Introduction
Long non-coding RNAs (lncRNAs) are a class of RNA
molecules, longer than 200 nucleotides, with no protein-coding
capacity (1,2) Previously, studies have identified that
lncRNAs are aberrantly expressed in different cancer types and are
closely associated with different disease processes (3,4). LncRNAs
have been explored as a prognostic tumor biomarker as they can
easily be detected in tissues and serum (5,6). Many
lncRNAs have been identified to serve a role as prognostic
biomarkers for various cancer types. Downregulation of lncRNA
SDPR-AS is associated with poor prognosis in renal cell carcinoma
(7). lncRNA CCAT1 promotes metastasis
and is associated with poor prognosis in epithelial ovarian cancer
(8). In addition, several molecular
tools, including reverse transcription-quantitative polymerase
chain reaction and RNA-fluorescent in situ hybridization,
are used for examining the lncRNA expression levels in the saliva,
plasma, urine, serum and tissues (5,9). Several
lncRNAs, including PCA3, are routinely used in the clinic as a
grade reclassification predictor in prostate cancer (10). Interest in therapeutic strategies
against cancer based on lncRNAs continues to increase.
Head and neck squamous cell carcinoma (HNSCC), the
sixth most common cancer worldwide, is an aggressive cancer type
that has a high morbidity (11,12). Early
detection and management of HNSCC may prevent progression of the
disease. A previous study has demonstrated that a panel of three
lncRNAs (KTN1-AS1, LINC00460 and RP5-894A10.6) may be a novel
biomarker for the accurate prediction of the prognosis of patients
with HNSCC (13).
In the present study, next-generation sequencing and
clinical data of patients with HNSCC from The Cancer Genome Atlas
(TCGA) were utilized. RNA-Sequencing (RNA-Seq) datasets for 43
paired tumor and adjacent normal tissues were downloaded from TCGA
and significant differences in lncRNA expression between normal and
cancer tissues were investigated. A novel lncRNA LOC541471 was
revealed to be significantly upregulated in HNSCC. The lncRNA
LOC541471 mRNA expression levels in various cases of HNSCC were
also analyzed to evaluate its prognostic value. Overall, the
potential of lncRNA LOC541471 as a biomarker and a therapeutic
target in high-risk HNSCC was investigated in the current
study.
Materials and methods
Patients and methods
The clinical and RNA-Seq expression data for HNSCC
were obtained from TCGA (https://tcga-data.nci.nih.gov) and the Gene Expression
Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). To investigate
differential lncRNA expression between normal and tumor tissues,
the expression profile analysis was based on 43 paired tumor and
adjacent normal tissues from TCGA (Table
I). The RNA-Seq data were matched with clinical data and used
to study expression profiles and search for lncRNAs associated with
HNSCC. Prior to analysis, the HNSCC RNA expression data downloaded
from TCGA were processed and normalized using the TCGA RNA-seq
system (version 2,TCGA) and the Illumina HiSeq (v7) and Illumina GA
microRNA (v7) sequencing platforms (Illumina, Inc., Hayward, CA,
USA). A total of 511 HNSCC samples were included in the analysis of
lncRNA LOC541471 and its association with clinicopathological
features and patient survival. Clinical characteristics of the
total HNSCC cohort used are provided in Table II. Patients were classified into two
groups (high and low expression) according to the median lncRNA
LOC541471 expression value. Known genes located close to LOC541741
were analyzed using the University of California, Santa Cruz (UCSC)
genome browser (http://genome.ucsc.edu).
 | Table I.The ID of 43 paired tumor and adjacent
normal tissues from TCGA. |
Table I.
The ID of 43 paired tumor and adjacent
normal tissues from TCGA.
