Introduction
Colorectal carcinoma (CRC) is a prevalent
gastrointestinal malignancy, and its morbidity ranks only second to
gastric and esophageal cancer (1).
The ranking of CRC patients was fifth in males and sixth in females
in the most common malignant tumor deaths in China (2). CRC is second only to lung cancer in
western developed countries, whereas its rate of morbidity in
different countries differs by almost 60 times (3). So far, the incidence of CRC is still
increasing, although various early detection methods and treatments
have made great progress. Consequently, developing a new biomarker
for diagnosis and clarifying the regulatory mechanism of the
occurrence and progression of CRC tumors are essential.
MicroRNAs (miRNAs) regulate target gene expression
through combining transcriptional mRNA (4). A previous study pointed out that miRNAs
took part in multiple cancer-related signaling pathways including
cell migration, invasion, proli-feration, apoptosis, and metastasis
(5). Importantly, miRNAs were found
to express abnormally which was relevant to CRC development. Among
them, miR-17, miR-21, miR-182 and miR-203 acted as oncogenes in
CRC, while miR-30a, miR-143, miR-145 and miR-195 were considered as
tumor suppressors (6,7). Recently, many scholars have investigated
the role of miR-140 in the process of tumor formation in various
cancers, such as glioma (8), cervical
cancer (9), gastric cancer (10) and breast cancer (11). Moreover, miR-140 has been considered
to be involved in colorectal tumorigenesis and progression through
regulating proliferation, apoptosis, differentiation, migration and
invasion (12–14). However, there are no previous studies
on the function of miR-140/SOX4 axis in CRC.
Sex-determining region Y-related high-mobility group
box 4 (SOX4) plays a role in cancer development. Moreover, SOX4 was
observed in many cancers regulated by miR-212 (15), miR-338 (16), miR-25 (17), and miR-132 (18). Vishnubalaji et al revealed that
miRNA-320 suppressed CRC by targeting SOX4, FOXM1, and FOXQ1
(19). In this study, miR-140-5p
expression and its clinicopathological significance in CRC were
investigated. Furthermore, the role of miR-140-5p was analyzed from
the perspective of cell proliferation and invasion in CRC at the
same time. SOX4 directly targeted miR-140-5p. This study aimed at
providing new therapeutic implications for the diagnosis of
CRC.
Materials and methods
Clinical tissues
Thirty-six surgical tumor specimens and adjacent
tissue samples were obtained from Shandong Provincial Third
Hospital (Jinan, Shandong) after receiving written informed
consent. None of the patients received treatment prior to the
operation. Human tissue was frozen in liquid nitrogen and then
stored at −80°C in a refrigerator for further use. This experiment
was approved by the Institutional Ethics Committee of Shandong
Provincial Third Hospital.
Cell culture and transfection
The human CRC cell lines HT29 (cat. no. HTB-38),
SW480 [ZK0200(XR)] and normal colorectal cell line NCM460 (cat. no.
BNF-3068) were used for this experiment. All the cell lines were
obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). All cells were
seeded in DMEM supplemented by 10% fetal bovine serum (FBS) and
cultured at 37°C with 5% CO2.
The miR-140-5p mimic and inhibitor, SOX4 siRNA
(si-SOX4) were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China) and then they were transferred into HT29 or
SW480 cells with Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer's instructions.
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied for extracting total RNA containing
miRNA to quantitate the miR-140-5p expression in CRC tissues and
cell lines. RT-qPCR was carried out through the SYBR-Green PCR kit
(Takara Bio, Inc., Otsu, Japan) on ABI 7500 Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster
City, CA, USA). The reaction conditions were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. U6 and
GAPDH were used as control for miR-140-5p and SOX4. The miR-145-5p
and TAGLN2 levels were analyzed using the 2−ΔΔCq method
(20).
