Introduction
Pulmonary large cell neuroendocrine carcinoma
(LCNEC) is categorized as a large cell carcinoma. The clinical and
biological characteristics of LCNEC are similar to those of small
cell lung carcinomas (SCLCs), and the disease exhibits aggressive
phenotypes of frequent recurrence and high metastatic potential
(1,2).
The optimal treatment strategies and molecular features of LCNEC
remain largely unknown. Therefore, to improve the prognosis of
patients with LCNEC, characterization of its molecular
characteristics is required (3,4).
Cluster of differentiation (CD)146 is a cell
adhesion molecule belonging to the immunoglobulin superfamily,
which is located on the human adipose-derived stem cell surface
(5,6).
CD146 has been reported to be involved in cell adhesion by binding
other cells or with the extracellular matrix (7). Moreover, abnormal CD146 expression has
been identified in several types of cancer, such as breast cancer
and prostate cancer, in which it was associated with cancer cell
motility, the state of epithelial-mesenchymal transition (EMT),
angiogenesis and prognosis (7,8). In
non-small cell lung cancer, CD146 overexpression is a useful marker
in predicting poor prognosis, though the reason for this remains
largely unknown; likewise, in the context of pulmonary LCNEC
(9,10).
In the present study, the role of CD146 in pulmonary
LCNEC was investigated. CD146 expression was detected in pulmonary
LCNEC cell lines (NCI-H460 and NCI-H810), and the association of
CD146 overexpression with migration and proliferation of the cells
was determined.
Materials and methods
Cell lines
The LCNEC cell lines, NCI-H460 and NCI-H810, were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA) (11). Human umbilical vein
endothelial cells (HUVECs) were obtained from Lonza (Walkersville,
MD, USA; cat. no. C2517A) and maintained in endothelial basal
medium-2 (Lonza). NCI-H460/H810 cells were maintained in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified environment
with 10% CO2.
Silencing of CD146 using small
interfering RNA (siRNA)
Gene silencing was performed using siRNAs (Qiagen
GmbH, Hilden, Germany) directed against human CD146 (8). The siRNA sequences were as follows:
siRNA-1 sense, 5′-GGGAGAGAAAUACAUCGAUTT-3′ and antisense,
5′-AUCGAUGUAUUUCUCUCCCTG-3′); siRNA-2 sense,
5′-GGAACUACUGGUGAACUAUTT-3′ and antisense,
5′-AUAGUUCACCAGUAGUUCCTG-3′. Qiagen AllStar siRNA (Qiagen GmbH) was
used as a negative control. Based on western blotting results,
NCI-H460 cells were selected for transfection with siRNA (20 nM)
using Lipofectamine 2000 (Qiagen GmbH, Hilden, Germany), according
to the manufacturer's protocol. All cells were used in subsequent
experiments at 24 h following transfection. Cell morphology means
to observe the change of cell-shape through a fluorescence
microscope (magnification, ×200; BZ-II analyser; Keyence, Osaka,
Japan) at 72 h following transfection, 20 cells were observed at a
randomly selected microscopic field of view.
Plasmid transfection
A CD146 expression plasmid, CD146-HaloTag vector,
was obtained from Promega Corporation (Madison, WI, USA). NCI-H460
and NCI-H810 cells were transiently transfected with this plasmid
(0.015 µg/µl) or a HaloTag (HT) control vector (0.015 µg/µl; cat.
no. G6591; Promega Corporation) using Fugene® HD
transfection reagent (Promega Corporation), according to the
manufacturer's protocol (8).
Migration assays
The migration capacity of cancer cells was assessed
by counting the number of cells migrating through Transwell
chambers (8 µm pore size; Corning Incorporated, Corning, NY, USA)
as described previously (12). Cells
were maintained in 10% FBS/Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc.) during these assays.
Cells were transfected with siRNAs or plasmids 48 h prior to
experimentation, and migration was determined at 24 h following
transfection.
Cell viability assay
A cell viability assay was performed as described
previously (8). Briefly, cancer cells
(1.5×103 cells/well) were seeded in 96-well plates 24 h
after transfection in the aforementioned culture conditions. Cell
viability was examined using a CellTiter-Glo Luminescent Cell
Viability assay kit (cat. no. G7570; Promega Corporation) with a
luminometer (Infinite 200, Tecan, Switzerland) at 24, 47, 72 and 96
h following transfection. Background was subtracted using the
values of wells containing only culture medium.
