Introduction
Colorectal cancer is a malignant tumor of the
digestive tract derived from mucous epithelium, including colon
cancer and rectal cancer (1). With
the changes in dietary structure and living habits, the incidence
rate of colorectal cancer ranks 3rd following lung cancer and
breast cancer, and its mortality rate shows a gradual increasing
trend (2). The 5-year survival rate
of early colorectal cancer can be up to 90%, so the early diagnosis
and treatment is extremely important (3). Radical surgery dominates the treatment
of early colorectal cancer, supplemented by chemotherapy. Patients
who can tolerate surgery with middle-advanced cancer and surgical
indications should undergo radical surgery. Good efficacy has been
obtained in most patients who have received radical surgery for
colorectal cancer (4). However,
radical surgery for colorectal cancer is difficult, and the
patient's immunity is low, so postoperative infection occurs easily
(5). The risk of infection is
increased due to the decline in the patient's defense after
surgery, and incision, pulmonary and abdominal infections often
occur after exogenous or endogenous surgical site infection
(6).
Interleukin-4 (IL-4) and IL-10 can activate and
regulate immune cells, and mediate the activation, proliferation
and differentiation of T and B cells, which play an important role
in inflammatory and immune responses (7). Adiponectin (APN) is an
insulin-sensitizing hormone, its level can predict the development
of inflammation and it has displayed anti-inflammatory potential in
clinical tests (8). Studies have
demonstrated that inflammation is involved in the occurrence and
development of postoperative infection, and there are significant
changes in the levels of serum IL-4, IL-10 and APN, which can
evaluate the postoperative infection and serve as indexes for the
clinical auxiliary diagnosis of postoperative infection of
malignant tumor (9). Currently, the
correlation of changes in serum IL-4, IL-10 and APN levels with
postoperative infection of colorectal cancer are rarely reported.
In this experiment, the data of patients with infection after
radical surgery for colorectal cancer were retrospectively
analyzed, the levels of serum IL-4, IL-10 and APN were compared in
patients with postoperative infection of colorectal cancer and
pulmonary infection, and their correlation with the stage of
colorectal cancer in patients with postoperative infection were
analyzed and explored, so as to provide references for the clinical
nursing and treatment after radical surgery for colorectal
cancer.
Patients and methods
Data of patients
The clinical data of 159 patients receiving radical
surgery for colorectal cancer in Xiangyang No. 1 People's Hospital,
Hubei University of Medicine (Xiangyang, China) from January 2014
to December 2017 were retrospectively analyzed. A total of 67
patients with postoperative infection were enrolled into the
infection group, including 39 males and 28 females aged 22–76 years
with an average age of 42.38±6.94 years, while the remaining 92
patients without infection were enrolled into the non-infection
group, including 54 males and 38 females aged 19–71 years with an
average age of 39.24±5.31 years. To ensure the accuracy and
reliability of experimental results, clinical data of patients were
compared between the two groups, and there were no significant
differences (P>0.05), proving that they were comparable between
the two groups. Basic data of patients are shown in Table I.
 | Table I.Basic data of 159 patients receiving
radical surgery for colorectal cancer [n (%)]. |
Table I.
Basic data of 159 patients receiving
radical surgery for colorectal cancer [n (%)].
