate. GAPDH was used as a negative
control. A positive plasmid containing such a sequence (synthesized
by Sangon Biotech Co., Ltd.) was employed as a positive control.
The cycle threshold (Cq) value was output from the instrument, and
experimental results were analyzed using the 2−ΔΔCq
method (9).
 | Table II.K-ras mutations in patients with
cardia cancer. |
Table II.
K-ras mutations in patients with
cardia cancer.
| Codon | Type of mutation | Amino acid
change | Case | Rate |
|---|
| 12 | 35G>A | Gly→Asp | 7 | 33.3 |
|
| 35G>T | Gly→Val | 5 | 23.8 |
|
| 35G>C | Gly→Cys | 5 | 23.8 |
|
| 34G>A | Gly→Ser | 3 | 14.3 |
| 13 | 34G>A | Gly→Asp | 1 | 4.8 |
 | Table I.Primer sequences of fluorescence
quantitative PCR. |
Table I.
Primer sequences of fluorescence
quantitative PCR.
| Genes | Primer sequences | Probe sequences |
|---|
| K-ras | F:
5′-TTCAAGCCCTCAGTCAGTTG-3′ | FAM:
5′-GGAGCTGGTGGCGTAGG-3′ |
|
| R:
5′-CACCGTCTCCAGTCAGCAGCTG-3′ | VIC:
5′-GGAGCTGATGGCGTAGG-3′(12,35G>A) |
|
|
|
5′-GGAGCTGTTGGCGTAGG-3′(12,35G>T) |
|
|
|
5′-GGAGCTGCTGGCGTAGG-3′(12,35G>C) |
|
|
|
5′-GGAGCTCGTGGCGTAGG-3′(12,34G>C) |
|
|
|
5′-GGAGCTGGTGACGTAGG-3′(13,35G>C) |
| Fascin | F:
5′-CCTGGACHCCAACCGCTCC-3′ | FAM:
5′-GACGGTGGGCAGTGACTCCG-3′ |
|
|
| R:
5′-CCACAGGAGTGTCGCCGCTG-3′ |
Detection of fascin expression in
cardia cancer cells via immunohistochemistry
Specimens were fixed with 10% formaldehyde at 20°C
for 16 h. Selected paraffin-embedded specimens were cut into
sections with 4 µm in thickness. Then, the sections were stained
using immunohistochemistry MaxVision two-step method blocked with
5% milk at 20°C for 2 h. After that, mouse anti-human fascin
monoclonal antibodies (dilution, 1:200; MAB-0228, clone no. FCN01)
and MaxVision immunohistochemistry kits (both from Fuzhou Maxim
Biotech, Inc., Fuzhou, China) were used. Fascin was expressed in
normal vascular endothelial cells and was used as a positive
control. Phosphate-buffered saline (PBS) was used as a negative
control instead of primary antibody. For each section, a total of
five representative high-power fields (×400) were selected for
observation.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
17.0 software package (SPSS, Inc., Chicago, IL, USA) was adopted
for statistical analyses. Measurement data were expressed as means
± SD, and t-test was used. Enumeration data were expressed as %,
and χ2 test was applied. P<0.05 indicated that the
difference was statistically significant.
Results
K-ras mutations in patients with
cardia cancer
A total of 21 out of 90 patients with cardia cancer
had mutations in K-ras gene, accounting for 23.3% of mutant
patients. Among these 21 patients, 20 patients had exon 12
mutation, accounting for 95.2%, and the mutations were mainly
detected in 35G>A, 35G>T, 35G>C, 34G>A and 34G>T.
One patient had exon 13 mutation, namely mutation 38G>A,
accounting for 4.8% of mutant patients (Table II).
Pearsons correlation analysis on risk
factors for K-ras mutations in cardia cancer
There were no statistically significant differences
in age, sex, weight and pathological stage between the 21 patients
with mutations and those without mutations (p>0.05), but the
mutation probability in patients with alcohol abuse was higher than
that in those without alcohol abuse, showing a statistically
significant difference (p<0.05) (Table III).
 | Table III.Correlation analyses on risk factors
for K-ras mutations. |
Table III.