Western blot analysis
Cancer cells were lysed in PRO-PREP™ Protein
Extraction Solution (iNtRON Biotechnology, Seongnam, Korea), and
proteins were separated on 12% SDS-polyacrylamide gels and
transferred onto mini polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked with 1X
TBST with 5% non-fat dry milk for 1 h at room temperature.
Membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-CD146 (1:10,000; cat. no. ab75769; Abcam,
Cambridge, UK), anti-epithelial (E)-cadherin (1:1,000; cat. no.
3195), anti-vimentin (1:1,000; cat. no. 5741), anti-Snail (1:1,000;
cat. no. 4719), anti-AKT (1:1,000; cat. no. 4691),
anti-phosphorylated AKT (1:2,000; cat. no. 4060; all from Cell
Signalling Technology, Inc., Danvers, MA, USA) and anti-β-actin
(1:5,000; cat. no. ab8227; Abcam). Membranes were then incubated
with Anti-rabbit/mouse IgG, HRP-linked Antibody (1:2,000; cat. no.
7074/7076; from Cell Signalling Technology, Inc.) for 1 h at room
temperature. An electrochemiluminescence western blotting analysis
system (Amersham Biosciences, Little Chalfont, UK) was used to
visualize the proteins, according to the manufacturer's protocol.
Densitometric analysis was performed with ImageJ 1.48v software
(National Institutes of Health, Bethesda, MD, USA). The protein
level of CD146 was also assessed in HUVECs as a positive control.