| Parameters | Infection group
(n=67) | Non-infection group
(n=92) | χ2 | P-value |
|---|
| Sex |
|
| 0.004 | 0.951 |
| Male | 39 (58.21) | 54 (58.70) |
|
|
|
Female | 28 (41.79) | 38 (41.30) |
|
|
| Age (years) |
|
| 0.070 | 0.791 |
|
<30 | 27 (40.30) | 39 (42.39) |
|
|
| ≥30 | 40 (59.70) | 53 (57.61) |
|
|
| Smoking |
|
| 0.222 | 0.637 |
| Yes | 42 (62.69) | 61 (66.30) |
|
|
| No | 25 (37.31) | 31 (33.70) |
|
|
| Dietary habit |
|
| 2.390 | 0.122 |
| Low
fiber | 19 (28.36) | 37 (40.22) |
|
|
| High
fiber | 48 (71.64) | 55 (59.78) |
|
|
| Intestinal
obstruction |
|
| 0.164 | 0.686 |
| Yes | 50 (74.63) | 66 (71.74) |
|
|
| No | 17 (25.37) | 26 (28.26) |
|
|
| Surgical mode |
|
| 0.258 | 0.611 |
|
Laparoscopic surgery | 42 (62.69) | 54 (58.70) |
|
|
|
Traditional laparotomy | 25 (37.31) | 38 (41.30) |
|
|
| Type of tumor |
|
| 0.421 | 0.810 |
| Right
hemicolon cancer | 15 (22.39) | 21 (22.83) |
|
|
| Left
hemicolon cancer | 19 (28.36) | 22 (23.91) |
|
|
| Rectal
cancer | 33(49.25) | 49 (53.26) |
|
|
| Pathological
stage |
|
| 0.272 | 0.965 |
| Stage
I | 17 (25.37) | 23 (25.00) |
|
|
| Stage
II | 19 (28.36) | 26 (28.26) |
|
|
| Stage
III | 24 (35.82) | 31 (33.70) |
|
|
| Stage
IV | 7
(10.45) | 12 (13.04) |
|
|
| Type of
infection |
|
| − | − |
| Incision
infection | 39 (58.21) | − |
|
|
| Abdominal
infection | 15 (22.39) | − |
|
|
| Pulmonary
infection | 13 (19.40) | − |
|
|
Exclusion and inclusion criteria
Inclusion criteria: patients whose examination
results of pathological section were in line with the
manifestations of colorectal cancer, patients aged above 18 years,
patients who were not treated in other hospitals, and patients who
underwent radical surgery for colorectal cancer in Xiangyang No. 1
People's Hospital Affiliated to Hubei University of Medicine.
Exclusion criteria: patients who did not cooperate in related
diagnosis and treatment, pregnant or lactating patients, patients
with other diseases that may be related to such cytokine expression
and changes, patients with genetic diseases, patients with tumors
other than colorectal cancer, or patients with communication
disorders or cognitive disorders. The subjects or their families
signed the informed consent and cooperated with medical workers in
related diagnosis and treatment.
This study was approved by the Ethics Committee of
Xiangyang No. 1 Peoples Hospital, Hubei University of Medicine
(Xiangyang, China).
Methods
After 4 ml fasting peripheral venous blood was drawn
from all patients in the early morning before surgery and at day 3
after surgery, the serum was isolated via centrifugation at 2,000 ×
g for 15 min at 4°C (Beckman Coulter, Inc., Brea, CA, USA) and
stored in a cryogenic refrigerator (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at −20°C. The levels of serum IL-4, IL-10 and APN
in both groups were detected via enzyme-linked immunosorbent assay
(ELISA) in strict accordance with instructions of the human IL-4
and IL-10 ELISA kits (Shanghai Kanglang Biological Technology Co.,
Ltd., Shanghai, China) and human APN ELISA kit (Shanghai MLbio Co.,
Ltd., Shanghai, China). The standard, blank and sample wells were
set. Then, 50 µl standard samples were accurately loaded, and the
sample well was added with 40 µl of sample diluent and then 10 µl
of sample to be detected and mixed evenly. The plate was sealed
with sealing membrane, followed by incubation at 37°C for 1 h. The
sealing membrane was uncovered. The solution was discarded and the
plate was dried. Each well was filled with 100 µl washing solution.
The sealing membrane was covered and the washing solution was
discarded after 30 sec. The above procedure was repeated 5 times,
and the plate was dried. The standard well and sample well were
added with 100 µl ELISA reagent. Color developing agents A (60 µl)
and B (60 µl) were added into each well and mixed evenly, and the
sealing membrane was covered, followed by color development in the
dark at 37°C for 30 min. Then 50 µl stop buffer was added into each
well to terminate the reaction. The optical density (OD) of each
well was immediately detected at a wavelength of 450 nm by using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA),
and the concentrations of serum IL-4, IL-10 and APN were
calculated.
