Introduction
Renal cancer is one of the most common clinical
urological tumors, accounting for 4% of adult malignancies and
80–90% of adult renal malignant diseases. In China, renal cancer
affects 453,000 people and causes 396,000 deaths every year
(1). Incidence of renal cancer is
higher in developed countries than in developing countries, and is
higher in males than in females (2).
Main factors leading to renal cancer include tobacco consumption,
alcohol abuse and obesity (3). Renal
cancer at early stages has no obvious symptoms, and patients
showing hematuria, pain, and mass are usually in advanced stages
with distant tumor metastasis (4). At
present, surgical treatment is still the main treatment for renal
cancer. However, recurrence and metastasis still occurs in 32%
patients with renal cancer after operation (5). Survival period of patients in advanced
stages is 6–12 months, and the 5-year survival rate is <10%.
Traditional treatment methods such as radiotherapy, chemotherapy,
and interferon are effective for only 10–30% patients (6).
In recent years, a large number of clinically open
phase III trials have demonstrated that targeted drugs are superior
to radiochemotherapy and interferon in the treatment of advanced
renal cancer, and have good tolerance and minimal adverse reactions
(7). Cancer treatment has entered the
age of targeted therapy. As a kinase inhibitor, sorafenib inhibits
many kinds of extracellular and intracellular kinases and has dual
antitumor and anti-angiogenic effects (8). It can inhibit MRK and RAF signaling
pathways as well as VECFR, PDGFR and tumor neovascularization, and
has become the first-line targeted drug for the treatment of
advanced renal cancer (9).
In this study, clinical data of patients with
advanced renal cancer treated with sorafenib in Shandong Provincial
Hospital Affiliated to Shandong University (Jinan, China) were
analyzed, and treatment efficacy, adverse events and prognosis were
analyzed as well. Our study provided references for the treatment
of advanced renal cancer.
Patients and methods
Clinical data
A total of 74 patients (41 males and 33 females,
median age 56.5 years) with advanced renal cancer treated with
sorafenib + interferon from January 2010 to August 2013 were
included as the observation group. Another 53 renal cancer patients
(29 males and 24 females, median age 58.2 years) treated with
interferon alone were included as the control group. Clinical data
of the patients were retrospectively analyzed.
Inclusion and exclusion criteria
Inclusion criteria: patients with AJCC stage VI
renal cancer; patients with measurable tumor lesions ≥2.0 cm in
diameter; patients without serious viral and bacterial infections;
patients received no systemic renal cancer treatment. Exclusion
criteria: patients with other tumors; patients with a history of
severe allergies; patients with major organ dysfunction; patients
who had received organ transplantation; patients with autoimmune
system disorders; patients with severe mental illness. The study
was approved by the Ethics Committee of Shandong Provincial
Hospital Affiliated to Shandong University. All the patients or
their families signed an informed consent.
Treatment
Sorafenib was purchased from Bayer AG, [Leverkusen,
Germany (HQ)]. All patients were treated with sorafenib in fasting
state with a dose of 400 mg, twice a day. Besides that, patients in
the interferon group were treated with another 300 MU every other
day through subcutaneous injection, 4 weeks for 1 treatment cycle.
Patients were not allowed to eat high-fat foods within 3 h after
taking the drug. If serious adverse reactions occurred during
medication, the dose was reduced. The normal dose was reused after
recovery. Treatment efficacy, no disease progression time and
adverse reactions were recorded. Blood pressure was measured every
other day. Related imaging examinations, blood routine tests,
electrocardiogram, liver and kidney function tests as well as
adverse reactions were evaluated every 4 weeks to evaluate the
safety of medication.
Efficacy evaluation and adverse
reactions
Efficacy evaluation was performed according to
Revised Evaluation Standards for Efficacy of Solid Tumors (10). Patients were divided into complete
remission (CR), partial remission (PR), stable disease (SD),
progression of disease (PD), objective response rate (RR) was CR +
PR and disease control rate (DCR) was CR + PR + SD. Adverse
reactions were evaluated according to the National Cancer Institute
(NCI-CTC) grading standard (version 4.03) (11).
Follow-up
A prospective follow-up was performed mainly through
telephone and out-patient visit every 3 months for 5 years. The
cause and time of death were recorded.