β-actin was used as the loading control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between multiple groups were conducted by one-way
analysis of variance (ANOVA) with Dunnet's post-hoc test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using JMP 11.0.0 software
(SAS Institute, Inc., Cary, NC, USA).
Results
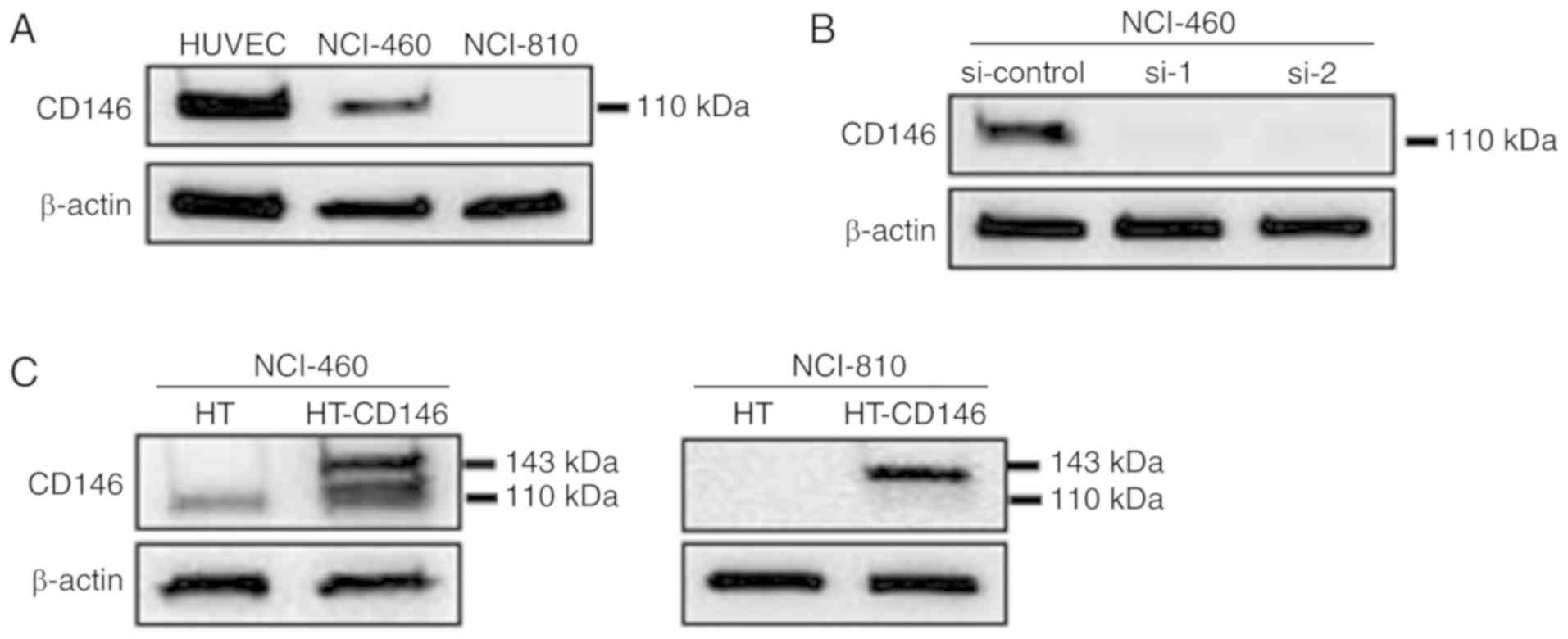
Analysis of CD146 expression in LCNEC
cells
The protein expression level of CD146 in two LCNEC
cell lines (NCI-H460 and NCI-H810) was analyzed by western
blotting. HUVECs were used as a positive control as they express
high levels of CD146 (8). High
protein levels of CD146 were detected in NCI-460 cells, but CD146
expression was not detected in NCI-810 cells (Fig. 1A). To investigate the function of
CD146, it was knocked down in NCI-460 cells and upregulated in
NCI-H460 and NCI-H810 cells using siRNA and plasmids. The
efficiencies of knockdown (Fig. 1B)
and overexpression (Fig. 1C) of CD146
were then confirmed. Since overexpression of CD146 was induced by
transfection with CD146-HaloTag vector, endogenous CD146 (110 kDa)
and exogenous CD146 (plus 33-kDa HaloTag) expressions occurred
simultaneously in the NCI-H460 cells.
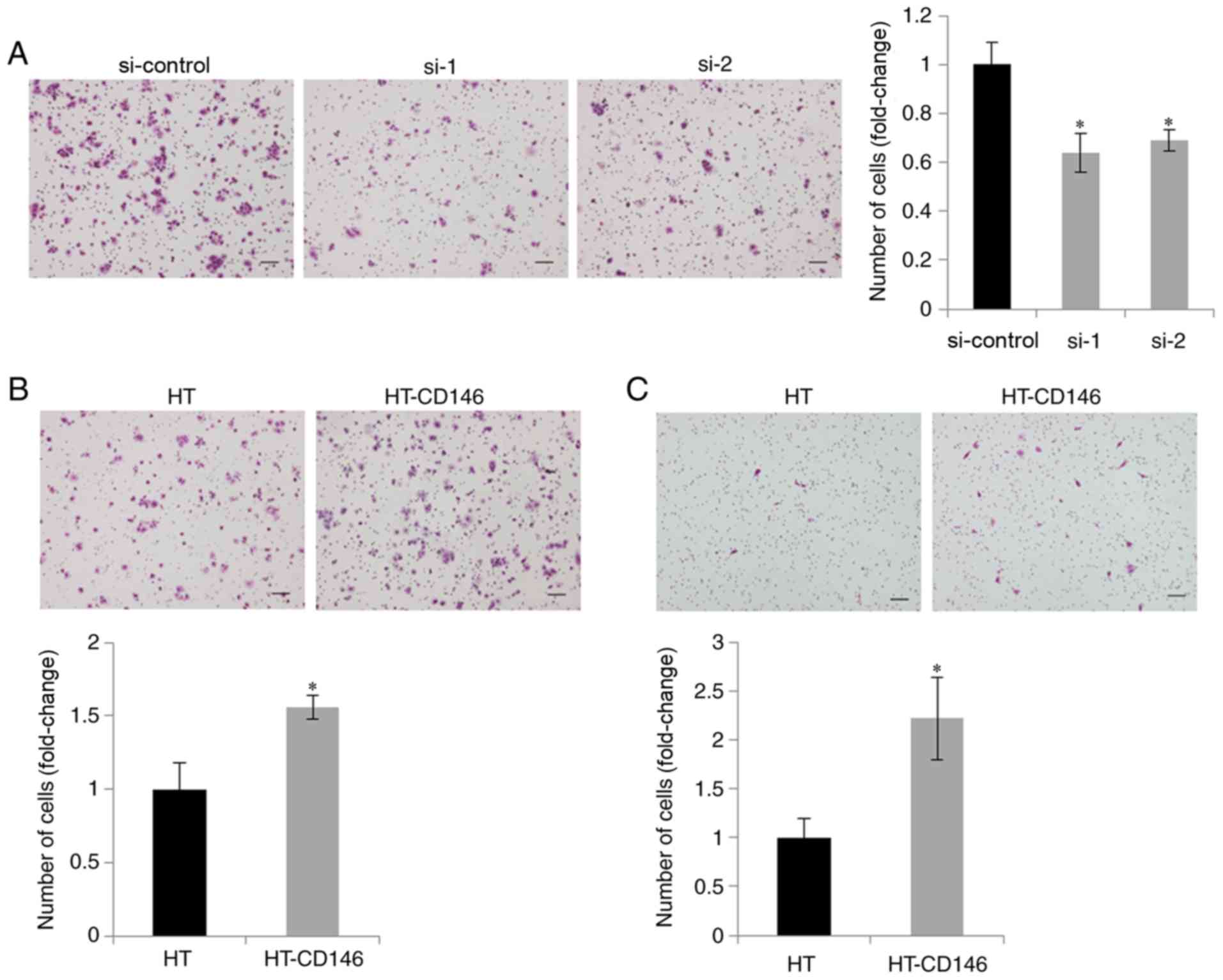
CD146 expression enhances the