Luciferase activity assay
TargetScan (http://www.targetscan.org/) was employed to predict
the biological targets of miRNAs (21). The wild or mutant type of 3′-UTR of
SOX4 was inserted into the pGL3 promoter vector (Invitrogen; Thermo
Fisher Scientific, Inc.) for luciferase reporter experiments. Then,
wild or mutant type of 3′-UTR of SOX4 and miR-140-5p mimic were
transfected into SW480 cells. After transfection for 48 h, the
Dual-Luciferase Reporter Assay (Promega Corp., Madison, WI, USA)
was applied to perform luciferase assays.
Cell proliferation (MTT) assay
The MTT assay was applied to measure cell
proliferation. Cells (2×103) were seeded onto 96-well
plates in medium containing 10% FBS. The cells containing
miR-140-5p mimic or inhibitor were incubated for 0–96 h. After
incubation, the cells added with MTT (Sigma-Aldrich; Merck KGaA,
St. Louis, MO, USA) were incubated for 4 h at 37°C. The absorbance
at 490 nm (OD=490 nm) was detected with a spectrophotometer
(Molecular Devices LLC., San Jose, CA, USA).
Cell invasion assay
Transwell assay was performed to measure cell
invasion. The cells were planted into the upper chambers (8 µm pore
size; Corning, Inc., Corning, NY, USA) and medium with 10% FBS was
added into the lower chamber. The cells were incubated for 24 h at
37°C in 5% CO2. Then the invasive cells on the lower
surface were fixed with 70% ethanol and stained with crystal violet
stain. Cells were counted by a light microscope (Olympus
Corporation, Tokyo, Japan).
Western blot analysis
The protein samples were obtained using RIPA lysis
buffer. Protein concentration was calculated using bicinchoninic
acid (BCA; Beyotime Institute of Biotechnology, Shanghai, China).
The 25 µl protein sample was added in the protein loaded per lane.
Proteins were separated through a 12% SDS-PAGE and then incubated
with 5% non-fat milk blocked membranes at room temperature for 2 h.
Next we incubated the membranes overnight at 4°C with anti-SOX4
rabbit polyclonal antibody (dilution, 1:1,000; cat. no. ab80261;
Abcam, Cambridge, MA, USA) anti-GAPDH mouse monoclonal antibody
(dilution, 1:1,000; cat. no. 60004-1-Ig; ProteinTech, Wuhan, China)
and subsequently incubated with goat anti-rabbit IgG H&L (HRP)
(dilution, 1:3,000; cat. no. ab6721; Abcam) secondary antibody.
Then, the protein expression levels were measured by a
chemiluminescent detection system (Pierce ECL Substrate Western
Blot Detection System; Pierce; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Protein expression levels were quantified using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The obtained data were shown as the mean ± SD.
Enumeration data were calculated according to ANOVA with
Tukey-Kramer post hoc test and χ2 test. Statistical
analysis was analyzed with GraphPad Prism 6.0 and SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). It was defined as
significant at P<0.05.