Statistical analysis
The data of this study were analyzed using SPSS 17.0
(Beijing Xinmeijiahong Technology Co., Ltd. Beijing, China)
software. Measurement data was expressed as mean ± standard
deviation and comparisons between the two groups were performed by
t-test. Enumeration data were expressed as (%) and compared using
χ2 test. Survival analysis was performed using the
Kaplan-Meier method and log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of general data
No significant differences in sex, age, ECOC score,
tumor size, and TNM stages were found between the two groups
(P>0.05) (Table I).
 | Table I.Comparison of general data (n, %). |
Table I.
Comparison of general data (n, %).
| Indexes | Observation
(n=74) | Control (n=53) | χ2 | P-value |
|---|
| Sex |
|
| 0.006 | 0.956 |
| Male | 41(55.41) | 29 (54.72) |
|
|
|
Female | 33 (44.59) | 24 (45.28) |
|
|
| Age |
|
| 0.164 | 0.592 |
| ≥55 | 38 (51.35) | 30 (56.60) |
|
|
|
<55 | 36 (48.65) | 23 (43.40) |
|
|
| ECOC score |
|
| 0.042 | 0.855 |
| 0 | 46 (62.16) | 32 (60.38) |
|
|
| 1 | 28 (37.84) | 21 (39.62) |
|
|
| Tumor size |
|
| 0.193 | 0.586 |
| ≥5
cm | 42 (56.76) | 33 (62.26) |
|
|
| <5
cm | 32 (43.24) | 20 (37.74) |
|
|
| TNM stage |
| T
stage |
|
T1 | 9 (12.16) | 5 (9.43) |
|
|
|
T2 | 13 (17.57) | 7 (13.21) |
|
|
|
T3 | 17 (22.97) | 13 (24.53) |
|
|
|
T4 | 35 (47.30) | 28 (52.83) |
|
|
| N
stage |
|
| 0.014 | 0.528 |
|
N0 | 23 (31.08) | 17 (32.08) |
|
|
|
N1 | 51 (68.92) | 36 (67.92) |
|
|
| M
stage |
|
| 0.228 | 0.561 |
|
M0 | 21 (28.38) | 18 (33.96) |
|
|
|
M1 | 53 (71.62) | 35 (66.04) |
|
|
Evaluation of treatment efficacy
The observation group included 4 cases of CR
(5.41%), 16 cases of PR (21.62%), 42 cases of SD (56.76%), 12 cases
of PD (16.22%), and 62 cases of DCR (83.78%). In the control group,
there were 2 cases of CR (3.77%), 11 cases of PR (20.75%), 20 cases
of SD (37.74%), 12 cases of PD (37.74%), and 33 cases of DCR
(62.26%). DCR in the observation group was significantly higher
than that in the control group (P<0.05) (Table II).
 | Table II.Evaluation of treatment efficacy (n,
%). |
Table II.
Evaluation of treatment efficacy (n,
%).
| Items | n | CR | PR | SD | PD | RR | DCR |
|---|
| Observation | 74 | 4 (5.41) | 16 (21.62) | 42 (56.76) | 12 (16.22) | 20 (27.03) | 62 (83.78) |
| Control | 53 | 2 (3.77) | 11 (20.75) | 20 (37.74) | 20 (37.74) | 13 (24.53) | 33 (62.26) |
| χ2 |
| 0.183 | 0.014 | 3.743 | 6.489 | 0.022 | 6.489 |
| P-value |
| 0.508 | 0.543 | 0.047 | 0.007 | 0.526 | 0.007 |
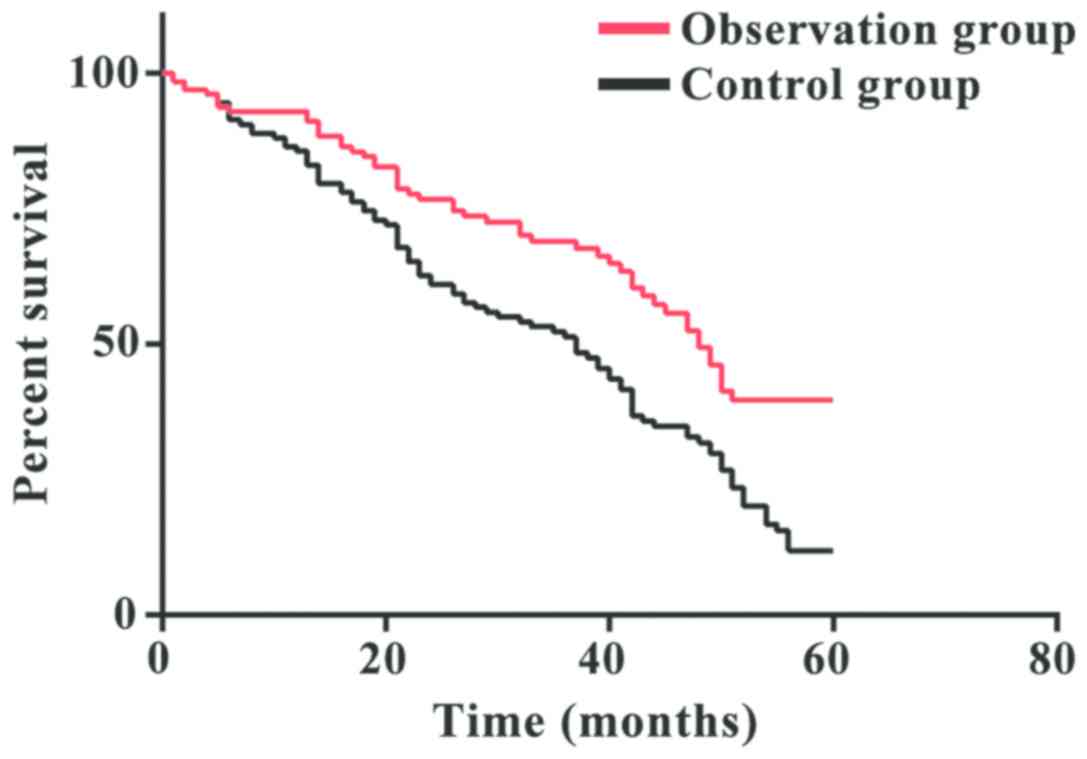