| Tumor tissue ID | Normal tissue ID |
|---|
| TCGA-CV-6933-01 | TCGA-CV-6933-11 |
| TCGA-CV-6934-01 | TCGA-CV-6934-11 |
| TCGA-CV-6935-01 | TCGA-CV-6935-11 |
| TCGA-CV-6936-01 | TCGA-CV-6936-11 |
| TCGA-CV-6938-01 | TCGA-CV-6938-11 |
| TCGA-CV-6939-01 | TCGA-CV-6939-11 |
| TCGA-CV-6943-01 | TCGA-CV-6943-11 |
| TCGA-CV-6955-01 | TCGA-CV-6955-11 |
| TCGA-CV-6956-01 | TCGA-CV-6956-11 |
| TCGA-CV-6959-01 | TCGA-CV-6959-11 |
| TCGA-CV-6960-01 | TCGA-CV-6960-11 |
| TCGA-CV-6961-01 | TCGA-CV-6961-11 |
| TCGA-CV-6962-01 | TCGA-CV-6962-11 |
| TCGA-CV-7091-01 | TCGA-CV-7091-11 |
| TCGA-CV-7097-01 | TCGA-CV-7097-11 |
| TCGA-CV-7101-01 | TCGA-CV-7101-11 |
| TCGA-CV-7103-01 | TCGA-CV-7103-11 |
| TCGA-CV-7177-01 | TCGA-CV-7177-11 |
| TCGA-CV-7178-01 | TCGA-CV-7178-11 |
| TCGA-CV-7183-01 | TCGA-CV-7183-11 |
| TCGA-CV-7235-01 | TCGA-CV-7235-11 |
| TCGA-CV-7238-01 | TCGA-CV-7238-11 |
| TCGA-CV-7242-01 | TCGA-CV-7242-11 |
| TCGA-CV-7245-01 | TCGA-CV-7245-11 |
| TCGA-CV-7250-01 | TCGA-CV-7250-11 |
| TCGA-CV-7252-01 | TCGA-CV-7252-11 |
| TCGA-CV-7255-01 | TCGA-CV-7255-11 |
| TCGA-CV-7261-01 | TCGA-CV-7261-11 |
| TCGA-CV-7406-01 | TCGA-CV-7406-11 |
|
TCGA-CV-7416-01 |
TCGA-CV-7416-11 |
|
TCGA-CV-7423-01 |
TCGA-CV-7423-11 |
|
TCGA-CV-7424-01 |
TCGA-CV-7424-11 |
|
TCGA-CV-7425-01 |
TCGA-CV-7425-11 |
|
TCGA-CV-7432-01 |
TCGA-CV-7432-11 |
|
TCGA-CV-7434-01 |
TCGA-CV-7434-11 |
|
TCGA-CV-7437-01 |
TCGA-CV-7437-11 |
|
TCGA-CV-7438-01 |
TCGA-CV-7438-11 |
|
TCGA-CV-7440-01 |
TCGA-CV-7440-11 |
|
TCGA-H7-A6C4-01 |
TCGA-H7-A6C4-11 |
|
TCGA-HD-8635-01 |
TCGA-HD-8635-11 |
|
TCGA-HD-A6HZ-01 |
TCGA-HD-A6HZ-11 |
|
TCGA-HD-A6I0-01 |
TCGA-HD-A6I0-11 |
|
TCGA-WA-A7GZ-01 |
TCGA-WA-A7GZ-11 |
 | Table II.Demographics and clinical
characteristics of the cohort of patients with head and neck
squamous cell carcinoma downloaded from The Cancer Genome Atlas
(n=487). |
Table II.
Demographics and clinical
characteristics of the cohort of patients with head and neck
squamous cell carcinoma downloaded from The Cancer Genome Atlas
(n=487).
|
Characteristics | Sample no, n |
|---|
| Sex |
|
|
Male | 356 |
|
Female | 131 |
| Age |
|
|
≤60 | 258 |
|
>61 | 229 |
| T
classification |
|
|
T1-2 | 180 |
|
T3-4 | 307 |
| N
classification |
|
|
N1-2 | 180 |
|
N3-4 | 307 |
| Clinical stage |
|
|
I–II | 115 |
|
III–IV | 372 |
Statistical analysis
A paired Student's t-test was used to identify any
significant differences in lncRNA LOC541471 expression between
tumor and tumor-adjacent normal tissues according to data from TCGA
and GEO, including 42 paired samples from TCGA and 24 paired
samples from GEO (one sample lacked the corresponding clinical
data). An independent sample t-test was used to identify
significant differences in lncRNA LOC541471 expression according to
data from patients with HNSCC with different lymph node metastasis
(N) classification and perineural invasion. Significant differences
in lncRNA LOC541471 expression between the groups were presented by
box plots (mean ± standard deviation). Univariate Cox regression
analysis was used to evaluate the overall survival (OS) and
relapse-free survival of patients with clinical characteristics as
categorical dependent variables. Furthermore, multivariate Cox
analysis was employed to assess the association of lncRNA LOC541471
expression with the OS and relapse-free survival of patients
together with other clinical factors, including age, sex, primary
tumor stage (T), N and clinical TNM staging. Survival curves were
constructed using Kaplan-Meier analysis and a log-rank test was
performed to assess differences between the groups. All data were
analyzed with SPSS software (version 20.0; IBM Corp., Armonk, NY,
USA) P<0.05 was considered to indicate a statistically
significant difference.