Results
Downregulation of miR-140-5p was
observed in CRC
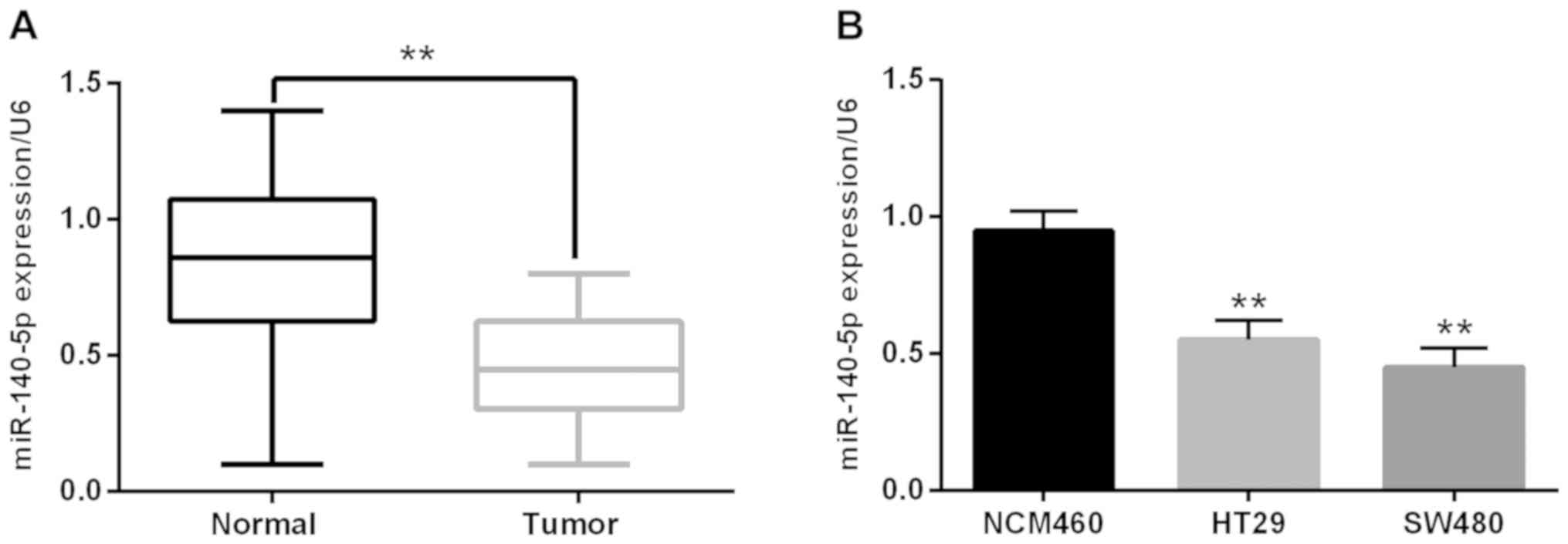
The miR-140-5p expression was detected to verify its
role in CRC carcinogenesis. Firstly, we analyzed the relationship
between the clinicopathological characteristics and aberrant
expression of miR-140-5p. The miR-140-5p downregulation was closely
related to tumor staging or metastasis (Table I). Additionally, the RT-qPCR
experiment suggested that the miR-140-5p expression was noticeably
declined in CRC tissues in contrast to the adjacent normal tissues
(Fig. 1A). Furthermore, we also found
that miR-140-5p expression was much lower in HT29 and SW480 cell
lines than that of NCM460 cells (control) (Fig. 1B). In brief, all results proved that
the miR-140-5p downregulation was related to carcinogenesis of
CRC.
 | Table I.Clinicopathological characteristics
and miR-140-5p expression in CRC. |
Table I.
Clinicopathological characteristics
and miR-140-5p expression in CRC.
|
|
| miR-140
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Cases (n=36) | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.3173 |
| ≥60 | 20 | 8 | 12 |
|
|
