Introduction
Women with a history of breast cancer are estimated
to have an annual probability of developing metachronous,
contralateral breast cancer (MCBC) of 0.5–0.75%, and according to
the Surveillance, Epidemiology, and End Results data, the
probabilities of developing MCBC within 10 and 20 years are 6 and
12%, respectively (1). As advances in
drug therapy have resulted in breast cancer patients surviving
longer, the number of women at risk of developing MCBC has
increased (2), and therefore, more
attention must be given to this disease.
MCBC is known to confer a high risk of distant
recurrence and poor prognosis in cases with a short interval
between the initial and second cancers. One reason is that if the
second cancer is not a primary tumour, it may be a metastasis from
the initial tumour. Previous studies comparing gene mutations in
initial and second cancers reported that in 6–12% of cases, the two
tumours present with similar genetic mutations (3,4). Another
reason is that the influence of long-term adjuvant therapy for the
initial cancer may affect the second cancer subtype, leading to a
more aggressive tumour or acquired treatment resistance.
In the case of MCBC, the second contralateral breast
cancer, unlike the initial cancer, has a history of being exposed
to drug treatment and is thus affected by this history. There have
been reports that the risk of developing a second cancer is
markedly affected by whether postoperative adjuvant hormone therapy
(HT) is administered for the initial cancer (5). This suggests that the development of a
second cancer may be prevented by HT. However, an
oestrogen-receptor (ER)-positive second cancer sometimes develops
during or soon after completion of postoperative adjuvant HT for
the initial cancer. It can therefore be suggested that the growth
of this second cancer occurs while under the influence of the HT
being administered; thus, despite the ER-positive nature of the
second cancer, standard HT may not be effective.
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian
target of rapamycin (mTOR) pathway is a cell growth pathway
mediated by oestrogen signalling, and abnormal activation of this
pathway has been shown to be associated with resistance to HT in
ER-positive breast cancer (6). In
addition, the mitogen-activated protein kinase (MAPK) pathway is
involved in non-oestrogen-dependent cell growth, differentiation,
survival, and infiltration, and is an important pathway in relation
to resistance to HT (7,8).
The objective of the present study was to study
HT-resistance of second cancers that arose during or after
completion of adjuvant HT for the initial cancer, by evaluating the
relationship between development of ER-positive second cancers and
the activation of the PI3K/Akt/mTOR and MAPK pathways, using
immunohistochemistry. The treatment-free interval, which is the
time between completion of adjuvant HT for the initial cancer and
development of the second cancer, was also included in the
analysis.
Materials and methods
Tumour specimens
Eighty-four patients underwent surgery for MCBC as a
second cancer at the Kindai University Faculty of Medicine between
January 2000 and June 2017. MCBC was defined as contralateral
breast cancer diagnosed >6 months after surgery for the initial
cancer; the second cancer was not evaluated to determine whether it
was primary or metastatic in nature. Three patients were excluded
from the analysis since surgery for the initial cancer was
performed at a different hospital and the details and duration of
the postoperative adjuvant therapy were unknown, and two were
excluded owing to the detection of local or distant metastases
before development of the second cancer. Of the 79 remaining
patients, 58 had an ER-positive second cancer. Postoperative
adjuvant HT was administered for the initial cancer in 41 patients,
who formed the subject group in the present study (HT group). No
adjuvant HT was administered for the initial cancer in 17 patients,
who formed the control group (no HT group). The present study was
retrospective in nature. Initially, the study subjects were
included from 2006 to 2015. This research plan was approved by the
Kindai University Faculty of Medicines Ethics Committee on 26th May
2017. However, since the number of cases was lesser than we
expected, we revised the study period, and subjects were included
from January 2000 to June 2017. This revised study period was
approved by the Ethics Committee on 20th February 2018.