Results
LncRNA LOC541471 upregulation in HNSCC
and association with perineural invasion
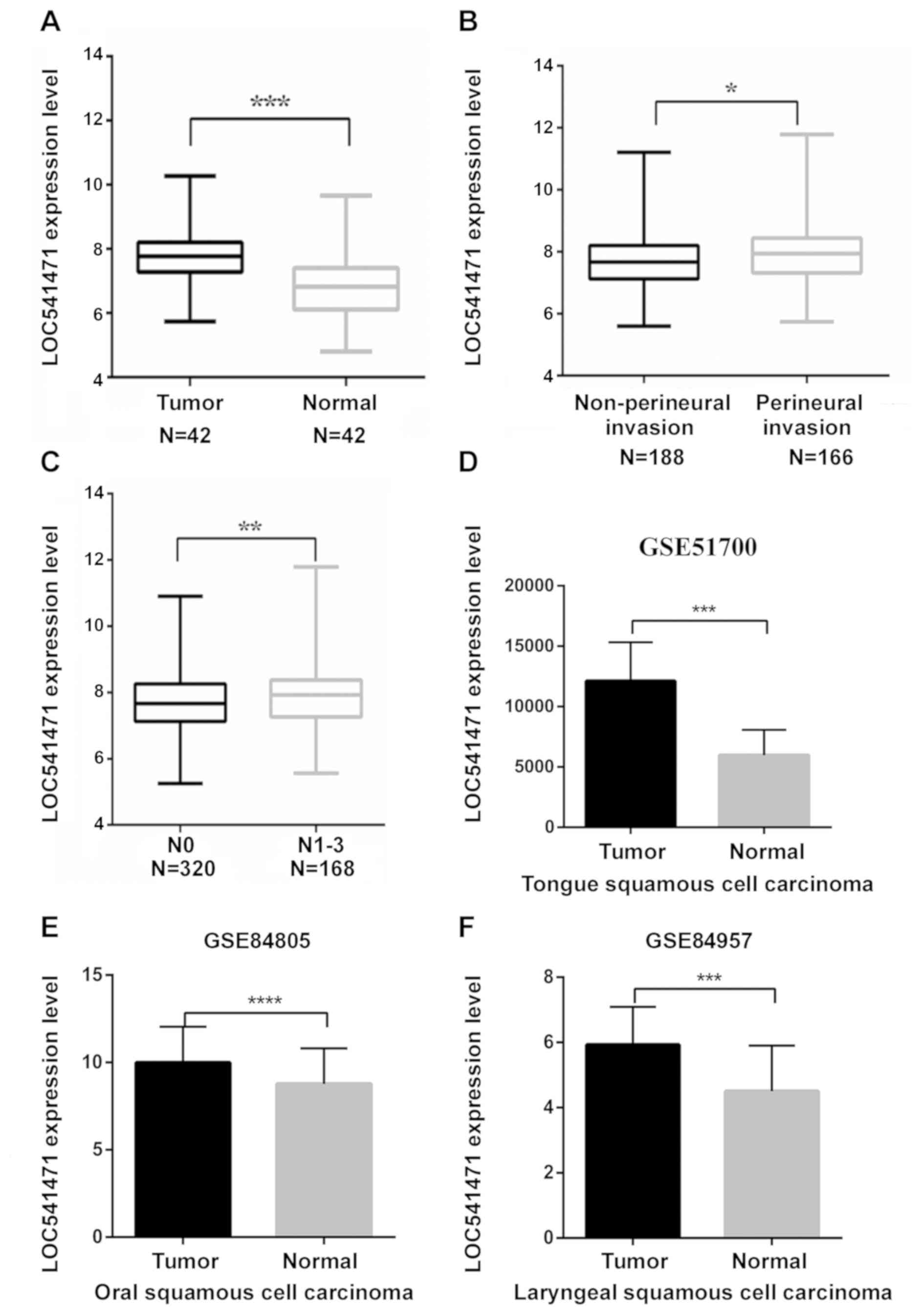
RNA-Seq datasets for 43 tumor-adjacent normal pairs
were downloaded and significant differences in lncRNA expression
were identified in HNSCC samples compared with healthy samples
(Table III). It was revealed that
lncRNA LOC541471 expression was significantly higher in the HNSCC
samples compared with the adjacent healthy samples, as presented in
Fig. 1A. In addition, lncRNA
LOC541471 was identified to be significantly upregulated in
patients with HNSCC and perineural invasion compared with patients
without perineural invasion from TCGA (Fig. 1B). Furthermore, high lncRNA LOC541471
expression was significantly associated with increased N
classification (Fig. 1C). Previously,
studies have revealed that perineural invasion and N classification
are prognostic factors for HNSCC (14,15).
Therefore, the current study analyzed whether lncRNA LOC541471 may
be a biomarker and potential therapeutic target for high-risk
HNSCC. Following clarification, HNSCC gene expression data and
corresponding clinical data were obtained from the GEO database.
The results revealed that lncRNA LOC541471 was significantly
upregulated in tongue squamous cell carcinoma (GSE51700, n=18),
oral squamous cell carcinoma (GSE84805, n=12) and laryngeal
squamous cell carcinoma (GSE84957, n=18), as presented in Fig. 1D-F, respectively.
 | Table III.Dysregulated long non-coding RNAs
identified in head and neck squamous cell carcinoma. |
Table III.
Dysregulated long non-coding RNAs
identified in head and neck squamous cell carcinoma.