<60 | 16 | 6 | 10 |
|
| Tumor size (cm) |
|
|
| 0.6276 |
| ≥5 | 19 | 9 | 10 |
|
|
<5 | 17 | 8 | 9 |
|
| Sex |
|
|
| 0.1213 |
| Male | 21 | 9 | 12 |
|
|
Female | 15 | 6 | 9 |
|
| TNM stage |
|
|
| 0.0005a |
| I+II | 24 | 7 | 17 |
|
|
III+IV | 12 | 5 | 7 |
|
| Lymph node
metastasis |
|
|
| 0.005a |
| Yes | 23 | 6 | 17 |
|
| No | 13 | 3 | 10 |
|
miR-140-5p inhibits CRC cell
proliferation and invasion
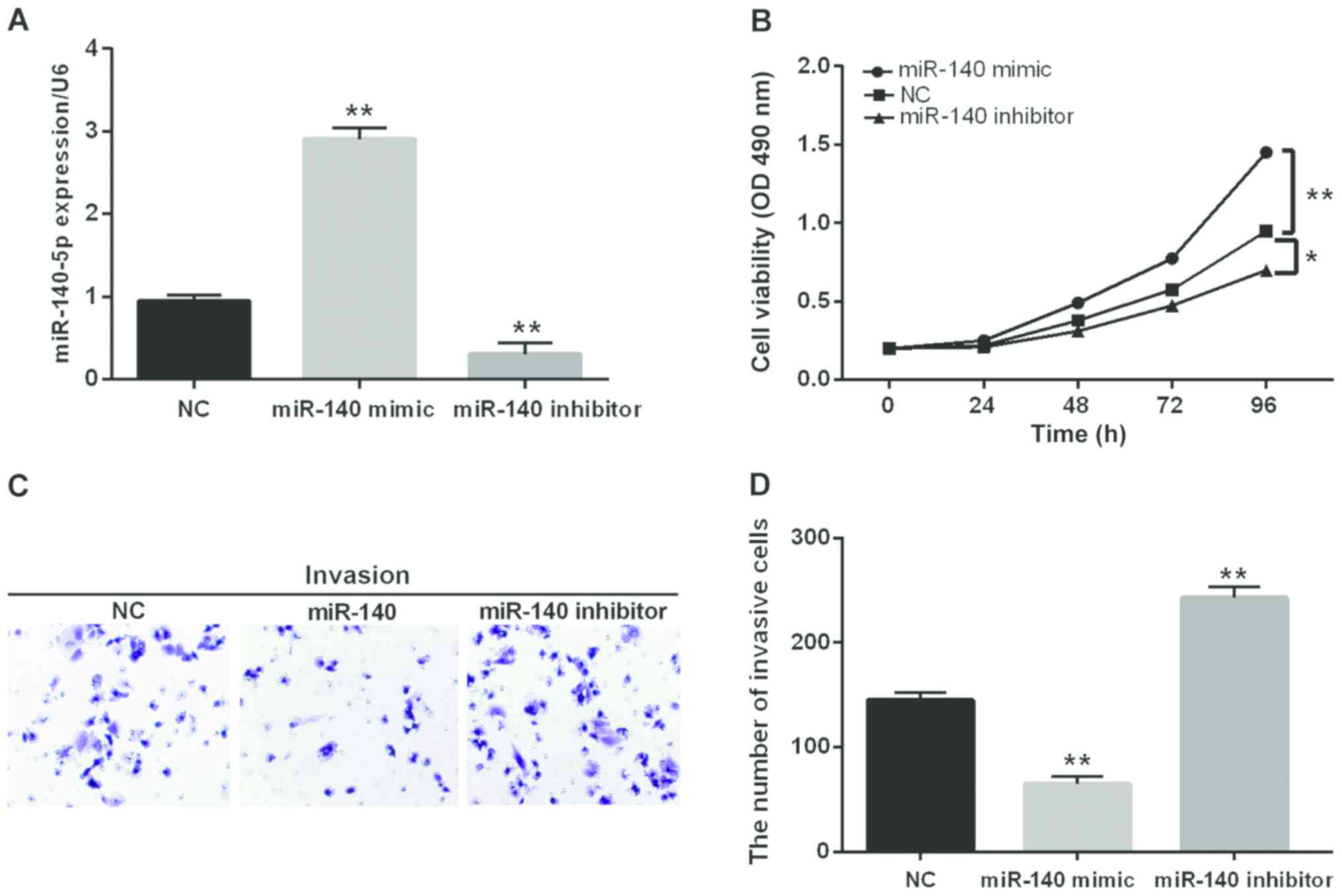
Then, we transfected miR-140-5p mimic or inhibitor
into SW480 cells to further confirm its function in CRC
progression. The transfection efficiency was detected through
RT-qPCR (Fig. 2A). Additionally, the
cell proliferation and invasion in transfected cells was
investigated by MTT and Transwell assay. The MTT analysis revealed
that miR-140-5p overexpression obviously inhibited cell
proliferation while the opposite effect was found in cells blocking
miR-140-5p (Fig. 2B). Similarly,
miR-140-5p overexpression markedly inhibited cell invasion while
blocking miR-140-5p inversely regulated cell invasion (Fig. 2C and D). From the above results, we
speculated that miR-140-5p had an inhibitory effect on CRC.