migration ability of LCNEC cells
As CD146 has been reported to be involved in the
migration of cancer cells (13),
migration assays were performed following knockdown of CD146 in
NCI-460 cells expressing high endogenous levels of CD146. It was
demonstrated that cell migration ability was decreased upon CD146
knockdown, when compared with cells transfected with negative
control siRNA (P<0.05; Fig. 2A).
Conversely, migration ability was increased upon overexpression of
CD146 in the two LCNEC cell lines, when compared with those cells
transfected with the HT control vector (P<0.05; Fig. 2B and C). These results suggest that
CD146 was involved in the migration of LCNEC cells.
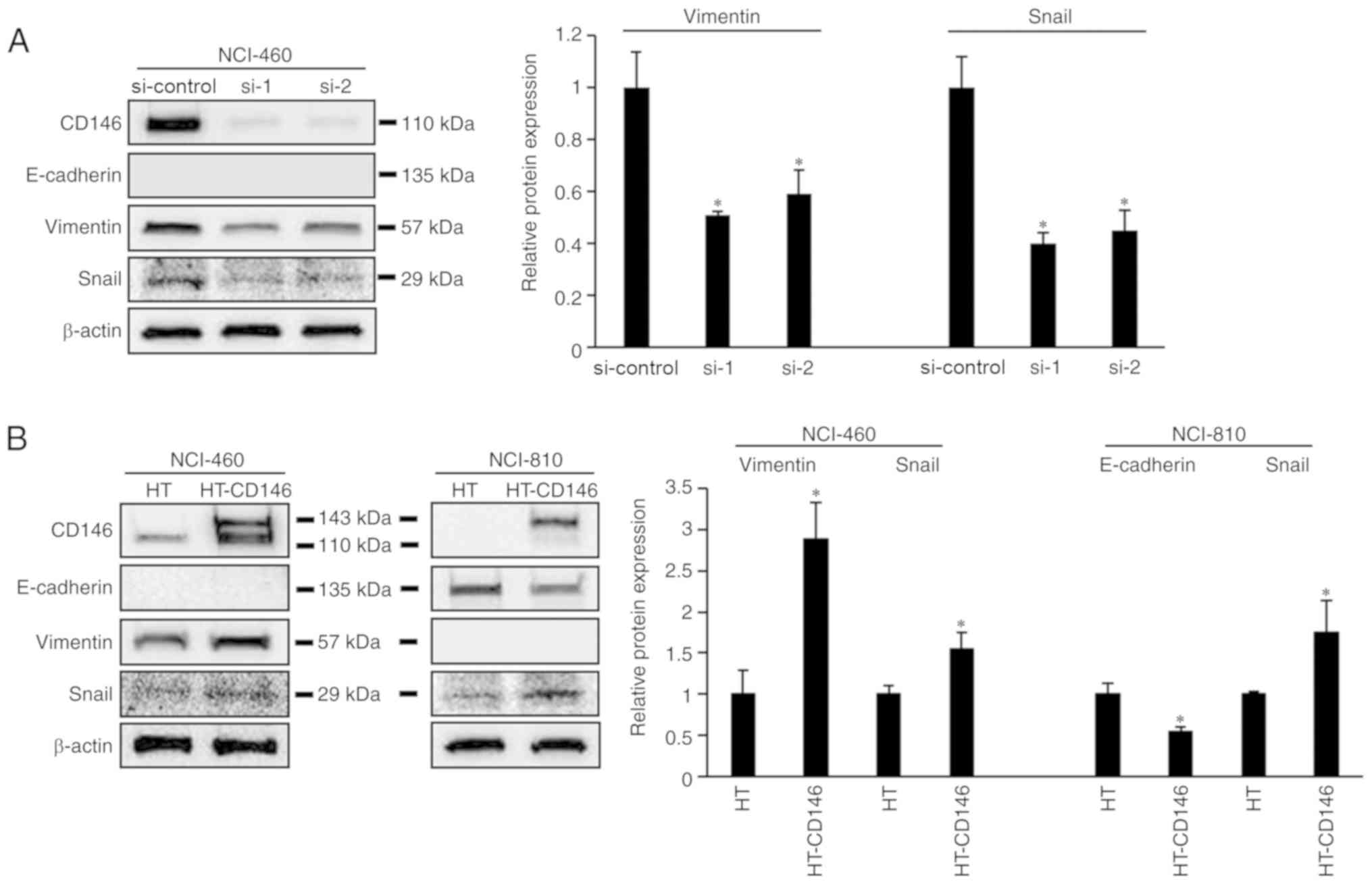
CD146 promotes EMT in LCNEC cells
The process of cancer cell migration requires
epithelial cancer cells to undergo EMT (14); therefore, the association between the
expression of CD146 and EMT markers in LCNEC cells was
investigated. It was demonstrated that expression of vimentin and
Snail was decreased in NCI-460 cells following knockdown of CD146
(P<0.05). Meanwhile, vimentin and Snail expression was increased
following overexpression of CD146 in NCI-460 cells (P<0.05;
Fig. 3B). In NCI-810 cells,
overexpression of CD146 resulted in downregulated E-cadherin