| GeneID | Symbol | Fold-change | Style | P-value | FDR |
|---|
| 112597 | LINC00152 | 2.07 | up | 0.0000036 | 0.000182 |
| 606 | NBEAP1 | 2.06 | up | 0.0002079 | 0.00649 |
| 440101 | FLJ12825 | 2.06 | up | 0.0000421 | 0.00165 |
| 29774 | POM121L9P | 2.05 | up | 0.0000261 | 0.00108 |
| 400508 | CRYM-AS1 | 2.05 | up | 0.0000048 | 0.000236 |
| 100128191 | TMPO-AS1 | 2.05 | up | 0.0000005 | 0.0000307 |
| 440081 | DDX12P | 2.04 | up | 0.0000001 | 0.00000747 |
| 3136 | HLA-H | 2.03 | up | 0.0000001 | 0.00000747 |
| 344967 | LOC344967 | 2.02 | up | 0.0000053 | 0.000258 |
| 541471 | LOC541471 | 2.02 | up | 0.0000001 | 0.00000747 |
| 2679 | GGT3P | 2.00 | up | 0.0009594 | 0.0227 |
| 10984 | KCNQ1OT1 | 1.99 | up | 0.0000067 | 0.000319 |
| 114043 | TSPEAR-AS2 | 1.96 | up | 0.0001926 | 0.00608 |
| 3139 | HLA-L | 1.95 | up | 0.0000018 | 0.0000971 |
| 54718 | BTN2A3P | 1.94 | up | 0.0000070 | 0.000332 |
| 145978 | LINC00052 | 1.94 | up | 0.0005988 | 0.0155 |
| 645166 | LOC645166 | 1.94 | up | 0.0000367 | 0.00146 |
| 692099 | FAM86DP | 0.66 | Down | 0.0000353 | 0.00113 |
| 729614 | FLJ37453 | 0.66 | Down | 0.0000694 | 0.00204 |
| 84981 | MIR22HG | 0.65 | down | 0.0011390 | 0.0251 |
| 284593 | FAM41C | 0.65 | down | 0.0004447 | 0.0107 |
| 84852 | ATP1A1-AS1 | 0.64 | down | 0.0000839 | 0.00241 |
| 197187 | SNAI3-AS1 | 0.64 | down | 0.0005752 | 0.0135 |
| 400752 | LINC01144 | 0.64 | down | 0.0015342 | 0.0329 |
| 100093630 | SNHG8 | 0.64 | down | 0.0000012 | 0.000065 |
| 57061 | HYMAI | 0.63 | down | 0.0000991 | 0.00279 |
| 282566 | LINC00515 | 0.63 | down | 0.0000965 | 0.00273 |
| 389741 | GLIDR | 0.63 | down | 0.0000010 | 0.000056 |
| 653162 | RPSAP9 | 0.63 | down | 0.0000372 | 0.00118 |
| 114814 | GNRHR2 | 0.62 | down | 0.0000001 | 0.00000149 |
| 645683 | RPL13AP3 | 0.62 | down | 0.0006937 | 0.016 |
| 84837 | ARHGAP5-AS1 | 0.61 | down | 0.0003332 | 0.00826 |
| 284835 | LINC00323 | 0.61 | down | 0.0011373 | 0.0251 |
| 574036 | SERTAD4-AS1 | 0.61 | down | 0.0014364 | 0.031 |
| 100128782 | LINC00476 | 0.61 | down | 0.0000001 | 0.00000889 |
| 84983 | FAM222A-AS1 | 0.6 | down | 0.0002945 | 0.00739 |
| 387066 | SNHG5 | 0.6 | down | 0.0000029 | 0.000135 |
LncRNA LOC541471 as an independent
predictor of OS and relapse-free survival of patients
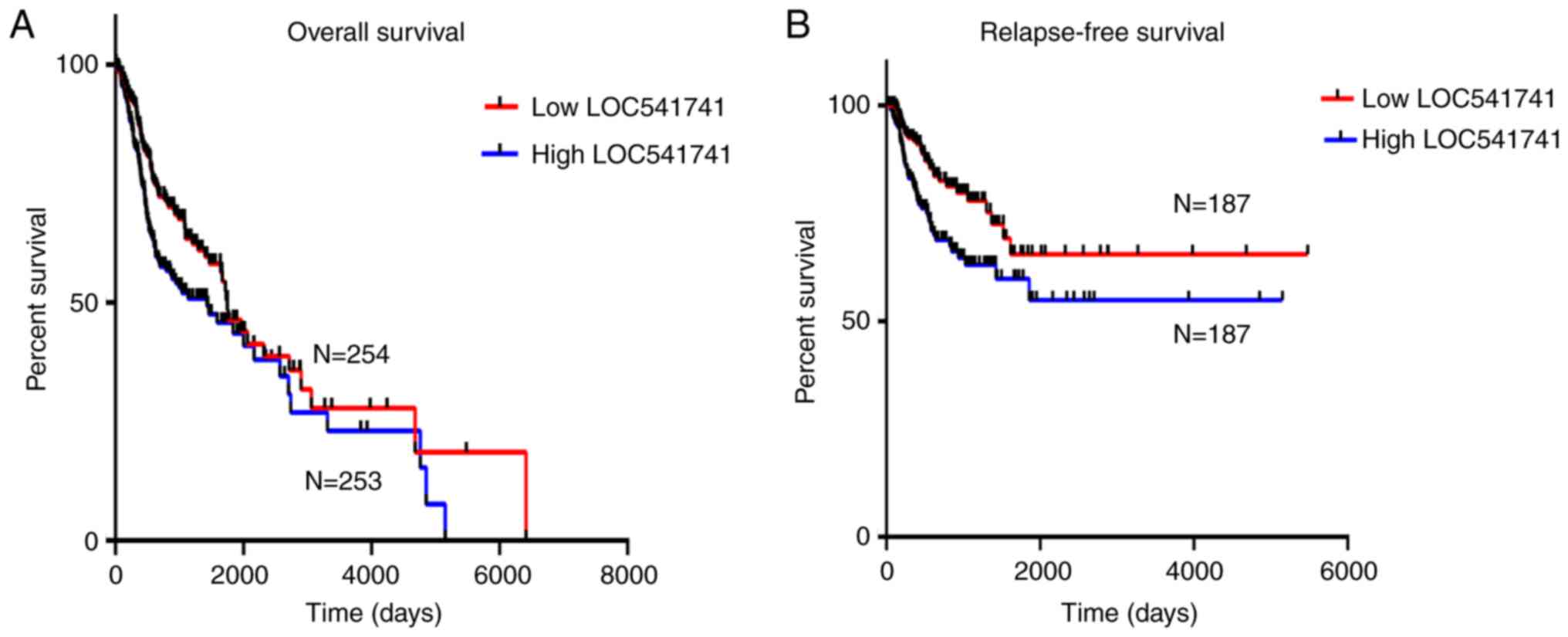
The lncRNA LOC541471 mRNA expression level was
analyzed in cases of HNSCC from TCGA and evaluated for its
prognostic value and potential as a prognostic biomarker for HNSCC.