Statistical analysis
SPSS 17.4 [AsiaAnalytics (formerly SPSS China)]
software system was used for statistical analysis. The basic
enumeration data of patients were expressed as percentage [n (%)],
and Chi-square test was performed. IL-4, IL-10 and APN levels were
expressed as mean ± standard deviation. t-test was adopted for the
comparison of differences between the two groups at different
time-points, and F analysis was used for the expression difference
among different types of infection. The correlation of IL-4, IL-10
and APN levels with the stage of colorectal cancer in patients with
postoperative infection of colorectal cancer was analyzed via
Spearmans correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in expression levels of serum
IL-4, IL-10 and APN in both groups
There were no differences in the expression levels
of IL-4, IL-10 and APN between the two groups before surgery
(P>0.05). The expression levels of IL-4 and IL-10 in the
infection group were significantly higher than those in the
non-infection group at day 3 after surgery (P<0.05), and they
were higher in both groups after surgery than those before surgery
(P<0.05), but the changes in the expression levels in the
infection group were more significant than those in the
non-infection group. There was no difference in the expression
level of APN between the two groups before surgery (P>0.05). The
expression level of APN in the infection group was lower than that
in the non-infection group at day 3 after surgery (P<0.05), and
it was lower in both groups after surgery than that before surgery
(P<0.05), but the change in the expression level was more
significant in the infection group than that in the non-infection
group (Table II).
 | Table II.Changes in expression levels of serum
IL-4, IL-10 and APN. |
Table II.
Changes in expression levels of serum
IL-4, IL-10 and APN.
|
| IL-4 (pg/l) | IL-10 (ng/l) | APN (pg/l) |
|---|
|
|
|
|
|
|---|
| Groups | Before surgery | 3 d after
surgery | Before surgery | 3 d after
surgery | Before surgery | 3 d after
surgery |
|---|
| Infection group
(n=67) | 162.78±13.76 |
357.64±16.48a | 153.67±29.78 |
219.41±52.97a | 146.50±37.53 |
107.26±26.61a |
| Non-infection group
(n=92) | 159.43±12.84 |
216.73±13.51a | 154.91±28.56 |
174.43±37.65a | 145.92±36.26 |
129.12±29.68a |
| t | 0.117 | 59.16 | 0.791 |
6.261 | 0.922 |
4.787 |
| P-value | 1.576 |
<0.001 | 0.266 | <0.001 | 0.098 | <0.001 |
Expression levels of serum IL-4, IL-10
and APN in different types of infection
The serum IL-4 level in pulmonary infection was
higher than that in incision infection and abdominal infection
(P<0.05), and it was also higher in abdominal infection than
that in incision infection, displaying no statistically significant
difference (P>0.05). The serum IL-10 level in pulmonary
infection was higher than that in incision infection and abdominal
infection, showing statistically significant differences
(P<0.05), and it was also higher in abdominal infection than
that in incision infection without a statistically significant
difference (P>0.05). The serum APN level in pulmonary infection
was lower than that in incision infection and abdominal infection,
showing statistically significant differences (P<0.05), and it
was also lower in abdominal infection than that in incision
infection without a statistically significant difference
(P>0.05; Table III).
 | Table III.Changes in expression levels of serum
IL-4, IL-10 and APN in different types of infection. |
Table III.
Changes in expression levels of serum
IL-4, IL-10 and APN in different types of infection.