Immunohistochemistry
When preparing tissue specimens, in order to ensure
the stability of phosphorylated proteins in the surgical samples,
excised tissue samples were fixed for 24 to 48 h with 10% buffered
formalin immediately after collection, after which they were
embedded in paraffin and sectioned to prepare 4-µm-thick slices. As
an index of PI3K/Akt/mTOR pathway activation, immunohistochemistry
was carried out for phospho-S6 ribosomal protein (pS6) expression
(9,10); pS6 is a protein which is
phosphorylated by S6 kinase (S6K; a downstream molecule in the
PI3K/Akt/mTOR signalling pathway), and is an important factor in
regulating translation (11,12). In addition, immunohistochemistry was
carried out for pMAPK expression as an index of MAPK pathway
activation (9,13). The primary antibodies used were
D57.2.2E [phospho-S6 ribosomal protein (Ser235/236), rabbit
monoclonal antibody; Cell Signaling Technology, Inc., Danvers, MA,
USA], and D13.14.4E [phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204),
XP® rabbit monoclonal antibody; Cell Signaling
Technology, Inc.]. After deparaffinisation of the sections, antigen
retrieval was carried out by heating the slides to 98°C for 40 min
in citric acid (pH 6.0). Endogenous peroxidase activity was blocked
by treatment with 0.3% hydrogen peroxide, and non-specific binding
was blocked by treatment for 10 min with 5% normal goat serum. The
primary antibodies were each diluted 400-fold and added to the
slides, which were incubated overnight at 4°C. After removal of the
primary antibodies, Histofine Simple Stain MAX-PO (Multi; Nichirei
Corporation, Tokyo, Japan) was added to the slides drop-wise,
followed by incubation for 30 min. Finally, we used the Liquid
DAB+, two-component system (Dako Corporation, Santa Clara, CA, USA)
for detection. We added 1 drop of the DAB Chromogen per ml of
substrate buffer, and staining was carried out for 1 min.
Counterstaining was also carried out according to the
manufacturer's instructions.
ER and progesterone-receptor (PgR) expressions were
analysed according to the new American Society of Clinical Oncology
guidelines; specimens with at least 1% of stained cells were
defined as positive. Specimens were defined as positive for human
epidermal growth factor receptor 2 (HER2) expression if they were
scored as 3+ by immunohistochemistry or as 2+ by
immunohistochemistry and were positive by fluorescent in
situ hybridisation. Whether Ki67 was high or low was based on a
14% positivity cut-off value (14).
The subtype of Luminal B was assumed to have high Ki67 or a
negative PgR status. Analysis of pS6 and pMAPK staining used the
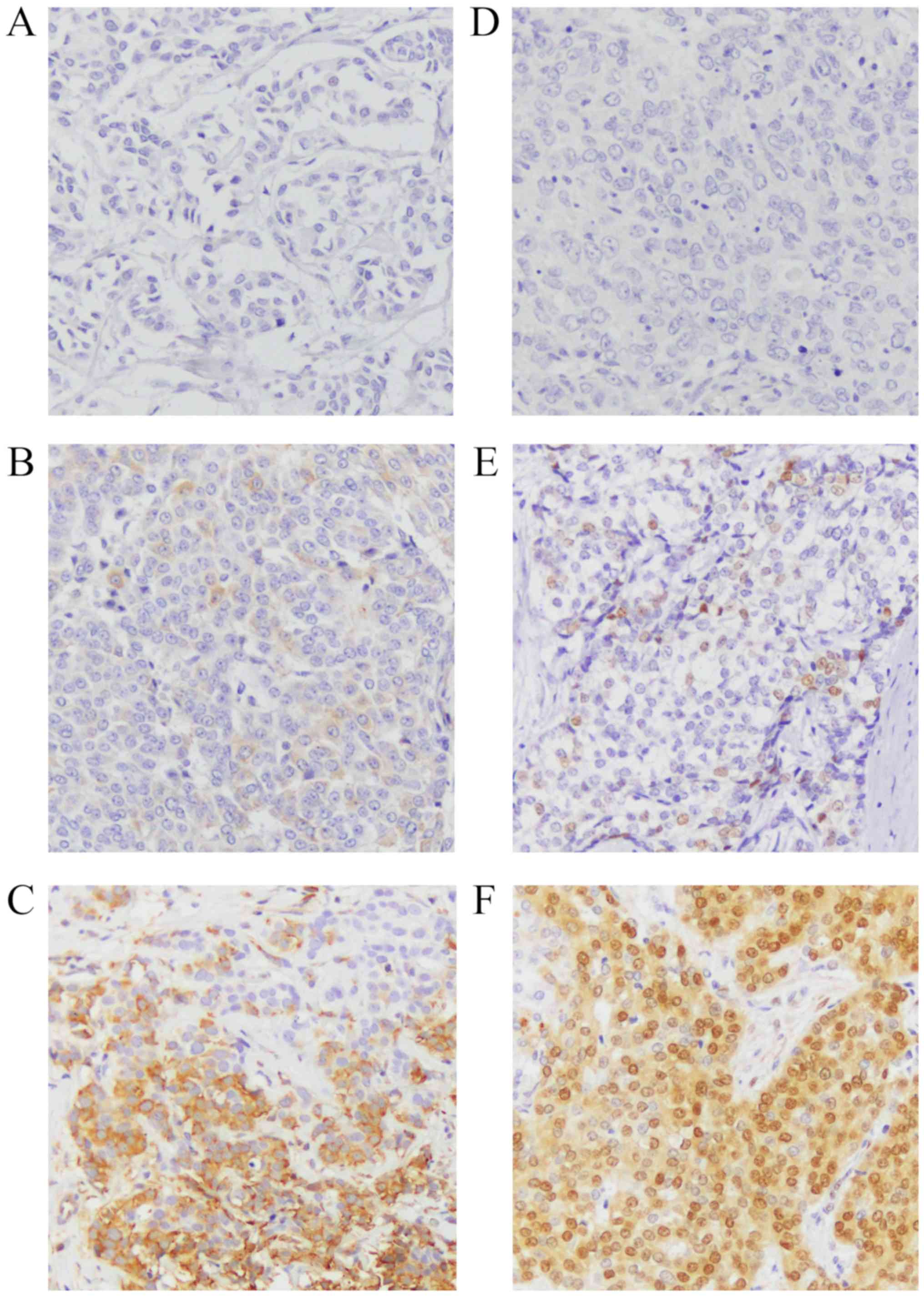
H-score, as reported previously (15–17).