Kaplan-Meier survival analysis was performed according to the
expression levels of lncRNA LOC541471. The results revealed that
high expression of lncRNA LOC541471 was negatively associated with
poor OS of patients compared with low expression of lncRNA
LOC541471 (Fig. 2A). Univariate Cox
regression analysis identified that lncRNA LOC541471 expression and
age significantly affected OS of patients. Meanwhile, multivariate
Cox regression analysis revealed that high lncRNA LOC541471
expression may be an independent predictor for reduced OS of
patients with HNSCC (Table IV).
Similarly, the results indicated that high lncRNA LOC541471
expression was negatively associated with relapse-free survival of
patients compared with high lncRNA LOC541471 expression (Fig. 2B). A univariate analysis and
multivariate Cox regression analysis also identified that high
lncRNA LOC541471 expression may be an independent predictor for
reduced relapse-free survival (Table
V). These results indicate that lncRNA LOC541471 may be an
independent predictor for OS and relapse-free survival of patients
with HNSCC.
 | Table IV.Univariate and multivariate analysis
of prognostic factors for overall survival using Cox proportional
hazards regression model (n=487). |
Table IV.
Univariate and multivariate analysis
of prognostic factors for overall survival using Cox proportional
hazards regression model (n=487).
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | HR | 95% CI |
|---|
| Age |
|
|
|
|
|
≤60 | 0.722a | 0.541–0.963 | 0.772 | 0.571–1.043 |
|
>60 | 1 |
| 1 |
|
| Sex |
|
|
|
|
|
Male | 0.753 | 0.555–1.023 | 0.801 | 0.583–1.102 |
|
Female | 1 |
| 1 |
|
| Primary tumor
stage |
|
|
|
|
|
T1-2 | 0.798 | 0.586–1.088 | 0.761 | 0.452–1.283 |
|
T3-4 | 1 |
| 1 |
|
| Lymph node
metastasis |
|
|
|
|
|
N0-1 | 0.807 | 0.591–1.101 | 0.753 | 0.533–1.064 |
|
N2-3 | 1 |
| 1 |
|
| Clinical TNM
staging |
|
|
|
|
|
I–II | 0.835 | 0.590–1.180 | 1.159 | 0.624–2.152 |
|
III–IV | 1 |
| 1 |
|
| LOC541471
expression level |
|
|
|
|
|
Low | 0.677a | 0.507–0.904 | 0.722a | 0.539–0.968 |
|
High | 1 |
| 1 |
|
 | Table V.Univariate and multivariate analysis
of prognostic factors for relapse-free survival using Cox
proportional hazards regression model (n=355). |
Table V.
Univariate and multivariate analysis
of prognostic factors for relapse-free survival using Cox
proportional hazards regression model (n=355).