Correlation analyses on risk factors
for K-ras mutations.
|
|
|
|
|
| Pathological stage
(%) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Groups | Case (n) | Age (years) | Sex (male/female,
%) | Weight (kg) | Tis | T1-T2 | T3-T4 | Alcohol abuse
(%) |
|---|
| Mutant | 21 | 57.21±11.21 | 53.2 | 68.23±13.52 | 18 | 68 | 14 | 45 |
| Non-mutant | 69 | 59.45±12.62 | 52.4 | 65.41±12.68 | 16 | 67 | 17 | 12 |
| χ2/t | – | 2.435 | 0.013 | 4.123 |
| 0.415 |
| 26.721 |
| P-value | – | 0.675 | 0.909 | 0.371 |
| 0.813 |
| <0.001 |
Distribution of mutant genotypes in
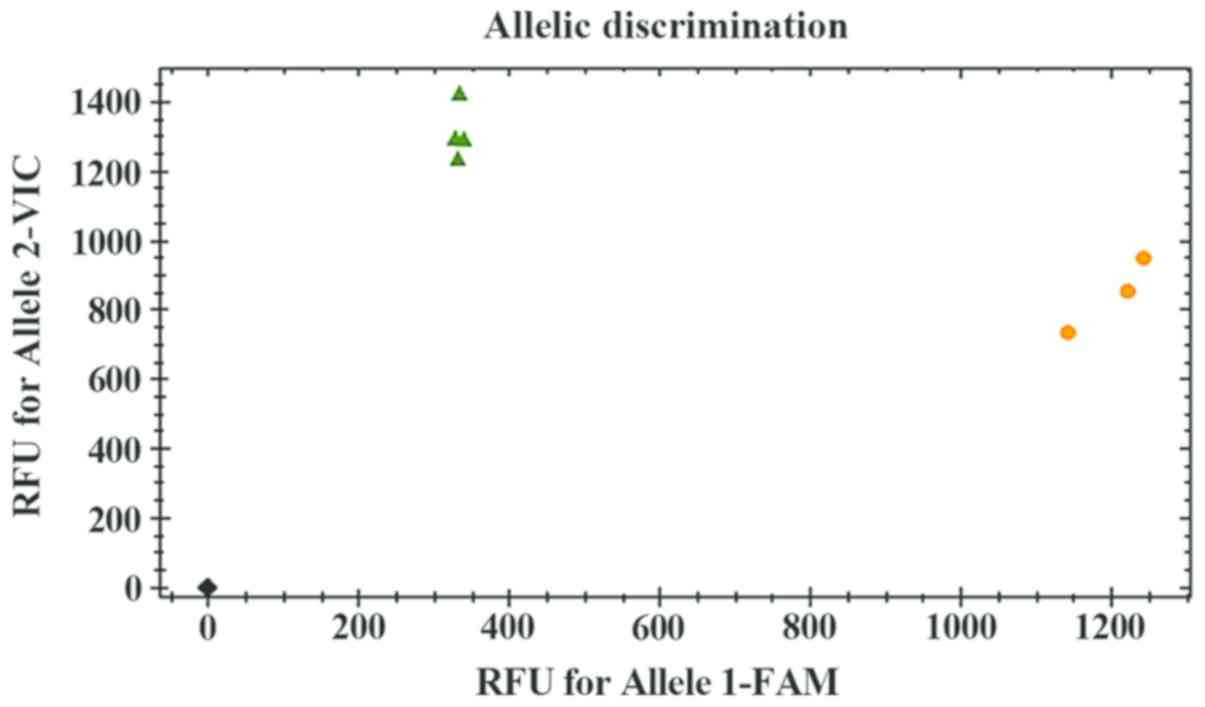
K-ras in cardia cancer
The genotypes containing mutant bases at each
mutation site were distributed as shown in Fig. 1. The difference in the mutation
probability between heterozygotes and homozygotes for four codon 12
mutations was not significant (p>0.05), but for one codon 13
mutation, the mutation probability of heterozygotes was higher than
that of homozygotes (p<0.05) (Table
IV).
 | Table IV.Distribution of mutant genotypes in
K-ras in cardia cancer (n, %). |
Table IV.