Briefly, 10 high-powered fields (magnification, ×200) were observed
using light microscopy, and the mean proportion of each field
occupied by positive cells was calculated and accorded a numeric
value as follows: 0: 0–5%; 1: 6–25%; 2: 26–50%; 3: 51–75%; or 4:
76–100%. In addition, the staining intensity was accorded a numeric
value as follows: 0: negative; 1: weak; 2: moderate; or 3: strong.
The numeric value for the proportion of positive cells was then
multiplied by that for staining intensity to obtain the H-score;
the scores were classified as follows: 0–1: negative; 2–4:
positive; and 5–12: strongly positive (Fig. 1). The positive controls used for
staining intensity were IHC controls #8101 for pS6 and #8103 for
pMAPK (SignalSlide®; Cell Signaling Technology, Inc.).
Immunohistochemical analysis was performed independently by two
doctors. Cases of H-score 1–4, for which a consensus can be
difficult, were additionally analysed by a pathologist. The slides
were numerically coded to ensure that the person performing the
analysis was blinded to the patient's clinical background.
Statistical analysis
The relationship between administration of adjuvant
HT for the primary cancer (HT group vs. no HT group) and
clinicopathological background factors was tested using the
Fisher's exact test. The clinicopathological characteristics
included age at diagnosis of the initial cancer, history of
chemotherapy or trastuzumab therapy for the initial cancer,
interval between the initial cancer and onset of the second cancer,
and characteristics of the initial and second cancer such as stage,
status of ER, PgR, HER2, and Ki67 expression, and subtype. A
P<0.05 defined a statistically significant difference. The
correlation between treatment-free interval or initial-to-second
period and the immunohistochemistry results was determined using
the Spearman's rank correlation coefficient. The treatment-free
interval of patients who developed a second cancer during hormonal
therapy was defined as 0 months. The initial-to-second period of
the control group was defined as the period from surgery for the
initial cancer to onset of the second cancer. The software used in
the analysis was JMP® 13 (SAS Institute, Inc., Cary, NC,
USA).
Results
We compared the clinicopathological background of
initial cancer between patients in the HT group (n=41) and those in
the no HT group (n=17) (Table I).
There was no difference in age at onset, T-stage, and lymph node
status of the initial cancer. ER status was positive in all cases
in the HT group. Though ER status of six patients in the no HT
group was positive, they did not receive HT. Of the six patients
who did not receive HT, two patients reported adverse events
immediately after the administration of the first dose of HT, so
did not receive the full therapy. Four other patients presented
with microinvasion (≤5 mm), and opted out of drug treatment for
this reason. PgR positivity was significantly higher in the HT
group. There was no difference in HER2 status and Ki67 status
between the two groups. Cancer subtype data for both the groups are
shown in Table I. Immunostaining was
not performed in two cases of first cancer in HT and no HT groups
due to insufficient amount of tissue. Regarding HT for the initial
cancer, most patients were treated with tamoxifen (TAM); while 19
patients received treatment for 2 years, 16 patients received
treatment for 5–10 years. Most of the patients who received
treatment only for 2 years were treated according to the
conventional standard of treatment. One patient was switched from
treatment with TAM (1 year) to that with an aromatase inhibitor
(AI) (4 years). Five patients were treated with AI (5 years). There
was also no difference in the frequency of adjuvant chemotherapy or
trastuzumab treatment for the initial cancer between the two
groups. We also compared the clinicopathological background of
second cancers (Table II). There was
no difference in age at second cancer onset between the two groups.