miR-140-5p directly targets SOX4 in
CRC cells
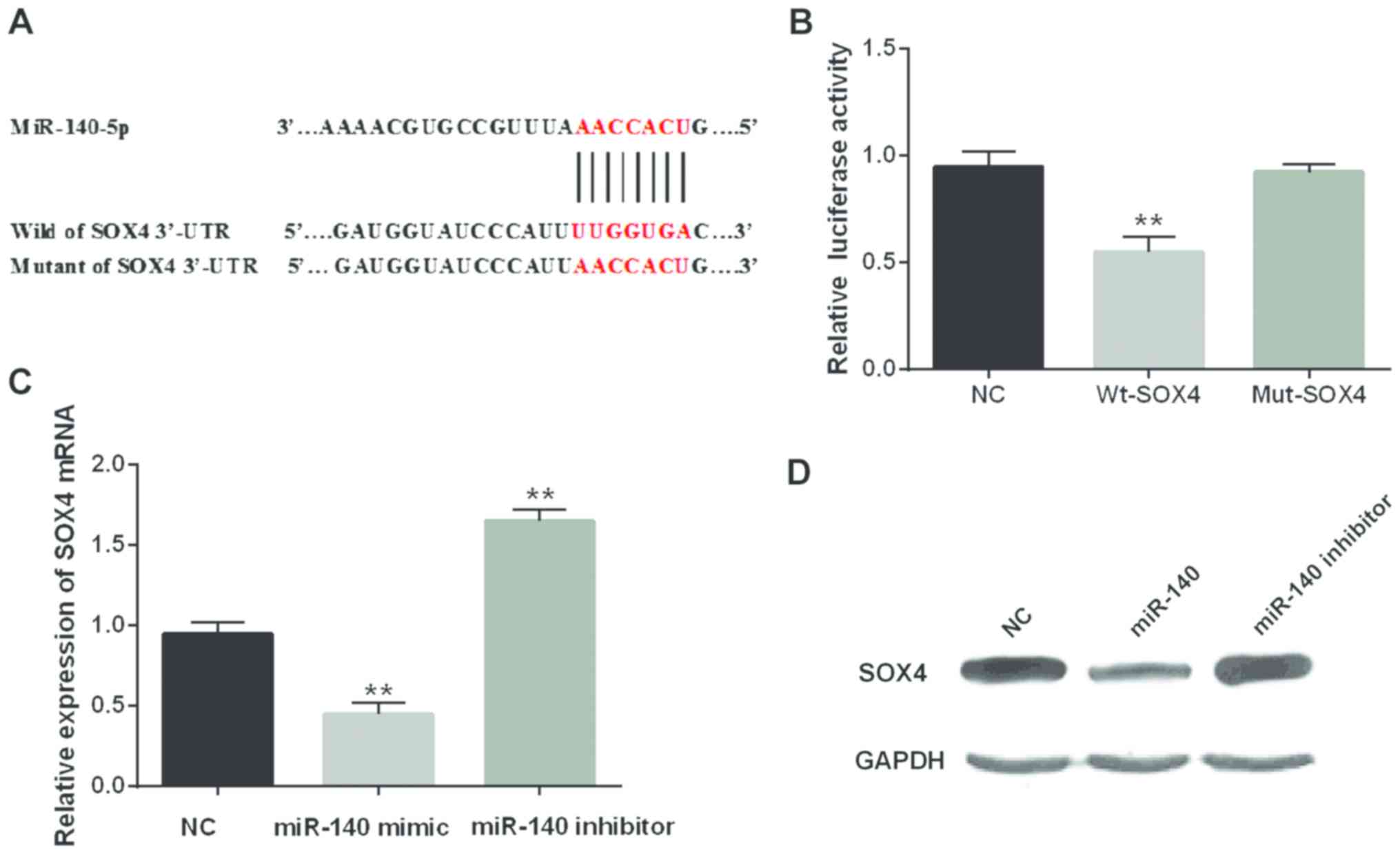
As a target gene, SOX4 was analyzed to elucidate the
regulated mechanisms in CRC. Among them, SOX4 as an oncogene
demonstrated that overexpression of SOX4 was strongly correlated
with the malignant diffusion of tumor in CRC (22). We presumed that miR-140-5p targeted
SOX4 (Fig. 3A). In order to verify
the above conclusion, we transfected Wt-SOX4 or Mut-SOX4 into SW480
cells containing miR-140-5p mimic. We found that miR-140-5p
overexpression obviously reduced the luciferase activity of Wt-SOX4
while there was almost no change in Mut-SOX4 (Fig. 3B). In addition, the overexpression of
miR-140-5p reduced the expression of mRNA and protein of SOX4.