Survival analysis
In the observation group, Kaplan-Meier survival
analysis showed a median overall survival (OS) of 15.3 months
(range, 9–60 months), and a median progression-free survival (PFS)
of 8.2 months (range, 2–36 months). The 1-year survival rate was
87.84%, the 3-year survival rate was 68.92%, and the 5-year
survival rate was 36.49%. In the control group, the median OS time
was 12.5 months (range, 8–60 months), and the median PFS time was
9.3 months (range, 2–40 months). The 1-year survival rate was
73.56%, the 3-year survival rate was 47.17%, and the 5-year
survival rate was 18.87%. The 5-year survival rate in the
observation group was significantly higher than that in the control
group (P<0.05) (Fig. 1).
Adverse reactions
Adverse reactions in the two groups mainly included
hand-foot skin reaction, fever, diarrhea, fatigue, rash, loss of
appetite, hypertension, hair loss, liver function abnormality, and
there was no statistical significance in incidence of adverse
reactions between the two groups (P>0.05) (Table III).
 | Table III.Adverse effects of sorafenib (%). |
Table III.
Adverse effects of sorafenib (%).
| Adverse
reactions | Observation
(n=74) | Control (n=53) | χ2 | P-value |
|---|
| Hand-foot skin
reaction | 18 (24.32) | 16 (30.19) | 0.284 | 0.543 |
| Fever | 6 (8.11) | 8 (15.09) | 0.907 | 0.257 |
| Diarrhea | 15 (20.27) | 13 (24.53) | 0.125 | 0.665 |
| Fatigue | 11 (14.86) | 10 (18.87) | 0.127 | 0.631 |
| Rash | 3 (4.05) | 5 (9.43) | 0.740 | 0.277 |
| Loss of
appetite | 10 (13.51) | 8 (15.09) | 0.063 | 0.802 |
| Hypertension | 10 (13.51) | 13 (24.53) | 1.838 | 0.160 |
| Hair loss | 4 (5.41) | 2 (3.77) | 0.183 | 0.508 |
| Abnormal liver
function | 5 (6.76) | 5 (9.43) | 0.048 | 0.741 |
Discussion
Primary renal cancer is one of the most common
malignancies in clinical practice. Since most of the patients are
diagnosed at advanced stages, treatment outcome and prognosis are
usually poor (12). Efficacy of
immunotherapy, radiotherapy and chemotherapy in the treatment of
renal cancer is low. In recent years, a variety of targeted drugs
have also been successfully used in the treatment of renal cancer,
and objective effectiveness, PFS and OS have improved significantly
(13). Sorafenib is an oral small
molecule multikinase inhibitor that inhibits the phosphorylation of
RAF/MEK/ERK by inhibiting the activity of c-RAF and b-RAF kinases
in tumor cells to inhibit the proliferation and growth of tumor
cells (14). Sorafenib is the
earliest targeted agent in the treatment of stage IV renal cancer.