Distribution of mutant genotypes in
K-ras in cardia cancer (n, %).
| Codon | Type of mutation | Genotype | Case (%) | χ2 | P-value |
|---|
| 12 | 35G>A | GA | 4 (19) | 0.796 | 0.372 |
|
|
| AA | 3 (14.3) |
|
|
|
| 35G>T | GT | 3 (14.3) | 1.099 | 0.294 |
|
|
| TT | 2 (9.5) |
|
|
|
| 35G>C | GC | 3 (14.3) | 1.099 | 0.294 |
|
|
| CC | 2 (9.5) |
|
|
|
| 34G>A | GA | 2 (9.5) | 1.664 | 0.197 |
|
|
| AA | 1 (4.8) |
|
|
| 13 | 34G>A | GA | 1 (4.8) | 4.918 | 0.027 |
|
|
| AA | 0 (0) |
|
|
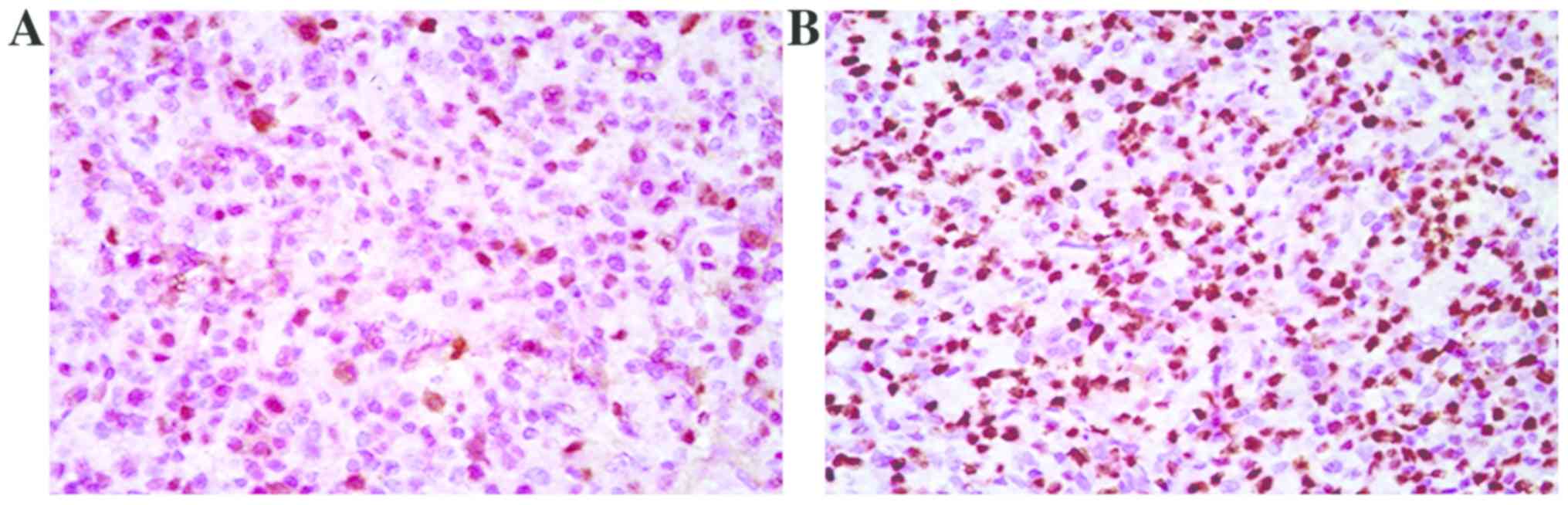
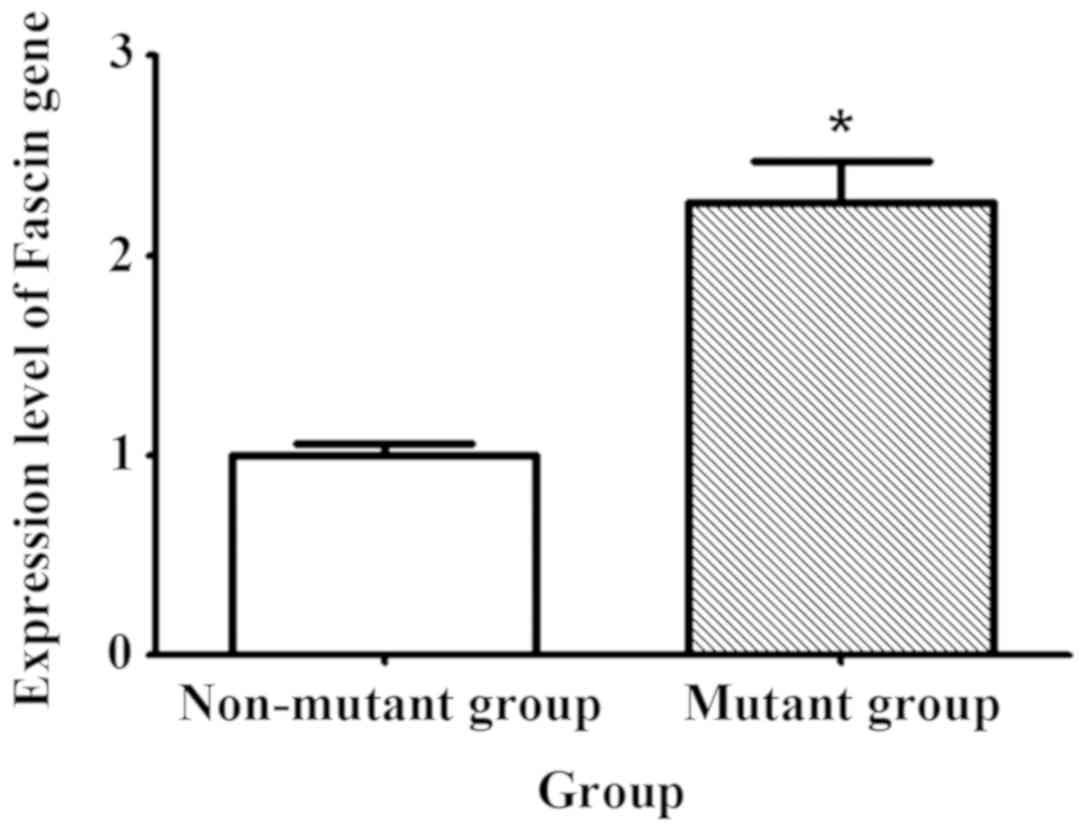
Comparison of fascin expression
between mutant and non-mutant groups
The expression level of fascin was compared between
the mutant and non-mutant groups at the molecular and protein
levels, respectively. Immunohistochemistry revealed that the number
of positive cells in the mutant group was increased compared with
that in the non-mutant group (p<0.05) (Fig. 2). qPCR results showed that the
expression level of fascin gene in the mutant group was 2.3 times
higher than that in the non-mutant group (Fig. 3).
Discussion
It has been reported that pathogenic factors for
cardia cancer are various, including diet, microbiota in the body
and external environmental stimuli. Studies on genetic inheritance
are rare, but with the development of sequencing and PCR
technologies, more and more attention is paid to the research on
the influence of genetic inheritance on cancers (10,11). K-ras
gene, as a member of ras gene, is now a hotspot gene in the
research on gastrointestinal tumors. Moreover, it has the greatest
impact on human cancers as a molecular switch, i.e., when it is
normal, it can control and regulate the path of cell growth, while
it leads to continuous grow and prevents self-destruction of cells
in case of abnormality (12). In
addition, K-ras is involved in intracellular signal transduction.