Conversely, the knockout of miR-140-5p promoted the SOX4 expression
(Fig. 3C and D). Hence, miR-140-5p
would directly target SOX4.
miR-140-5p regulates the progression
of CRC through affecting SOX4 expression
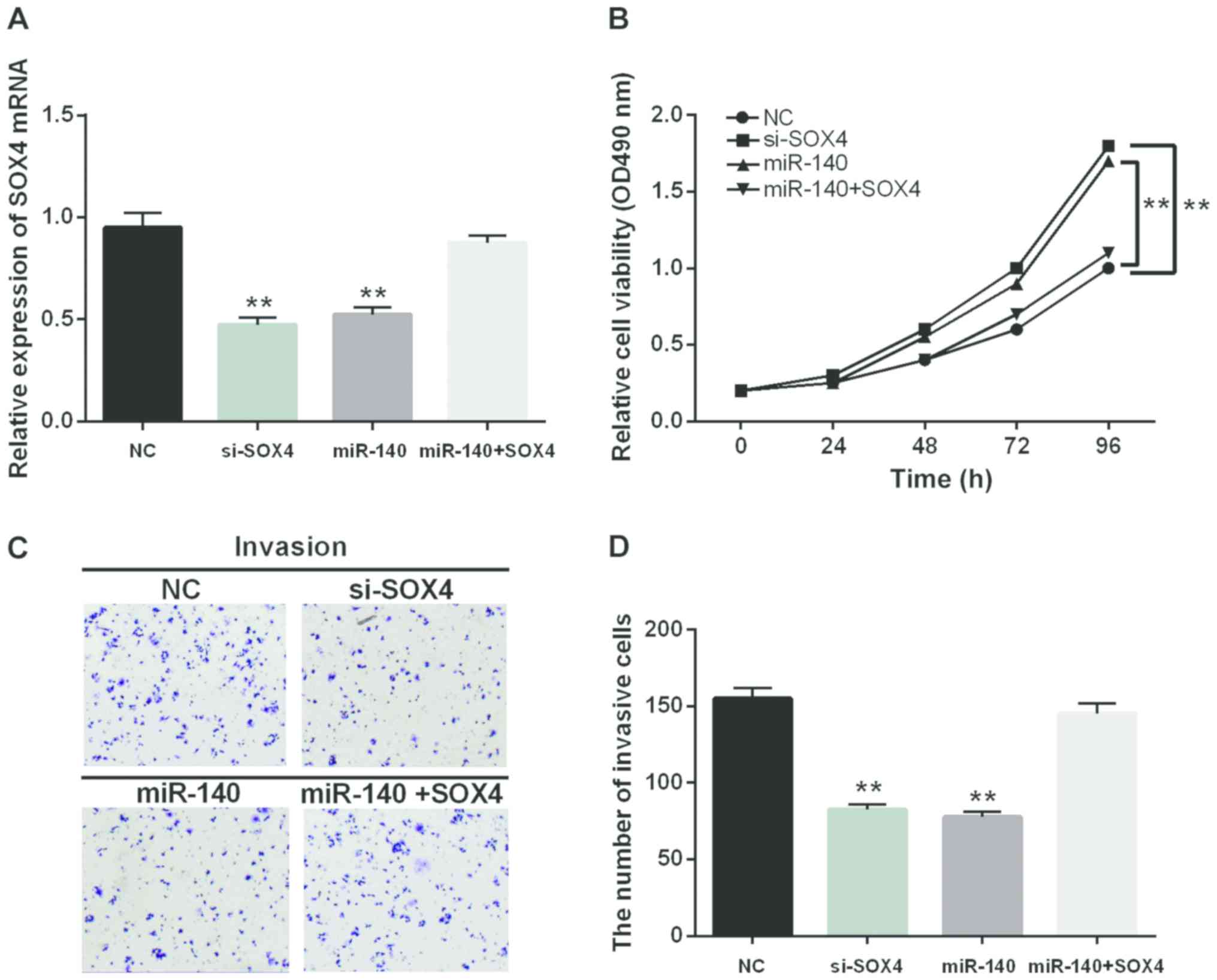
For better understanding the interaction between
miR-140-5p and SOX4, the si-SOX4 was applied to suppress the
expression of SOX4 so as to identify its function in CRC. Moreover,
the negative control or SOX4 expression vector was transfected into
SW480 cells, which overexpressed miR-140-5p to further explore
their function. The expression of SOX4 in the transfected cells
were measured by RT-qPCR (Fig. 4A).
Consistent with the expected result, the decrease of SOX4
expression suppressed cell proliferation and invasion (Fig. 4B-D). A similar result was identified
in the cells containing miR-140-5p mimic as well. Furthermore, the
SOX4 overexpression could conversely change the inhibition of cell
proliferation and invasion induced by miR-140-5p (Fig. 4B-D). Thus, miR-140-5p inhibited the
proliferation and invasion of CRC cells by regulating SOX4
expression.
Discussion
CRC patients still suffer a diagnosis in late stage
and a high mortality, although the treatment for CRC has made great
progress. Hence, exploiting novel biomarkers and applying them to
early diagnosis, as well as improved cure rate will be the main
tasks for us in the future. Moreover, CRC tumors occur through
multiple steps to activate the oncogene or inactivate tumor
suppressor that impact different regulatory pathways in CRC tumors
(14). Opportunely, miRNAs have been
found to take part in various biological processes in different
tumors, which regulate mRNA stability or prevent mRNA translation
by combining with its target gene (23). In addition, miRNA has been observed as
an oncogene or a suppressor in previous studies. Among them, the
different roles of miR-140-5p were identified in various cancers
(8–12). Nevertheless, there were no sufficient
investigations about the effect of miR-140-5p on the proliferation
and invasion of CRC cells.
In this study, miR-140-5p inhibited the
proliferation and invasion of CRC cells. In addition, the decreased
expression of miR-140-5p was found in CRC which was consistent with
other cancers (8–11). It proved that miR-140-5p had an
inhibiting effect on tumor progression. Furthermore, we confirmed
that low miR-140-5p expression was closely related to advanced
clinical stage and lymph node metastasis which was similar to the
previous study (12). These
experimental results indicated that overexpression of miR-140-5p
suppressed the tumorige-nesis and progression of CRC.