Multiple clinical studies have confirmed the value of sorafenib in
the treatment of advanced renal cancer (15,16).
Results of this study showed that the control group
had an objective RR of 24.53%, a DCR of 62.26%, a median OS of 15.3
months (range, 9–60 months), and a median PFS of 8.2 months (range,
2–36 months), while the 1-year survival rate was 73.56%, the 3-year
survival rate was 47.17%, and the 5-year survival rate was 18.87%.
The observation group had an objective RR of 27.03%, a DCR of
83.78%, a median OS of 12.5 months (range, 8–60 months), and a
median PFS of 9.3 months (range, 2–40 months), while the 1-year
survival rate was 87.84%, the 3-year survival rate was 68.92%, and
the 5-year survival rate was 36.49%. DCR in the observation group
was significantly higher than that in the control group
(P<0.05). The 5-year survival rate in the observation group was
significantly higher than that in the control group (P<0.05).
Compared with the control group, targeted therapy can reduce the
drug resistance of sorafenib and prolong the survival of patients.
Targeting therapy with sorafenib can inhibit tumor growth-related
signaling pathways. After cytotoxic sorafenib act on tumor cells,
tumor cell apoptosis will be induced and tumor size will be reduced
(17). On the other hand, SD reflects
cancer cell growth inhibition and has important value in evaluation
of targeted therapy (18). Consistent
with related reports by Yang et al (19), it was pointed out that sorafenib can
directly induce tumor cell apoptosis, indicating that sorafenib can
delay SD and prolong the OS of patients.
Consistent with the findings reported by Galluzzi
et al (20), it was confirmed
that targeted treatment with sorafenib can effectively prolong the
survival of patients with renal cancer. Adverse reactions were
observed in both groups and no significant differences in incidence
of adverse reactions were found between the two groups (P>0.05).
Most adverse reactions are controlled and tolerated through drug
reduction, drug suspension or symptomatic treatment. Adverse
reactions of Nexavar mainly include hand-foot skin reactions, skin
rashes, hypertension, and vomiting, which are basically the same as
reported by previous studies (21).
Sorafenib targeted therapy can be used to treat
renal cancer that is not suitable for surgery or have distant
metastasis. Sorafenib can effectively prolong a patient's survival
and efficacy is stable. This is a retrospective study with a small
sample size, and regional differences cannot be avoided. Our future
studies will attempt to solve these problems.