Although all patients in the no HT group had T1 stage second
cancer, there was no significant difference in stage between the
two groups. In addition, there was no difference in the
pathological background (ER, PgR, HER2, Ki67 status) regarding the
subtype of the second cancer or the presence or absence of lymph
node metastasis. The median interval between the initial cancer and
the second cancer onset was 104 months (9–261 months) for the HT
group and 84 months (6–234 months) for the no HT group. In the HT
group, the median treatment-free interval was 48 months, and the
range was 0–241 months. There were seven patients diagnosed with a
second tumour during postoperative adjuvant HT for the initial
tumour.
 | Table I.Association of clinicopathological
background with initial cancer between patients in the HT group and
those in the no HT group. |
Table I.
Association of clinicopathological
background with initial cancer between patients in the HT group and
those in the no HT group.
| Clincopathological
criteria | HT group (n=41) | No HT group
(n=17) | P-value |
|---|
| Age at diagnosis,
years |
|
<50 | 25 | 8 | 0.390 |
| ≥50 | 16 | 9 |
|
| T-stage |
| T1 | 24 | 13 | 0.241 |
| T2-4 | 17 | 4 |
|
| Lymph node
status |
|
Absence | 34 | 15 | 1.000 |
|
Presence | 7 | 2 |
|
| ER status |
|
Positive | 41 | 6 | 0.001 |
|
Negative | 0 | 9 |
|
| N/A |
| 2 |
|
| PgR status |
|
Positive | 39 | 6 | 0.001 |
|
Negative | 2 | 9 |
|
| N/A |
| 2 |
|
| HER2 status |
|
Positive | 6 | 4 | 0.438 |
|
Negative | 33 | 11 |
|
| N/A | 2 | 2 |
|
| Ki67 status (%) |
|
<14 | 35 | 14 | 1.000 |
| ≥14 | 4 | 1 |
|
| N/A | 2 | 2 |
|
| Subtype |
| Luminal
A | 28 | 5 |
|
| Luminal
B | 5 | 0 |
|
| HER2
enriched | 6 | 4 |
|
| Basal
like | 0 | 6 |
|
| N/A | 2 | 2 |
|
| HT |
| TAM (2
years) | 19 | – | – |
| TAM (5–10
years) | 16 | – |
|
| Switch
from | 1 | – |
|
| TAM to
AI |
| AI (5
years) | 5 | – |
|
| Chemotherapy |
|
Yes | 21 | 7 | 0.570 |
| No | 20 | 10 |
|
| Trastuzumab |
|
Yes | 2 | 2 | 0.573 |
| No | 39 | 15 |
|
 | Table II.Association of clinicopathological
background with second cancer between patients in the HT group and
those in the no HT group. |
Table II.
Association of clinicopathological
background with second cancer between patients in the HT group and
those in the no HT group.
| Clincopathological
criteria | HT group
(n=41) | No HT group
(n=7) | P-value |
|---|
| Age at diagnosis,
years |
|
<50 | 12 | 7 | 0.540 |
|
≥50 | 29 | 10 |
|
| T-stage |
| T1 | 34 | 17 | 0.093 |
|
T2-4 | 7 | 0 |
|
| Lymph node |
|
Absence | 30 | 14 | 0.523 |
|
Presence | 11 | 3 |
|
| PgR status |
|
Positive | 36 | 17 | 0.307 |
|
Negative | 5 | 0 |
|
| HER2 status |
|
Positive | 6 | 3 | 1.000 |
|
Negative | 35 | 14 |
|
| Ki67 status |
|
<14% | 25 | 14 | 0.137 |
|
≥14% | 16 | 3 |
|
| Subtype |
| Luminal
A | 20 | 13 | – |
| Luminal
B | 15 | 1 |
|
| HER2
enriched | 6 | 3 |
|
We compared the immunostaining results of initial
and second cancers in the HT group (Table III). Although there was no
difference in the frequency of PgR and HER2 positivity, the
incidence of a Ki67 positive status was significantly higher in the
second cancers. As a result, the incidence of the luminal B subtype
was large in the second cancers. The pS6 positivity of the second
cancers increased slightly compared to that of the initial cancers,
though the difference was not significant. There was no difference
in MAPK positivity between the initial and second cancers as
well.
 | Table III.Association of immunostaining with
initial and second cancer in the HT group. |
Table III.