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | HR | 95% CI |
|---|
| Age |
|
|
|
|
|
≤60 | 0.937 | 0.604–1.454 | 0.974 | 0.617–1.536 |
|
>60 | 1 |
| 1 |
|
| Sex |
|
|
|
|
|
Male | 1.026 | 0.619–1.703 | 1.075 | 0.637–1.814 |
|
Female | 1 |
| 1 |
|
| Primary tumor
stage |
|
|
|
|
|
T1-2 | 0.956 | 0.603–1.518 | 0.836 | 0.425–1.642 |
|
T3-4 | 1 |
| 1 |
|
| Lymph node
metastasis |
|
|
|
|
|
N0-1 | 0.685 | 0.437–1.072 | 0.626 | 0.375–1.047 |
|
N2-3 | 1 |
| 1 |
|
| Clinical TNM
staging |
|
|
|
|
|
I–II | 1 | 0.585–1.712 | 1.462 | 0.620–3.447 |
|
III–IV | 1 |
| 1 |
|
| LOC541471
expression level |
|
|
|
|
|
Low | 0.563a | 0.360–0.881 | 0.574a | 0.365–0.904 |
|
High | 1 |
| 1 |
|
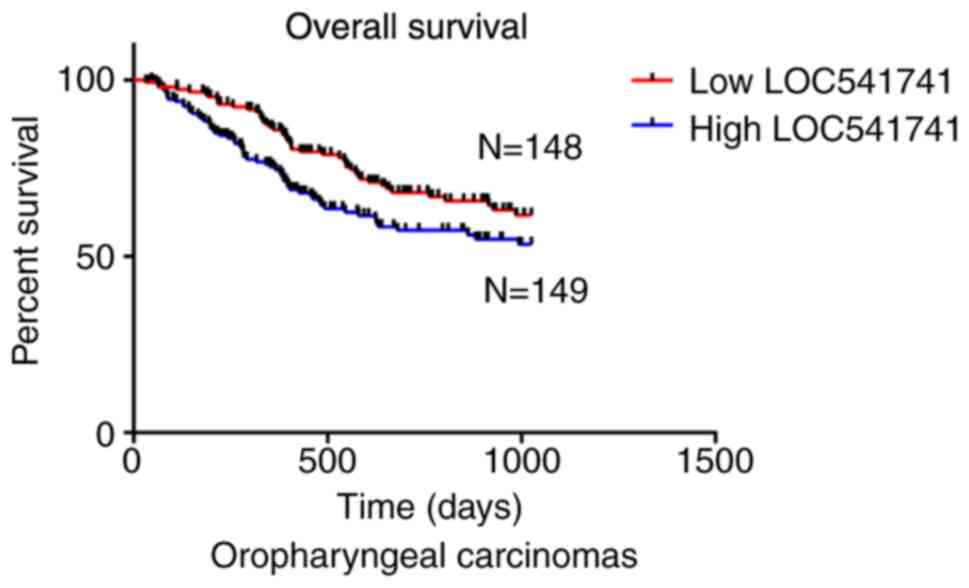
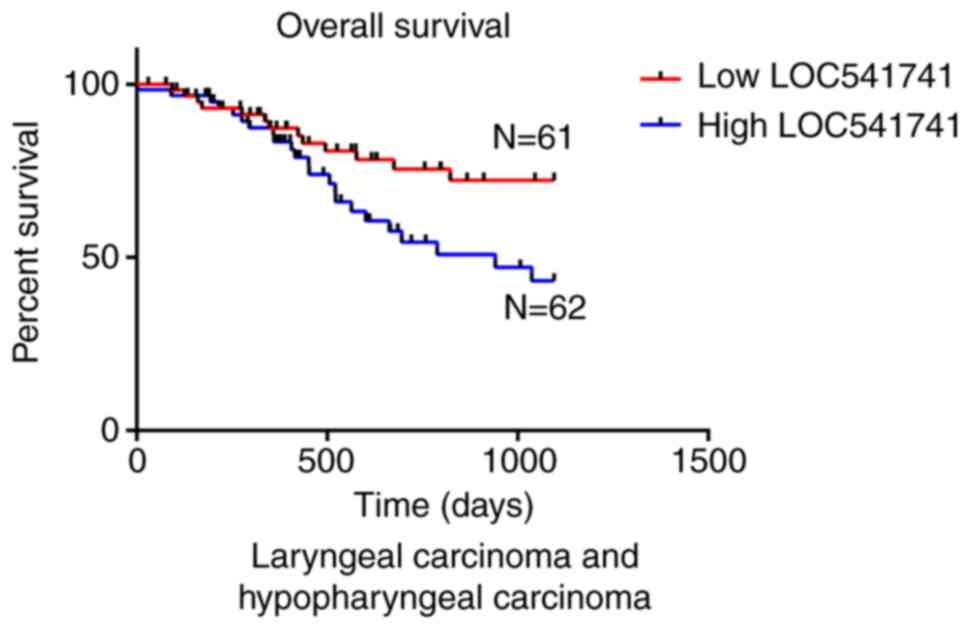
Significant association of lncRNA LOC541471 with
patient survival in subtypes of HNSCC. According to the anatomic
neoplasm subdivision, 500 HNSCC samples from TCGA were classified
as oropharyngeal, oral, laryngeal and hypopharyngeal carcinomas. On
the basis of lncRNA LOC541471 expression levels, Kaplan-Meier
survival analysis was performed for each subtype. The results
revealed that high lncRNA LOC541471 expression was associated with
poor OS compared with low lncRNA LOC541471 expression for all
classified subtypes of HNSCC (Figs.
3–5).
Discussion
Recent studies have revealed that up to 70% of the
mammalian transcriptome is transcribed into lncRNAs, while coding
transcripts only account for 2% of the genome (16,17).
Previously, lncRNAs have been regarded as nonfunctional transcripts
due to uncertainty of their function (17,18).
However, recent studies have identified that lncRNAs serve an
important role in tumor initiation and progression (19,20). In
addition, previous studies have revealed that lncRNAs serve as
vital participants in tumor cell proliferation, differentiation and
apoptosis (9,21). Furthermore, lncRNAs exhibit structural
stereospecificity and tissue selectivity (5,22).