In conclusion, treatment with sorafenib achieved
longer OS and PFS in patients with advanced renal cancer. DCR of
sorafenib is high and adverse reactions can be controlled and
tolerated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ drafted the manuscript. JQ and DL were mainly
devoted to collecting and interpreting the general data. ZY, WJ and
QZ helped with efficacy evaluation and adverse reactions. NL, WD
and KD were responsible for follow-up. All authors read and
approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Provincial Hospital Affiliated to Shandong University
(Jinan, China). Signed informed consents were obtained from the
patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends - An update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goday A, Barneto I, García-Almeida JM,
Blasco A, Lecube A, Grávalos C, Martínez de Icaya P, de las Peñas
R, Monereo S, Vázquez L, et al: Obesity as a risk factor in cancer:
A national consensus of the Spanish Society for the Study of
Obesity and the Spanish Society of Medical Oncology. Clin Transl
Oncol. 17:763–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eble JN and Delahunt B: Emerging entities
in renal cell neoplasia: Thyroid-like follicular renal cell
carcinoma and multifocal oncocytoma-like tumours associated with
oncocytosis. Pathology. 50:24–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santoni M, Conti A, Partelli S, Porta C,
Sternberg CN, Procopio G, Bracarda S, Basso U, De Giorgi U, Derosa
L, et al: Surgical resection does not improve survival in patients
with renal metastases to the pancreas in the era of tyrosine kinase
inhibitors. Ann Surg Oncol. 22:2094–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choueiri TK, Halabi S, Sanford BL, Hahn O,
Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ,
et al: Cabozantinib versus sunitinib as initial targeted therapy
for patients with metastatic renal cell carcinoma of poor or
intermediate risk: The alliance A031203 CABOSUN trial. J Clin
Oncol. 35:591–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiao Y, Xin BT, Zhang Y, Wu J, Lu X, Zheng
Y, Tang W and Zhou X: Design, synthesis and evaluation of novel
2-(1H-imidazol-2-yl) pyridine Sorafenib derivatives as potential
BRAF inhibitors and anti-tumor agents. Eur J Med Chem. 90:170–183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai B, Jiang X, He C, Zhao D, Ma L, Xu L,
Jiang H and Sun X: Arsenic trioxide potentiates the anti-cancer
activities of sorafenib against hepatocellular carcinoma by
inhibiting Akt activation. Tumour Biol. 36:2323–2334. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flaherty KT, Manola JB, Pins M, McDermott
DF, Atkins MB, Dutcher JJ, George DJ, Margolin KA and DiPaola RS:
BEST: A randomized phase II study of vascular endothelial growth
factor, RAF kinase, and mammalian target of rapamycin combination
targeted therapy with bevacizumab, sorafenib, and temsirolimus in
advanced renal cell carcinoma - A trial of the ECOG-ACRIN cancer
research group (E2804). J Clin Oncol. 33:2384–2391. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shahabi V, Postow MA, Tuck D and Wolchok
JD: Immune-priming of the tumor microenvironment by radiotherapy:
Rationale for combination with immunotherapy to improve anticancer
efficacy. Am J Clin Oncol. 38:90–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HH, Feng WC, Lu LC, Shao YY, Hsu CH
and Cheng AL: Inhibition of the Wnt/β-catenin signaling pathway
improves the anti-tumor effects of sorafenib against hepatocellular
carcinoma. Cancer Lett. 381:58–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raman R and Vaena D: Immunotherapy in
metastatic renal cell carcinoma: A comprehensive review. BioMed Res
Int. 2015:3673542015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: MiR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma X, Shen D, Li H, Zhang Y, Lv X, Huang
Q, Gao Y, Li X, Gu L, Xiu S, et al: MicroRNA-185 inhibits cell
proliferation and induces cell apoptosis by targeting VEGFA
directly in von Hippel-Lindau-inactivated clear cell renal cell
carcinoma. Urol Oncol. 33:169.e1–169.e11. 2015. View Article : Google Scholar
|
|
17
|
Bil J, Zapala L, Nowis D, Jakobisiak M and
Golab J: Statins potentiate cytostatic/cytotoxic activity of
sorafenib but not sunitinib against tumor cell lines in vitro.
Cancer Lett. 288:57–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi Y, Mai M, Taguchi T, Urushizaki
I and Nishioka K: Prolonged stable disease effects survival in
patients with solid gastric tumor: Analysis of phase II studies of
doxifluridine. Int J Oncol. 17:285–289. 2000.PubMed/NCBI
|
|
19
|
Yang GW, Jiang JS and Lu WQ: Ferulic acid
exerts anti-angiogenic and anti-tumor activity by targeting
fibroblast growth factor receptor 1-mediated angiogenesis. Int J
Mol Sci. 16:24011–24031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunological effects of conventional chemotherapy
and targeted anticancer agents. Cancer Cell. 28:690–714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eichelberg C, Vervenne WL, De Santis M,
Fischer von Weikersthal L, Goebell PJ, Lerchenmüller C, Zimmermann
U, Bos MM, Freier W, Schirrmacher-Memmel S, et al: SWITCH: A
randomised, sequential, open-label study to evaluate the efficacy
and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the
treatment of metastatic renal cell cancer. Eur Urol. 68:837–847.
2015. View Article : Google Scholar : PubMed/NCBI
|