| Items | Incision infection
(n=39) | Abdominal infection
(n=15) | Pulmonary infection
(n=13) | F | P-value |
|---|
| IL-4 (pg/l) | 327.84±24.51 | 344.52±21.46 |
355.75±28.54a | 7.122 | 0.002 |
| IL-10 (ng/l) | 197.55±48.72 | 209.24±52.63 | 225.61±49.25 | 1.605 | 0.209 |
| APN (pg/l) | 135.61±27.64 | 126.91±23.97 |
108.24±24.39a | 5.315 | 0.007 |
Correlation of serum IL-4, IL-10 and
APN with stage of colorectal cancer in patients in infection
group
The IL-4 and IL-10 levels in patients with
colorectal cancer in the infection group at day 3 after surgery had
a significant positive correlation with the stage of colorectal
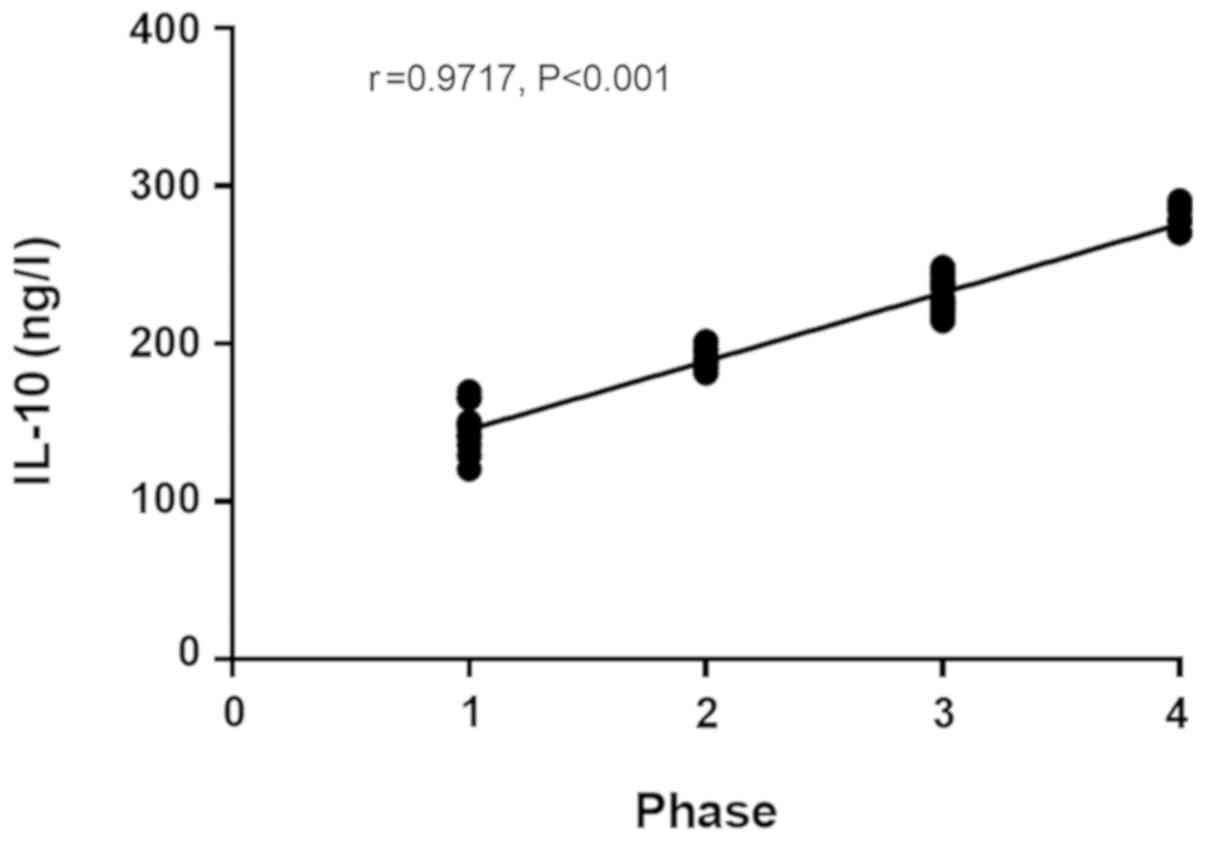
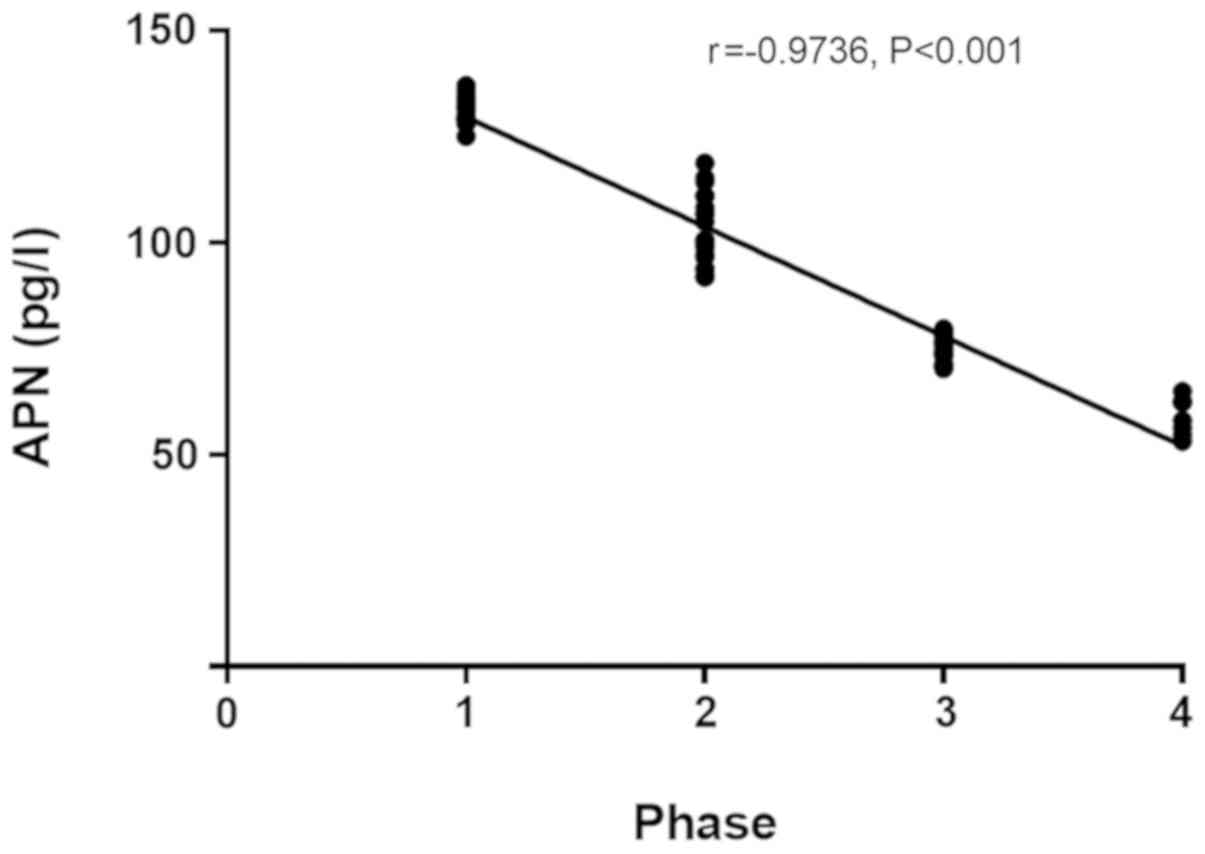
cancer (r=0.9357, P<0.001) (r=0.9717, P<0.001; Figs. 1 and 2),
and the APN level in patients with colorectal cancer in the
infection group at day 3 after surgery had a significant negative
correlation with the stage of colorectal cancer (r=−0.9736,
P<0.001; Fig. 3).
Discussion
The large intestine functions to absorb the liquid
in the food debris and to make the food debris feces, which is an
important component of the digestive system and the lower part of
the digestive tract (10). At the
same time, the large intestine also secretes mucin, so that the
feces can be excreted easily and the intestinal wall can be
protected from the mechanical damage (11). Colorectal cancer is divided into colon
cancer and rectal cancer. There are no obvious symptoms in the
early stage of colorectal cancer, and it has developed into
advanced colorectal cancer if the lesion metastasizes to the
stomach, liver and extra-territorial lymph nodes (12,13). The
position of colorectal cancer is low, so it can be diagnosed via
digital rectal examination and colonoscopy. However, the large
intestine is located deep in the pelvic cavity, so surgery is
difficult and incomplete, and the postoperative recurrence rate and
infection rate are extremely high (14). After radical surgery for colorectal
cancer, pulmonary infection can occur in patients due to
malnutrition, hypoproteinemia and anemia, abdominal infection can
also be caused by increased abdominal pressure formed due to
special functions of the large intestine, and there are various
abdominal fats and the incision is prone to effusion, thus leading
to incision infection (15–17).