Association of immunostaining with
initial and second cancer in the HT group.
| Clincopathological
criteria | Initial cancer | Second cancer | P-value |
|---|
| PgR status |
|
Positive | 39 | 36 | 0.432 |
|
Negative | 2 | 5 |
|
| HER2 status |
|
Positive | 6 | 6 | 1.000 |
|
Negative | 35 | 35 |
|
| Ki67 status |
|
<14% | 35 | 25 | 0.004 |
|
≥14% | 4 | 16 |
|
|
N/A | 2 | 0 |
|
| Subtype |
| Luminal
A | 28 | 20 | – |
| Luminal
B | 5 | 15 |
|
| HER2
enriched | 6 | 6 |
|
|
N/A | 2 | 0 |
|
| pS6 status |
|
Positive | 6 | 9 | 0.570 |
|
Negative | 33 | 32 |
|
|
N/A | 2 |
|
|
| pMAPK status |
|
Positive | 11 | 10 | 0.801 |
|
Negative | 28 | 31 |
|
|
N/A | 2 |
|
|
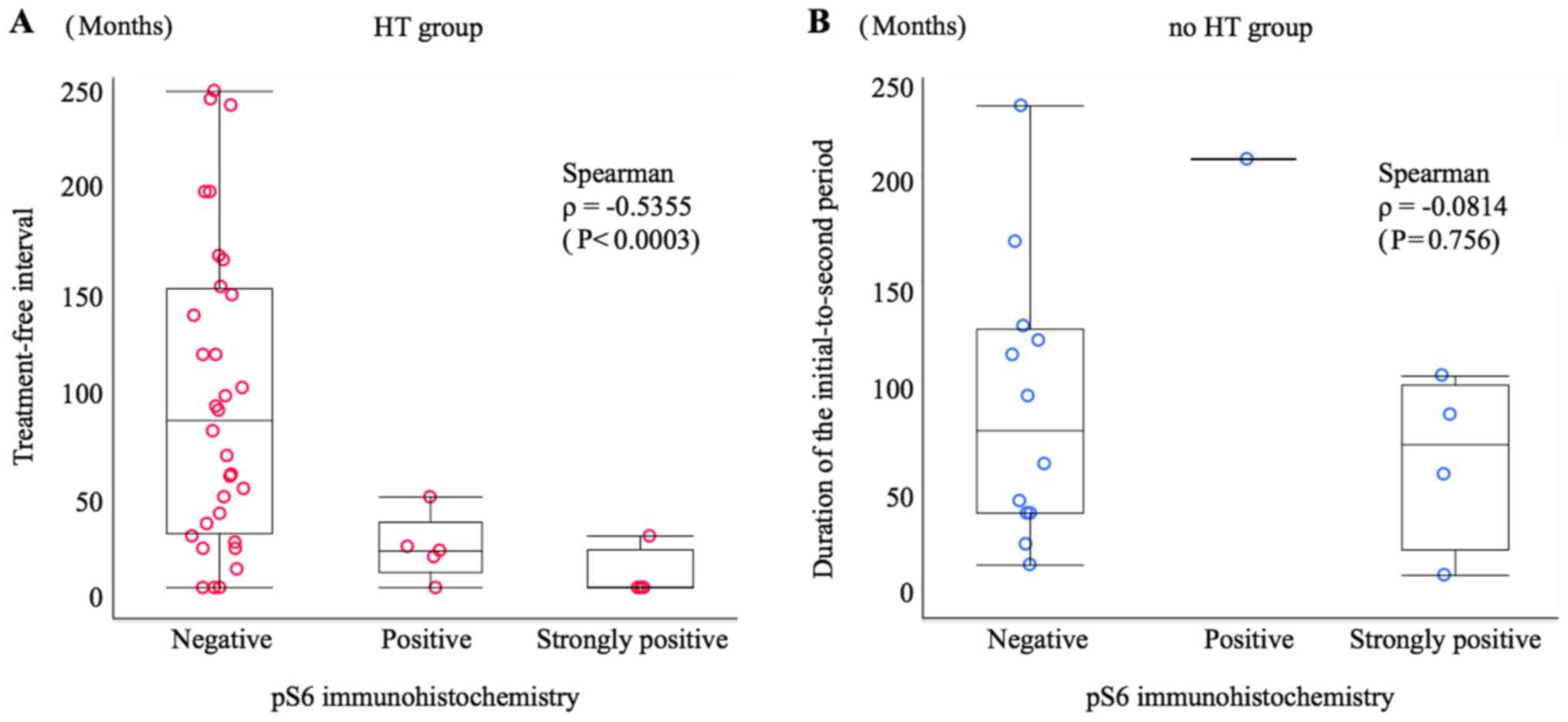
In order to ascertain the relationship between
treatment-free interval or initial-to-second period duration and