expression and upregulated Snail expression (P<0.05; Fig. 3B). However, changes in cell morphology
were not observed following knockdown or overexpression of CD146
(data not shown).
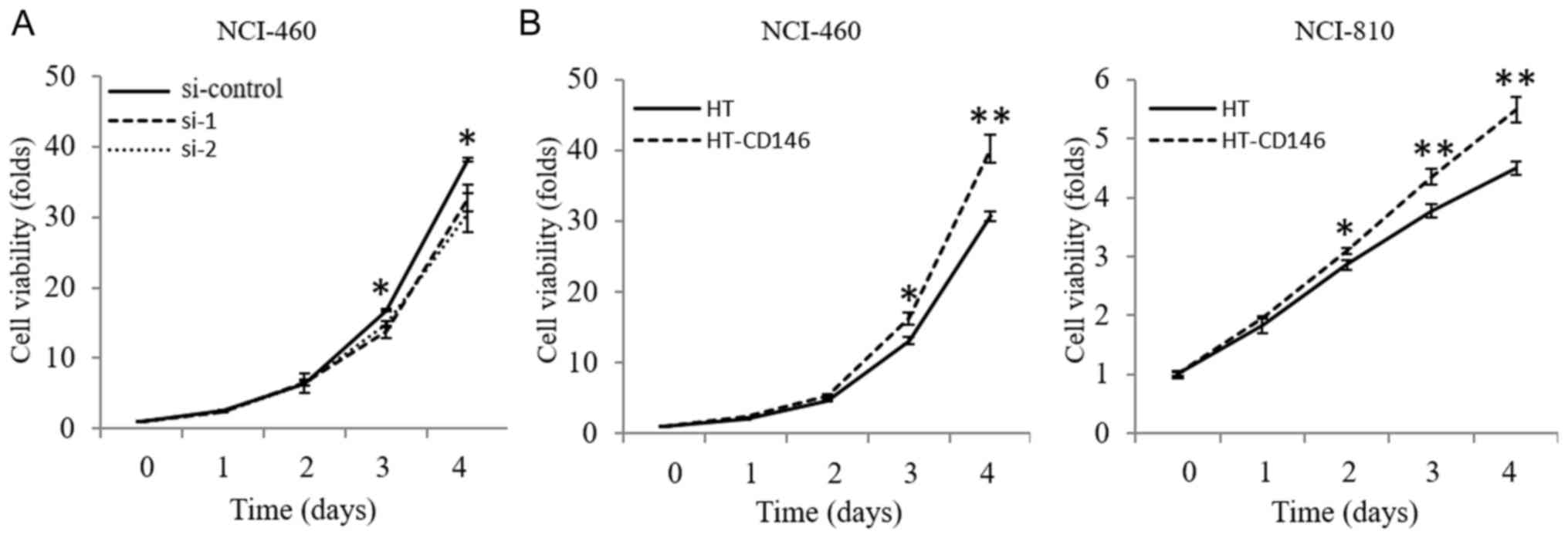
CD146 increases the proliferative
ability of LCNEC cells
The effect of CD146 on the proliferation of LCNEC
cells was also examined via a cell viability assay. The results
revealed that cell viability was significantly decreased following
knockdown of CD146 in NCI-460 cells by day 4 post-transfection
(P<0.05; Fig. 4A) and that cell
viability was increased upon overexpression of CD146 in NCI-460 and
NCI-810 cells by ≥2 days post-transfection (P<0.05; Fig. 4B). Moreover, the level of
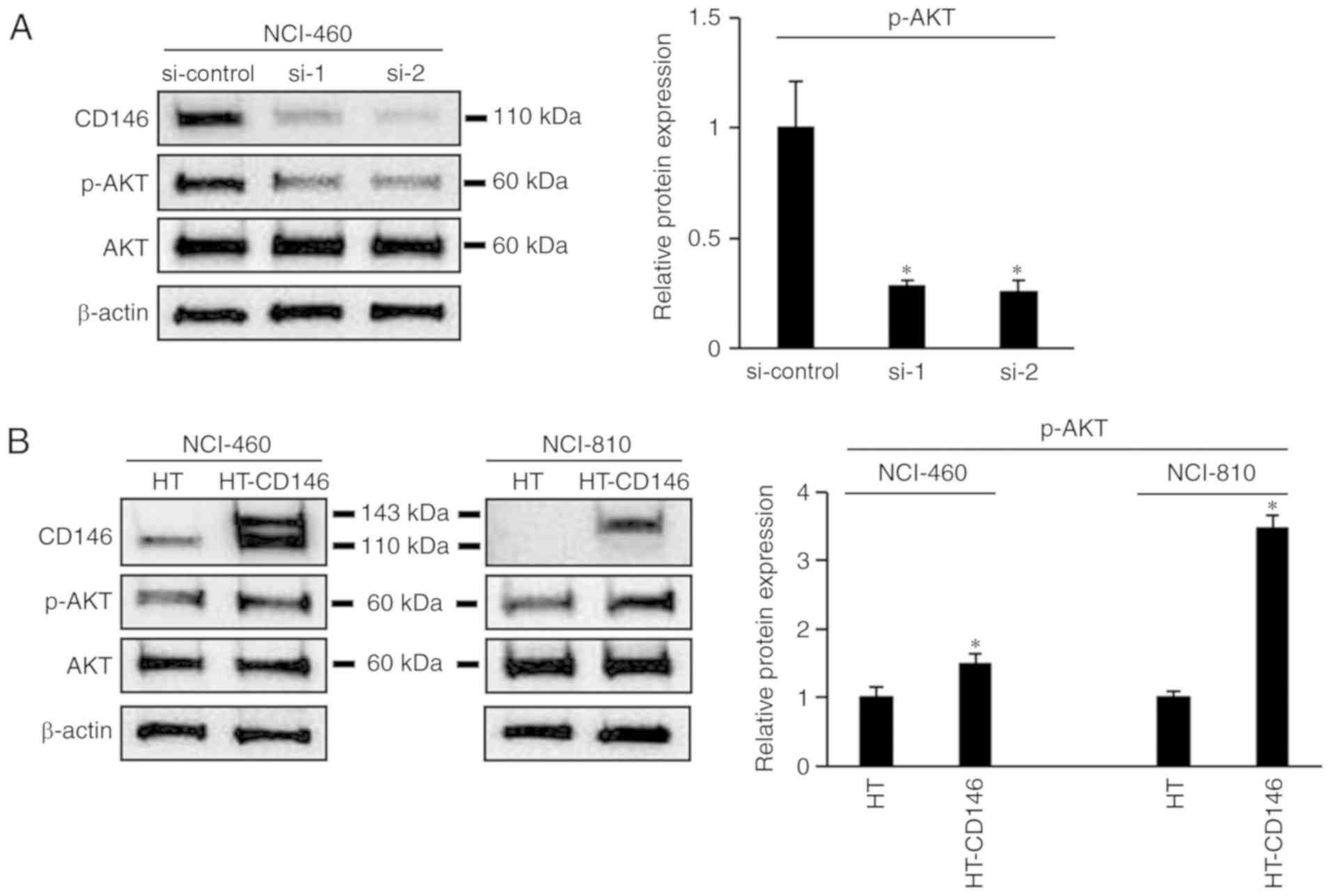
phosphorylation of AKT decreased following knockdown of CD146 in
NCI-460 cells (P<0.05; Fig. 5A).
The opposite result was apparent following overexpression of CD146
in NCI-460 and NCI-810 cells (Fig.
5B).
Discussion
CD146, also known as melanoma cell adhesion
molecule, is a transmembrane glycoprotein belonging to the
immunoglobulin superfamily (15). The
expression of CD146 has been detected in multiple types of human
carcinoma (7), and its overexpression
has been associated with poor overall survival in non-small cell
lung cancer (9). In the present
study, the role of CD146 in LCNEC cell lines (NCI-460 and NCI-810)