After infection, the tissues in the patient's body
will be stimulated by pain, trauma and stress, thereby releasing a
large number of inflammatory factors. Both IL-4 and IL-10 are
anti-inflammatory factors, which can exert an immunomodulatory
effect in the large intestine through stimulating B cells,
mastocytes, macrophages and T cells (18). IL-4 and IL-10 can inhibit the
secretion of TNF, IL-1 and IL-6 and promote the body's immune
response through downregulating inflammatory mediators (19). In the post-operative infection of
patients with colorectal cancer, the activation process of Th1 and
Th2 cells can be delayed under the combined action of IL-4 and
IL-10, thereby enhancing the Th-type response and improving the
body repair (20). APN,
anti-inflammatory factor, is consumed due to the aggravation of
inflammation in patients with postoperative infection of colorectal
cancer, leading to significant decline in APN in the body. At the
same time, the low-level APN can accelerate the occurrence of
inflammatory response (21).
This study revealed that there were no differences
in the expression levels of IL-4, IL-10 and APN between the two
groups before surgery (P>0.05). The expression levels of IL-4
and IL-10 in the infection group were significantly higher than
those in the non-infection group at day 3 after surgery
(P<0.05), and they were higher in both groups after surgery than
those before surgery (P<0.05), but the changes in expression
levels in the infection group were more significant than those in
the non-infection group. The expression level of APN in the
infection group was lower than that in the non-infection group at
day 3 after surgery (P<0.05), and it was lower in both groups
after surgery than that before surgery (P<0.05), but the change
in the expression level was more significant in the infection group
than that in the non-infection group. The levels of
anti-inflammatory factors IL-4 and IL-10 in the infection group
were higher than those in the non-infection group, indicating that
in the progression of postoperative infection of colorectal cancer,
the anti-inflammatory effect of the patient's body is enhanced, and
the decline in APN can strengthen the anti-inflammatory effect. The
serum IL-4 level in pulmonary infection was higher than that in
incision infection and abdominal infection (P<0.05), and it was
also higher in abdominal infection than that in incision infection
(P>0.05). The serum IL-10 level in pulmonary infection was
higher than that in incision infection and abdominal infection
(P<0.05), and it was also higher in abdominal infection than
that in incision infection (P>0.05). The serum APN level in
pulmonary infection was lower than that in incision infection and
abdominal infection (P<0.05), and it was also lower in abdominal
infection than that in incision infection (P>0.05). The
expression levels of anti-inflammatory factors were different among
different types of infections. Moreover, the IL-4 and IL-10 levels
in patients with colorectal cancer in the infection group at day 3
after surgery had a significant positive correlation with the stage
of colorectal cancer, and the APN level in patients with colorectal
cancer in the infection group at day 3 after surgery had a
significant negative correlation with the stage of colorectal
cancer. It is reported (22) that the
body immunity of patients with advanced colorectal cancer is poorer
than that of patients with early colorectal cancer, which are
consistent with the results in this study, and the expression
levels of IL-4 and IL-10 in patients with advanced colorectal
cancer were higher than those in patients with early colorectal
cancer, while the expression level of APN was lower than that in
patients with early colorectal cancer.
In this study, the sample size was small due to the
limited medical resources in Xiangyang No. 1 People's Hospital
Affiliated to Hubei University of Medicine, so there might be a
certain contingency in the results. In the future, subjects in this
study will be followed up for survey for a longer time, to obtain
optimal results.