immunohistochemistry results, a scatter graph was prepared. The
Spearman's rank correlation coefficient (ρ) for pS6
immunohistochemistry and treatment-free interval in the HT group
was ρ=−0.5355 (P=0.0003), indicating a negative correlation, i.e. a
tendency towards a high to strong frequency of pS6 positivity with
a short treatment-free interval. However, this trend was not
observed in the relationship between pS6 immunohistochemistry and
initial-to-second period duration in the no HT group (ρ=−0.0814;
P=0.756) (Fig. 2). There was no
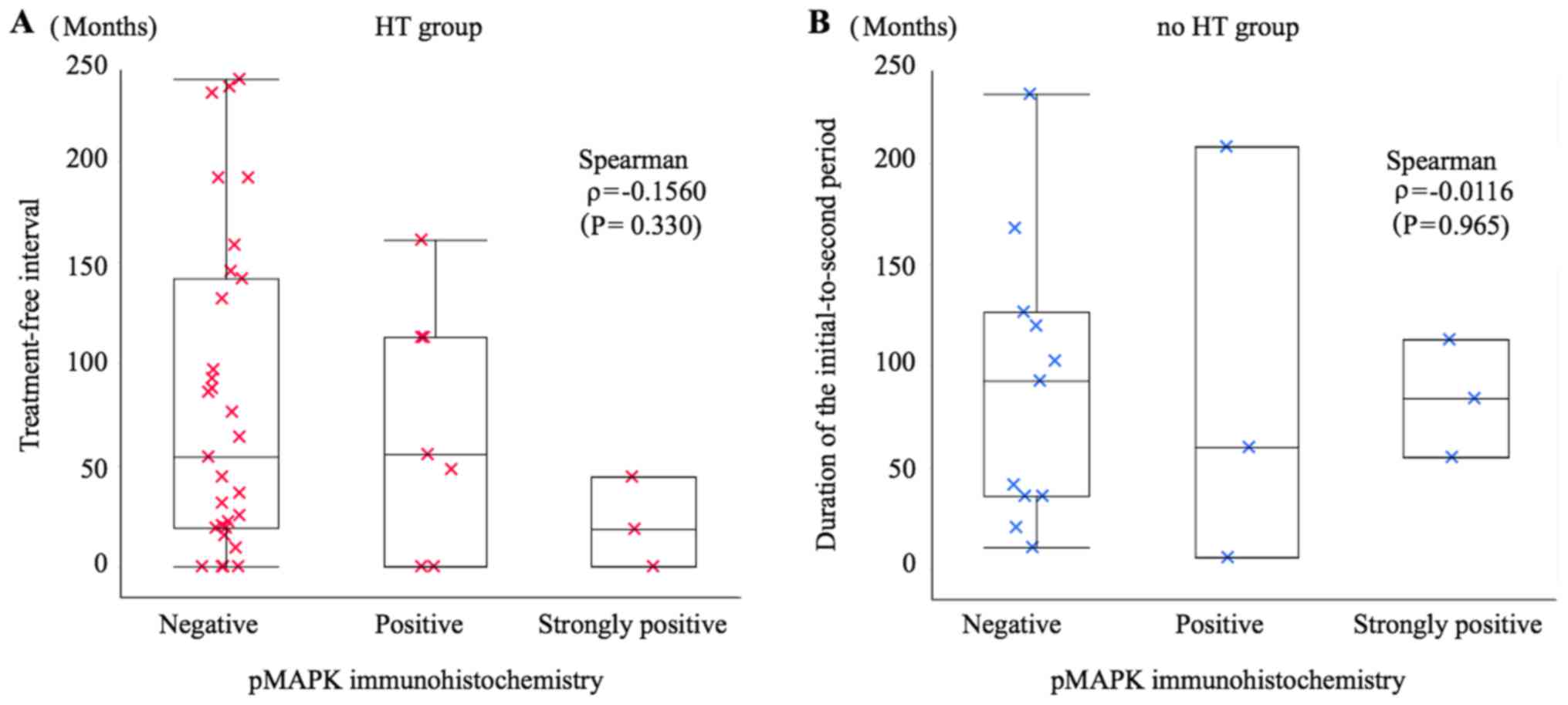
correlation between pMAPK signalling and the treatment-free
interval in the HT group (ρ=−0.1560; P=0.330) or the
initial-to-second period duration in the no HT group (ρ=−0.0116;
P=0.965) (Fig. 3).