Therefore, dysregulated expression of lncRNAs has been identified
as a biological marker in various cancer types. The lncRNAs,
MALAT-1 and thymosin β-4, predict metastasis and survival in
early-stage non-small cell lung cancer (23). The current study demonstrated that
lncRNA LOC541471 expression was upregulated in tumor tissues and
positively associated with increasing N classification and
perineural invasion in patients with HNSCC. Previously, studies
have revealed that perineural invasion and N classification are
prognostic factors for HNSCC (15,24).
Therefore, the current study analyzed the potential of lncRNA
LOC541471 as a prognostic biomarker for HNSCC.
To further validate the hypothesis that lncRNA
LOC541471 is a prognostic biomarker for HNSCC, clinical
characteristics were downloaded from TCGA and analyzed. It was
revealed that upregulation of lncRNA LOC541471 was associated with
poor OS and poor relapse-free survival of patients. In addition, a
multivariate Cox regression analysis revealed that lncRNA LOC541471
expression levels may be an independent predictor for the OS and
relapse-free survival of patients. To determine whether lncRNA
LOC541471 has similar prognostic potential in various subtypes of
HNSCC, a total of 500 samples were classified as oropharyngeal,
oral, laryngeal and hypopharyngeal. It was identified that
upregulation of lncRNA LOC541471 was associated with poor survival
outcomes in all subtypes of HNSCC studied. Therefore, it is
proposed that lncRNA LOC541471 may have sufficient reliability and
validity to serve as a prognostic biomarker for HNSCC.
The current study has indicated that LOC541471 may
be considered an unfavorable prognostic factor for HNSCC. However,
the specific mechanism by which LOC541471 participates in HNSCC
development requires further investigation. Notably, lncRNAs can
target RNA-binding proteins (RBPs) or competing endogenous RNA
(ceRNA) (25). In future studies, the
specific function of LOC541471, including targeting RBPs or ceRNA,
may be investigated in vitro or in vivo. Identifying
known genes located close to lncRNA could provide a basis for
understanding its mechanism (26).
Therefore, known genes located close to LOC541741 were analyzed
using the University of California, Santa Cruz (UCSC) genome
browser (http://genome.ucsc.edu). A selection of
these analysis results is provided in Table VI. Several of these genes have
previously been identified to be associated with tumors. Low
budding uninhibited by benzimidazoles 1 (BUB1) expression is an
adverse prognostic marker in gastric adenocarcinoma. Tumors with
low BUB1 expression have been associated with larger tumor size
(27). Bcl-2-like protein 11 belongs
to the Bcl-2 family, and acts as a central regulator of the
intrinsic apoptotic cascade and mediates cell apoptosis (28). Proto-oncogene tyrosine-protein kinase
MER inhibition combined with radiation therapy has a therapeutic
effect in a subset of glioblastoma (29).
 | Table VI.A selection of known genes located
close to LOC541741. |
Table VI.
A selection of known genes located
close to LOC541741.
|
Ensembl_Gene_ID | Gene symbol | Gene_biotype |
|---|
|
ENSG00000256671 | LIMS4 | protein_coding |
|
ENSG00000183054 | RGPD6 | protein_coding |
|
ENSG00000169679 | BUB1 | protein_coding |
|
ENSG00000153093 | ACOXL | protein_coding |
|
ENSG00000153094 | BCL2L11 | protein_coding |
|
ENSG00000153107 | ANAPC1 | protein_coding |
|
ENSG00000153208 | MERTK | protein_coding |
|
ENSG00000153214 | TMEM87B | protein_coding |
|
ENSG00000144152 | FBLN7 | protein_coding |
|
ENSG00000144161 | ZC3H8 | protein_coding |
|
ENSG00000235881 | AC114776.1 | lincRNA |
|
ENSG00000236330 | RPL5P9 |
processed_pseudogene |
|
ENSG00000204581 | ACOXL-AS1 | antisense_RNA |
|
ENSG00000230499 | AC108463.2 | lincRNA |
|
ENSG00000263881 | MIR4436B2 | miRNA |
|
ENSG00000266139 | MIR4435-2 | miRNA |
|
ENSG00000266063 | MIR4771-2 | miRNA |
The current study preliminarily concludes that
lncRNA LOC541471 is significantly associated with OS and
relapse-free survival of patients and may be considered a potential
unfavorable prognostic factor for HNSCC. However, information
regarding the function of lncRNA LOC541471 is limited. Therefore,
further studies are necessary to determine the involvement of
lncRNA LOC541471 in mediating tumor biology.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81774266).
Availability of data and materials
All the data and materials used in the present study
are freely accessible in GEO (https://www.ncbi.nlm.nih.gov/geo/) and TCGA
(https://cancergenome.nih.gov/).
Authors' contributions
HW performed the statistical analysis and wrote the
manuscript. DY performed the statistical analysis. MW and TH
designed the research, collected the data, and submitted the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
TCGA
|
The Cancer Genome Atlas
|
|
lncRNA
|
long non-coding RNA
|
References
|
1
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nohata N, Abba MC and Gutkind JS:
Unraveling the oral cancer lncRNAome: Identification of novel
lncRNAs associated with malignant progression and HPV infection.