was evaluated. Endogenous CD146 expression was detected in NCI-460
cells, and exogenous overexpression was demonstrated to enhance
migratory ability in NCI-460 and NCI-810 cells. Previous studies
have reported that CD146 promotes breast cancer progression via
induction of EMT due to upregulated expression of the EMT
transcription factor, Slug (13,16). In
this study, CD146 was also demonstrated to regulate the expression
of EMT markers, namely vimentin, E-cadherin and Snail. These
findings suggest that CD146 expression is associated with cell
migration via regulation of EMT in LCNEC cells.
The effect of CD146 on cell proliferation was also
investigated, which revealed that CD146 increased the viability of
LCNEC cells and increased AKT phosphorylation. The AKT kinases are
key members of various signaling pathways that regulate cellular
processes, involved in control of cell growth, proliferation and
survival (17). A previous study
reported that CD146 promotes tumor proliferation and survival
through the phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/AKT pathway, and that the expression level of CD146 is
reciprocally regulated by PI3K/AKT signaling in melanoma (18). Taken together, these data suggest that
CD146 promotes LCNEC cell proliferation and may be involved in
modulation of the AKT pathway.
Although the exact mechanism underlying the
regulation of AKT activity by CD146 remains unclear, the
association between CD146 and AKT may indicate how CD146 increases
the viability of LCNEC cells. Improving the existing understanding
of CD146 function in signal transduction will require further study
of its crosstalk with members of other signaling pathways (7), including those in EMT induction. The
clinical significance of CD146 expression in LCNEC was not
investigated in the present study, and should be a focus of future
study.
In conclusion, the present study determined that
CD146 served a critical role in controlling the migration and
proliferation of pulmonary LCNEC cells. Further exploration of the
molecular mechanisms underlying the interaction between CD146 and
AKT signaling, and EMT, in LCNEC cells may aid the development of
novel therapies for LCNEC. Further investigation is required to
elucidate the association between CD146 expression and the
clinicopathological characteristics of pulmonary LCNEC, as well as
prognosis.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Heilongjiang
Postdoctoral Science Foundation (grant no. LBH-Z16157) and the
Medical Scientific Research Foundation of Guangdong Province, China
(grant no. A2018015).