Discussion
The results of the present study show that in
ER-positive MCBCs in patients with a history of HT, the frequency
of pS6 positivity increases with decreasing treatment-free
interval. This suggests that, despite the second cancer being
ER-positive, the PI3K/Akt/mTOR pathway is activated in these
tumours owing to the effect of HT for the initial cancer, resulting
in resistance to HT.
According to a previous study, pS6 expression was
elevated in a murine model of ER- and PgR-positive breast cancer
treated with preoperative adjuvant HT, and the expression level was
linked to the efficacy of mTOR inhibition (18). In addition, there have recently been
reports that the effects of HT include development of resistance to
the therapy. According to studies of metastatic foci of breast
cancer, patients who are administered adjuvant HT have metastatic
foci that are significantly more positive for Akt, mTOR, and
phosphorylated S6K1 than do patients not treated with HT,
suggesting activation of the PI3K/Akt/mTOR signalling pathway
through oestrogen blockade (19). It
has also been reported that S6K-positive patients, in comparison to
S6K-negative patients, did not show reduced risk of cancer
recurrence, even when administered tamoxifen as adjuvant therapy
(20). In our study, there was no
increase in the frequency of pS6 positivity due to endocrine
treatment history; however, our results showed a significant
correlation between the time to diagnosis of a second cancer after
completion of HT and the frequency and intensity of pS6 expression.
We consider that this correlation may potentially affect the
success of treatment regimens and should be used to guide treatment
decisions for second cancers with short treatment-free
intervals.
No relationship was found between MAPK pathway
activation and time to diagnosis of a second cancer after HT. The
results of an in vitro study suggested that long-term
survival of a cancer cell line cultured under conditions of
oestrogen depletion is more markedly dependent on the PI3K/Akt/mTOR
pathway than it does on the MAPK pathway (21,22), which
is in agreement with our findings.
One limitation to the present study is that since
MCBC is a relatively uncommon disease, our cohort was small; thus,
we could not make definitive conclusions based on our study
results. The trends seen in our findings are significant and
interesting, but the correlation coefficients were not strong.
Moreover, in this case, since the follow-up period from the onset
of the second cancer was short and the number of relapses and
deaths was small, we could not determine the prognosis of the cases
that were positive for pS6. In addition, the type and duration of
endocrine treatment for the first cancer could affect pS6
expression of the second cancer. However, in our case, there were
several treatment periods and types, so we could not analyse
that.
In conclusion, we found that in patients with
ER-positive breast cancer treated with HT, rapid development of an
ER-positive second cancer correlated with a higher frequency of pS6
positivity. With this type of second cancer, the likelihood of
activating the PI3K/Akt/mTOR pathway is high, and selection of
adjuvant therapy regimens and follow-up should be performed
accordingly.
Acknowledgements
The authors would like to thank Professor Takao Sato
((Department of Pathology, Kindai University Faculty of Medicine)
for their help in analysing immunostaining spcimens. The
preliminary result of the present study was presented at The 11th
European Breast Cancer Conference in March 2018.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK designed the study. HK analysed the data and
wrote the paper. YK and HK analysed the immunostained specimens.
CH, YT, MH, WS, TA, YH, HI and TH collected the data, critically
evaluated the data analysis and analysis of immunostained
specimens, and contributed to the construction of important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Research
Ethics Committee of the Kindai University (Osakasayama, Japan). We
explained to all patients who underwent an operation that we may
use the resected tissues for research purposes. In the present
study, all patients agreed to this. We also explained the present
study to the outpatients and obtained their consent for
participation. We provided a means to opt out to patients who
discontinued their visits and to the families of deceased patients.
An explanatory document (research document) on the research
approved by the Ethics Committee was published on the website
(http://www.kindai-geka.jp/). Questions
and consultation concerning the present study (subjects such as the
implementation plan, analysis results, etc.) from patients and
families were accepted at any time on the website, and the
opportunity to refuse participation was provided.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MCBC
|
metachronous, contralateral breast
cancer
|
|
ER
|
oestrogen receptor
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
MAPK
|
mitogen-activated protein kinase
|
|
pS6
|
phospho-S6 ribosomal protein
|
|
HT
|
hormone therapy
|
|
S6K
|
S6 kinase
|
|
PgR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
TAM
|
tamoxifen
|
|
AI
|
aromatase inhibitor
|
References
|
1
|
Gao X, Fisher SG and Emami B: Risk of
second initial cancer in the contralateral breast in women treated
for early-stage breast cancer: A population-based study. Int J
Radiat Oncol Biol Phys. 56:1038–1045. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jobsen JJ, van der Palen J, Ong F,
Riemersma S and Struikmans H: Bilateral breast cancer, synchronous
and metachronous; differences and outcome. Breast Cancer Res Treat.