Oral Oncol. 59:58–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hammerman PS, Hayes DN and Grandis JR:
Therapeutic insights from genomic studies of head and neck squamous
cell carcinomas. Cancer Discov. 5:239–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandra Gupta S and Nandan Tripathi Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortez VS, Cervantes-Barragan L, Robinette
ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E,
Sun JC, et al: Transforming growth factor-β signaling guides the
differentiation of innate lymphoid cells in salivary glands.
Immunity. 44:1127–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ni W, Song E, Gong M, Li Y, Yao J and An
R: Downregulation of lncRNA SDPR-AS is associated with poor
prognosis in renal cell carcinoma. Onco Targets Ther. 10:3039–3047.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Y, Shi H, Ren F, Jia Y and Zhang R:
Long non-coding RNA CCAT1 promotes metastasis and poor prognosis in
epithelial ovarian cancer. Exp Cell Res. 359:185–194. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi P, Zhou XY and Du X: Circulating long
non-coding RNAs in cancer: Current status and future perspectives.
Mol Cancer. 15:392016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fenner A: Prostate cancer: PCA3 as a grade
reclassification predictor. Nat Rev Urol. 14:3902017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sacco AG and Cohen EE: Current treatment
options for recurrent or metastatic head and neck squamous cell
carcinoma. J Clin Oncol. 33:3305–3313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao W, Liu JN, Liu Z, Wang X, Han ZG, Ji
T, Chen WT and Zou X: A three-lncRNA signature derived from the
Atlas of ncRNA in cancer (TANRIC) database predicts the survival of
patients with head and neck squamous cell carcinoma. Oral Oncol.
65:94–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyzas PA, Loizou KT and Ioannidis JP:
Selective reporting biases in cancer prognostic factor studies. J
Natl Cancer Inst. 97:1043–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunne AA, Müller HH, Eisele DW, Kessel K,
Moll R and Werner JA: Meta-analysis of the prognostic significance
of perinodal spread in head and neck squamous cell carcinomas
(HNSCC) patients. Eur J Cancer. 42:1863–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu S, Wang P, You Z, Meng H, Mu G, Bai X,
Zhang G, Zhang J and Pang D: The long non-coding RNA EPB41L4A-AS2
inhibits tumor proliferation and is associated with favorable
prognoses in breast cancer and other solid tumors. Oncotarget.
7:20704–20717. 2016.PubMed/NCBI
|
|
17
|
Zou AE, Zheng H, Saad MA, Rahimy M, Ku J,
Kuo SZ, Honda TK, Wang-Rodriguez J, Xuan Y, Korrapati A, et al: The
non-coding landscape of head and neck squamous cell carcinoma.
Oncotarget. 7:51211–51222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu JW, Kong Y and Sun X: Long noncoding
RNA NEAT1 is an unfavorable prognostic factor and regulates
migration and invasion in gastric cancer. J Cancer Res Clin Oncol.
142:1571–1579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Lena PG, Paz-Gallardo A, Paramio JM and
Garcia-Escudero R: Clusterization in head and neck squamous
carcinomas based on lncRNA expression: Molecular and clinical
correlates. Clin Epigenetics. 9:362017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Salvo TG: Evolving targeted therapies
for right ventricular failure. Expert Opin Biol Ther. 15:1263–1283.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji P, Diederichs S, Wang W, Boing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fanelli MF, Oliveira TB, Braun AC, Corassa
M, Abdallah EA, Nicolau UR, da Silva Alves V, Garcia D, Calsavara
VF, Kowalski LP and Chinen LTD: Evaluation of incidence,
significance, and prognostic role of circulating tumor microemboli
and transforming growth factor-β receptor I in head and neck
cancer. Head Neck. 39:2283–2292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang T, Huang W, Lu H, Zhang BY, Ma J,
Zhao D, Wang YJ, Yu DH and He X: Identification and validation a
TGF-β-associated long non-coding RNA of head and neck squamous cell
carcinoma by bioinformatics method. J Transl Med. 16:462018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du M, Huang T, Wu J, Gu JJ, Zhang N, Ding
K, Qian LX, Lu ZW, Zhang WJ, Tian XK, et al: Long non-coding RNA
n326322 promotes the proliferation and invasion in nasopharyngeal
carcinoma. Oncotarget. 9:1843–1851. 2017.PubMed/NCBI
|
|
27
|
Stahl D, Braun M, Gentles AJ, Lingohr P,
Walter A, Kristiansen G and Gütgemann I: Low BUB1 expression is an
adverse prognostic marker in gastric adenocarcinoma. Oncotarget.
8:76329–76339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Duan J, Qu Y, Deng T, Liu R,
Zhang L, Bai M, Li J, Ning T, Ge S, et al: Onco-miR-24 regulates
cell growth and apoptosis by targeting BCL2L11 in gastric cancer.
Protein Cell. 7:141–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Frady LN, Bash RE, Cohen SM,
Schorzman AN, Su YT, Irvin DM, Zamboni WC, Wang X, Frye SV, et al:
MerTK as a therapeutic target in glioblastoma. Neuro Oncol.
20:92–102. 2018. View Article : Google Scholar : PubMed/NCBI
|