In conclusion, the serum IL-4, IL-10 and APN levels
have a certain correlation with the presence or absence of
postoperative infection of colorectal cancer, the type of infection
and the stage of colorectal cancer, which are worthy of clinical
promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and ZW performed ELISA. SZ analyzed the general
data of patients and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xiangyang No. 1 People's Hospital, Hubei University of Medicine
(Xiangyang, China). Patients who participated in this study, signed
the informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bibbins-Domingo K, Grossman DC, Curry SJ,
Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM,
Kemper AR, Krist AH, et al: US Preventive Services Task Force:
Screening for colorectal cancer: US preventive services task force
recommendation statement. JAMA. 315:2564–2575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JS, Piper MA, Perdue LA, Rutter CM,
Webber EM, O'Connor E, Smith N and Whitlock EP: Screening for
colorectal cancer: updated evidence report and systematic review
for the US preventive services task force. JAMA. 315:2576–2594.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: a
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McSorley ST, Watt DG, Horgan PG and
McMillan DC: Postoperative systemic inflammatory response,
complication severity, and survival following surgery for
colorectal cancer. Ann Surg Oncol. 23:2832–2840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tei M, Wakasugi M, Kishi K, Tanemura M and
Akamatsu H: Incidence and risk factors of postoperative delirium in
elderly patients who underwent laparoscopic surgery for colorectal
cancer. Int J Colorectal Dis. 31:67–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakellariou S, Fragkou P, Levidou G,
Gargalionis AN, Piperi C, Dalagiorgou G, Adamopoulos C, Saetta A,
Agrogiannis G, Theohari I, et al: Clinical significance of AGE-RAGE
axis in colorectal cancer: associations with glyoxalase-I,
adiponectin receptor expression and prognosis. BMC Cancer.
16:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lasry A, Zinger A and Ben-Neriah Y:
Inflammatory networks underlying colorectal cancer. Nat Immunol.
17:230–240. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kojima M, Ikeda K, Saito N, Sakuyama N,
Koushi K, Kawano S, Watanabe T, Sugihara K, Ito M and Ochiai A:
Neuroendocrine tumors of the large intestine: clinicopathological
features and predictive factors of lymph node metastasis. Front
Oncol. 6:1732016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bettington M, Walker N, Rosty C, Brown I,
Clouston A, McKeone D, Pearson SA, Klein K, Leggett B and Whitehall
V: Serrated tubulovillous adenoma of the large intestine.
Histopathology. 68:578–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sinicrope FA, Okamoto K, Kasi PM and
Kawakami H: Molecular biomarkers in the personalized treatment of
colorectal cancer. Clin Gastroenterol Hepatol. 14:651–658. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mármol I, Sánchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: a
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18:1972017. View Article : Google Scholar
|
|
14
|
Tariq K and Ghias K: Colorectal cancer
carcinogenesis: a review of mechanisms. Cancer Biol Med.
13:120–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koelzer VH, Zlobec I and Lugli A: Tumor
budding in colorectal cancer-ready for diagnostic practice? Hum
Pathol. 47:4–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka K, Kumamoto T, Nojiri K, Matsuyama
R, Takeda K and Endo I: Impact of postoperative morbidity on
long-term survival after resection for colorectal liver metastases.
Ann Surg Oncol. 23 Suppl 5:929–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mokutani Y, Mizushima T, Yamasaki M,
Rakugi H, Doki Y and Mori M: Prediction of postoperative
complications following elective surgery in elderly patients with
colorectal cancer using the comprehensive geriatric assessment. Dig
Surg. 33:470–477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Watt DG, Roxburgh CS, Horgan PG
and McMillan DC: Colorectal cancer, systemic inflammation, and
outcome: staging the tumor and staging the host. Ann Surg.
263:326–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landy J, Ronde E, English N, Clark SK,
Hart AL, Knight SC, Ciclitira PJ and Al-Hassi HO: Tight junctions
in inflammatory bowel diseases and inflammatory bowel disease
associated colorectal cancer. World J Gastroenterol. 22:3117–3126.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brennan CA and Garrett WS: Gut microbiota,
inflammation, and colorectal cancer. Annu Rev Microbiol.
70:395–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Passardi A, Scarpi E, Cavanna L,
Dall'Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F,
Tamberi S, Brandes AA, et al: Inflammatory indexes as predictors of
prognosis and bevacizumab efficacy in patients with metastatic
colorectal cancer. Oncotarget. 7:33210–33219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moriarity A, O'Sullivan J, Kennedy J,
Mehigan B and McCormick P: Current targeted therapies in the
treatment of advanced colorectal cancer: A review. Ther Adv Med
Oncol. 8:276–293. 2016. View Article : Google Scholar : PubMed/NCBI
|