When K-ras gene is mutated, it is permanently activated and cannot
produce normal ras proteins, resulting in disordered intracellular
signal transduction and uncontrolled cell proliferation, thus
leading to carcinogenesis (13). At
present, there are some studies on K-ras gene in colorectal,
gastric and pancreatic cancer (14–16). A
study by Stewart and Crook (17)
found that K-ras gene mutations are frequently detected in female
patients with colorectal cancer and lymph node metastasis, which
are expected to be important indicators determining the prognosis
of colorectal cancer. Lee et al (18) pointed out in a study on gastric cancer
that the probability of K-ras mutations in distant metastasis group
is higher than that in the non-distant metastasis group. The
expression function of the gene can be affected by many factors
including the roles of non-coding regions and various regulatory
factors. Spontaneous SNPs of the gene change the structure, affect
the realization of translation function and indirectly influence
the health of the body, thus resulting in various diseases
(19). This study proved that the
probability of K-ras mutations in patients with cardia cancer was
23.3%. Most mutations occurred at codon 12, but there was no
significant difference in the mutation probability between
heterozygotes and homozygotes for four mutations at codon 12. The
mutation probability of heterozygotes at codon 13 was higher than
that of homozygotes at codon 13, but the number of cases was small.
Therefore, the sample size should be increased for further
confirmation.
Fascin is able to reduce the matrix resistance
between cells to promote cell migration, thus facilitating the
infiltration and metastasis of tumor cells. Fascin exists in three
forms in the human body, namely, fascin-1, −2 and −3. Among them,
fascin-1 is the dominant. A study suggested that fascin is often
lowly expressed when the body is in normal condition, but the
expression of fascin is increased in tumor cells (20). A study of Omran and Al Sheeha
(21) discovered that the expression
level of fascin is diverse in different tumors. Studies have
indicated that fascin expression in gastric cancer tissue is
significantly higher than that in normal gastric mucosa and is
related to lymph node and distant metastasis in gastric cancer. In
this study, immunohistochemistry revealed that the number of
positive cells in the mutant group was greater than that in the
non-mutant group, and the results of qPCR showed that the
expression level of fascin gene in the mutant group was 2.3 times
higher than that in the non-mutant group, indicating that the
expression of fascin is still high in cardia cancer cells and is
positively correlated with K-ras gene mutations.
In summary, the mutation probability of codon 12 is
high in K-ras gene in patients with cardia cancer, and the
expression of fascin is high in mutant patients and positively
related to the mutations in K-ras gene.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW wrote the manuscript. LW and HC helped with the
extraction of genomic DNA and qPCR. SH was responsible for
immunohistochemistry. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jining First People's Hospital (Jining, China) and informed
consents were signed by the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Y, Kang Y, Hong L and Yao H:
Hypoglycemia caused by co-secretion of insulin from lung tumor and
cardia cancer: First case report. Sao Paulo Med J. Nov
17–2017.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Guo W, Lv P, Liu S, Xu F, Guo Y, Shen S,
Liang J, Kuang G and Dong Z: Aberrant methylation-mediated
downregulation of long noncoding RNA C5orf66-AS1 promotes the
development of gastric cardia adenocarcinoma. Mol Carcinog. Mar
22–2018.(Epub ahead of print]). View
Article : Google Scholar
|
|
3
|
Barra WF, Moreira FC, Cruz Pereira AM,
Khayat AS, Calcagno DQ, Dos Santos Carneiro NP, Junior Mascarenhas
RW, Araújo Thomaz TM, Ishak G, Demachki S, et al: GEJ cancers:
Gastric or esophageal tumors? searching for the answer according to
molecular identity. Oncotarget. 8:104286–104294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Binh TT, Tuan VP, Dung HDQ, Tung PH, Tri