Availability of data and materials
The datasets generated and/or analysed during this
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ designed the research. YP, HG and YG performed
the research. HG and ZQ contributed to data collection and
statistical analysis. YP, YG and BZ wrote the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iyoda A, Hiroshima K, Nakatani Y and
Fujisawa T: Pulmonary large cell neuroendocrine carcinoma: Its
place in the spectrum of pulmonary carcinoma. Ann Thorac Surg.
84:702–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bodey B, Bodey B Jr, Groger AM, Siegel SE
and Kaiser HE: Invasion and metastasis: The expression and
significance of matrix metalloproteinases in carcinomas of the
lung. In Vivo. 15:175–180. 2001.PubMed/NCBI
|
|
3
|
Makino T, Mikami T, Hata Y, Otsuka H,
Koezuka S, Isobe K, Tochigi N, Shibuya K, Homma S and Iyoda A:
Comprehensive biomarkers for personalized treatment in pulmonary
large cell neuroendocrine carcinoma: A comparative analysis with
adenocarcinoma. Ann Thorac Surg. 102:1694–1701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyoshi T, Umemura S, Matsumura Y, Mimaki
S, Tada S, Makinoshima H, Ishii G, Udagawa H, Matsumoto S, Yoh K,
et al: Genomic profiling of large-cell neuroendocrine carcinoma of
the lung. Clin Cancer Res. 23:757–765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okumura S, Kohama K, Kim S, Iwao H, Miki N
and Taira E: Induction of gicerin/CD146 in the rat carotid artery
after balloon injury. Biochem Biophys Res Commun. 313:902–906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohsen-Kanson T, Hafner AL, Wdziekonski B,
Villageois P, Chignon-Sicard B and Dani C: Expression of cell
surface markers during self-renewal and differentiation of human
adipose-derived stem cells. Biochem Biophys Res Commun.
430:871–875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z and Yan X: CD146, a
multi-functional molecule beyond adhesion. Cancer Lett.
330:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng B, Ohuchida K, Chijiiwa Y, Zhao M,
Mizuuchi Y, Cui L, Horioka K, Ohtsuka T, Mizumoto K, Oda Y, et al:
CD146 attenuation in cancer-associated fibroblasts promotes
pancreatic cancer progression. Mol Carcinog. 55:1560–1572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kristiansen G, Yu Y, Schluns K, Sers C,
Dietel M and Petersen I: Expression of the cell adhesion molecule
CD146/MCAM in non-small cell lung cancer. Anal Cell Pathol.
25:77–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oka S, Uramoto H, Chikaishi Y and Tanaka
F: The expression of CD146 predicts a poor overall survival in
patients with adenocarcinoma of the lung. Anticancer Res.
32:861–864. 2012.PubMed/NCBI
|
|
11
|
Odate S, Nakamura K, Onishi H, Kojima M,
Uchiyama A, Nakano K, Kato M, Tanaka M and Katano M: TrkB/BDNF
signaling pathway is a potential therapeutic target for pulmonary
large cell neuroendocrine carcinoma. Lung Cancer. 79:205–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng B, Ohuchida K, Cui L, Zhao M, Shindo
K, Fujiwara K, Manabe T, Torata N, Moriyama T, Miyasaka Y, et al:
TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma
is involved in migration and invasion of cancer cells. Int J Oncol.
47:490–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Q, Li W, Lu D, Wu Z, Duan H, Luo Y,
Feng J, Yang D, Fu L and Yan X: CD146, an epithelial-mesenchymal
transition inducer, is associated with triple-negative breast
cancer. Proc Natl Acad Sci USA. 109:1127–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lehmann JM, Riethmuller G and Johnson JP:
MUC18, a marker of tumor progression in human melanoma, shows
sequence similarity to the neural cell adhesion molecules of the
immunoglobulin superfamily. Proc Natl Acad Sci USA. 86:9891–9895.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imbert AM, Garulli C, Choquet E, Koubi M,
Aurrand-Lions M and Chabannon C: CD146 expression in human breast
cancer cell lines induces phenotypic and functional changes
observed in epithelial to mesenchymal transition. PLoS One.
7:e437522012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li G, Kalabis J, Xu X, Meier F, Oka M,
Bogenrieder T and Herlyn M: Reciprocal regulation of MelCAM and AKT
in human melanoma. Oncogene. 22:6891–6899. 2003. View Article : Google Scholar : PubMed/NCBI
|