153:277–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klevebring D, Lindberg J, Rockberg J,
Hilliges C, Hall P, Sandberg M and Czene K: Exome sequencing of
contralateral breast cancer identifies metastatic disease. Breast
Cancer Res Treat. 151:319–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Begg CB, Ostrovnaya I, Geyer FC,
Papanastasiou AD, Ng CKY, Sakr RA, Bernstein JL, Burke KA, King TA,
Piscuoglio S, et al: Contralateral breast cancers: Independent
cancers or metastases? Int J Cancer. 142:347–356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaapveld M, Visser O, Louwman WJ,
Willemse PH, de Vries EG, van der Graaf WT, Otter R, Coebergh JW
and van Leeuwen FE: The impact of adjuvant therapy on contralateral
breast cancer risk and the prognostic significance of contralateral
breast cancer: A population based study in the Netherlands. Breast
Cancer Res Treat. 110:189–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller TW, Balko JM and Arteaga CL:
Phosphatidylinositol 3-kinase and antiestrogen resistance in breast
cancer. J Clin Oncol. 29:4452–4461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
9
|
Yanai A, Inoue N, Yagi T, Nishimukai A,
Miyagawa Y, Murase K, Imamura M, Enomoto Y, Takatsuka Y, Watanabe
T, et al: Activation of mTOR/S6K but not MAPK pathways might be
associated with High Ki-67, ER+, and HER2 breast cancer.
Clin Breast Cancer. 15:197–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fingar DC, Richardson CJ, Tee AR, Cheatham
L, Tsou C and Blenis J: mTOR controls cell cycle progression
through its cell growth effectors S6K1 and 4E-BP1/eukaryotic
translation initiation factor 4E. Mol Cell Biol. 24:200–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mamane Y, Petroulakis E, LeBacquer O and
Sonenberg N: mTOR, translation initiation and cancer. Oncogene.
25:6416–6422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bose S, Chandran S, Mirocha JM and Bose N:
The Akt pathway in human breast cancer: A tissue-array-based
analysis. Mod Pathol. 19:238–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song CH, Park SY, Eom KY, Kim JH, Kim SW,
Kim JS and Kim IA: Potential prognostic value of heat-shock protein
90 in the presence of phosphatidylinositol-3-kinase overexpression
or loss of PTEN, in invasive breast cancers. Breast Cancer Res.
12:R202010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brewster AM and Parker PA: Current
knowledge on contralateral prophylactic mastectomy among women with
sporadic breast cancer. Oncologist. 16:935–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Yun R, Yu X, Hu H, Huang G, Tan B
and Chen T: Overexpression of Notch3 and pS6 is associated with
poor prognosis in human ovarian epithelial cancer. Mediators
Inflamm. 2016:59534982016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polo ML, Riggio M, May M, Rodríguez MJ,
Perrone MC, Stallings-Mann M, Kaen D, Frost F, Goetz M, Boughey J,
et al: Activation of PI3K/Akt/mTOR signaling in the tumor stroma
drives endocrine therapy-dependent breast tumor regression.
Oncotarget. 6:22081–22097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beelen K, Hoefnagel LD, Opdam M, Wesseling
J, Sanders J, Vincent AD, van Diest PJ and Linn SC: PI3K/AKT/mTOR
pathway activation in initial and corresponding metastatic breast
tumors after adjuvant endocrine therapy. Int J Cancer.
135:1257–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim EK, Kim HA, Koh JS, Kim MS, Kim KI,
Lee JI, Moon NM, Ko E and Noh WC: Phosphorylated S6K1 is a possible
marker for endocrine therapy resistance in hormone
receptor-positive breast cancer. Breast Cancer Res Treat.
126:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ray S and Darbre PD: Crosstalk with
insulin and dependence on PI3K/Akt/mTOR rather than MAPK pathways
in upregulation of basal growth following long-term oestrogen
deprivation in three human breast cancer cell lines. Horm Mol Biol
Clin Investig. 5:53–65. 2011.PubMed/NCBI
|
|
22
|
Martin LA, Farmer I, Johnston SR, Ali S,
Marshall C and Dowsett M: Enhanced estrogen receptor (ER) alpha,
ERBB2, and MAPK signal transduction pathways operate during the
adaptation of MCF-7 cells to long term estrogen deprivation. J Biol
Chem. 278:30458–30468. 2003. View Article : Google Scholar : PubMed/NCBI
|