TD, Thuan NPM, Khien VV, Hoan PQ, Suzuki R, Uchida T, et al:
Advanced non-cardia gastric cancer and Helicobacter pylori
infection in Vietnam. Gut Pathog. 9:462017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roviello F, Polom K, D'Ignazio A, Pascale
V and Marrelli D: K-RAS mutation in gastric cancer and its link
with microsatellite instability status. Eur J Cancer. 92 Suppl
2:S62018. View Article : Google Scholar
|
|
6
|
Sekita-Hatakeyama Y, Nishikawa T, Takeuchi
M, Morita K, Takeda M, Hatakeyama K, Nakai T, Uchiyama T, Itami H,
Fujii T, et al: K-ras mutation analysis of residual liquid-based
cytology specimens from endoscopic ultrasound-guided fine needle
aspiration improves cell block diagnosis of pancreatic ductal
adenocarcinoma. PLoS One. 13:e01936922018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin YJ and Haigis KM: Brother's keeper:
Wild-type mutant K-ras dimers limit oncogenesis. Cell. 172:645–647.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimbo K, Yokoyama K, Ogawa M, Hirano M,
Ochi K, Kobayashi M, Yusa N, Shimizu E, Kawamata T, Yasui H, et al:
Autologous peripheral blood stem cell transplantation for
double-refractory myeloma with K-RAS and N-RAS mutations. Rinsho
Ketsueki. 58:2380–2385. 2017.(In Japanese). PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddiqui I, Erreni M, Kamal MA, Porta C,
Marchesi F, Pesce S, Pasqualini F, Schiarea S, Chiabrando C,
Mantovani A, et al: Differential role of Interleukin-1 and
Interleukin-6 in K-Ras-driven pancreatic carcinoma undergoing
mesenchymal transition. OncoImmunology. 7:e13884852017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Wang J, Chen F, Zhong Z and Qi L:
Detection of K-ras gene mutations in feces by magnetic nanoprobe in
patients with pancreatic cancer: A preliminary study. Exp Ther Med.
15:527–531. 2018.PubMed/NCBI
|
|
12
|
Zhou Y and Hancock JF: A novel
prenyl-polybasic domain code determines lipid-binding specificity
of the K-Ras membrane anchor. Small GTPases. 15:1–5. 2018.
View Article : Google Scholar
|
|
13
|
Son BK, Kim DH, Min KW, Kim EK and Kwon
MJ: Smad4/Fascin index is highly prognostic in patients with
diffuse type EBV-associated gastric cancer. Pathol Res Pract.
214:475–481. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koçer NE and Kayaselçuk F: Is availability
of anti-EGFR therapy for the colorectal adenocarcinomas showing
fascin expression limited? Target Oncol. 9:171–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang MT, Holderfield M, Galeas J,
Delrosario R, To MD, Balmain A and McCormick F: K-Ras promotes
tumorigenicity through suppression of non-canonical Wnt signaling.
Cell. 163:1237–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu W, Wang Z, Zhang W, Qian K, Li H, Kong
D, Li Y and Tang Y: Mutated K-ras activates CDK8 to stimulate the
epithelial-to-mesenchymal transition in pancreatic cancer in part
via the Wnt/β-catenin signaling pathway. Cancer Lett. 356:(2 Pt B).
613–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart CJ and Crook ML: Fascin expression
in undifferentiated and dedifferentiated endometrial carcinoma. Hum
Pathol. 46:1514–1520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee LY, Chen YJ, Lu YC, Liao CT, Chen IH,
Chang JT, Huang YC, Chen WH, Huang CC, Tsai CY, et al: Fascin is a
circulating tumor marker for head and neck cancer as determined by
a proteomic analysis of interstitial fluid from the tumor
microenvironment. Clin Chem Lab Med. 53:1631–1641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao W, Gao J, Wu J, Liu QH, Wang ZG, Li
HL and Xing LH: Expression of Fascin-1 on human lung cancer and
paracarcinoma tissue and its relation to clinicopathological
characteristics in patients with lung cancer. Onco Targets Ther.
8:2571–2576. 2015.PubMed/NCBI
|
|
20
|
Abrams JA, Gonsalves L and Neugut AI:
Diverging trends in the incidence of reflux-related and
Helicobacter pylori-related gastric cardia cancer. J Clin
Gastroenterol. 47:322–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Omran OM and Al Sheeha M: Cytoskeletal
focal adhesion proteins fascin-1 and paxillin are predictors of
malignant progression and poor prognosis in human breast cancer. J
Environ Pathol Toxicol Oncol. 34:201–212. 2015. View Article : Google Scholar : PubMed/NCBI
|