In addition, we paid more attention to SOX4 based on
the prediction software databases in CRC. Previous studies reported
that abnormal expression of many transcription factors, such as
CBFB, SMARCC1 and SOX4, were all related to the occurrence of CRC
(24). In this study, we silenced the
SOX4 gene in SW480 cells and further explored its function in CRC
progression. We found that the SOX4 overexpression promoted cell
proliferation and invasion in CRC. The same result for cell
proliferation and invasion was identified in esophageal tumor as
well (25). Moreover, the SOX4
overexpression has been found to enhance cell migrated and invasive
abilities in renal cell carcinoma (26). Some research indicated that SOX4 could
accelerate malignant progression of CRC. Moreover, the interactions
of miR-140-5p and SOX4 were investigated thoroughly in this study
and miR-140-5p overexpression inversely regulated the SOX4
expression. Therefore, the miR-140-5p/SOX4 axis was considered to
provide an effective biomarker for CRC diagnosis.
In conclusion, this study first revealed that
miR-140-5p inhibited the SOX4 expression and contributed to the
proli-feration and invasion of CRC cells. The miR-140-5p/SOX4 axis
may provide new therapeutic implications for CRC. miR-140-5p
performed as a tumor suppressor through downregulating SOX4 which
can be developed as an effective therapeutic path for the treatment
of CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ contributed significantly to the data analysis
and wrote the manuscript. WL performed the data analyses. JL
contributed to the conception of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Provincial Third Hospital (Jinan, Shandong). Signed
informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng ZX, Zheng RS, Zhang SW and Chen WQ:
Colorectal cancer incidence and mortality in China, 2010. Asian Pac
J Cancer Prev. 15:8455–8460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harris TJ and McCormick F: The molecular
pathology of cancer. Nat Rev Clin Oncol. 7:251–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng WJ, Yang L, Ma Q, Zhang H, Adell G,
Arbman G, Wang ZQ, Li Y, Zhou ZG and Sun XF: MicroRNA expression
profile reveals miR-17-92 and miR-143-145 cluster in synchronous
colorectal cancer. Medicine (Baltimore). 94:e12972015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun G, Cheng YW, Lai L, Huang TC, Wang J,
Wu X, Wang Y, Huang Y, Wang J, Zhang K, et al: Signature miRNAs in
colorectal cancers were revealed using a bias reduction small RNA
deep sequencing protocol. Oncotarget. 7:3857–3872. 2016.PubMed/NCBI
|
|
8
|
Hu Y, Li Y, Wu C, Zhou L, Han X, Wang Q,
Xie X, Zhou Y and Du Z: MicroRNA-140-5p inhibits cell proliferation
and invasion by regulating VEGFA/MMP2 signaling in glioma. Tumour
Biol. 39:10104283176975582017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su Y, Xiong J, Hu J, Wei X, Zhang X and
Rao L: MicroRNA-140-5p targets insulin like growth factor 2 mRNA
binding protein 1 (IGF2BP1) to suppress cervical cancer growth and
metastasis. Oncotarget. 7:68397–68411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou J and Xu Y: MicroRNA-140 inhibits cell
proliferation in gastric cancer cell line HGC-27 by suppressing
SOX4. Med Sci Monit. 22:2243–2252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Qin T, Li J, Wang L, Zhang Q, Jiang
Z and Mao J: MicroRNA-140-5p inhibits invasion and angiogenesis
through targeting VEGF-A in breast cancer. Cancer Gene Ther.
24:386–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Zou C, Pan L, Xu Y, Qi W, Ma G,
Hou Y and Jiang P: MicroRNA-140-5p inhibits the progression of
colorectal cancer by targeting VEGFA. Cell Physiol Biochem.
37:1123–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai H, Fesler A, Ba Y, Wu S and Ju J:
Inhibition of colorectal cancer stem cell survival and invasive
potential by hsa-miR-140-5p mediated suppression of Smad2 and
autophagy. Oncotarget. 6:19735–19746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu L, Lu Y, Han X, Zhao W, Li J, Mao J,
Wang B, Shen J, Fan S, Wang L, et al: microRNA-140-5p inhibits
colorectal cancer invasion and metastasis by targeting ADAMTS5 and
IGFBP5. Stem Cell Res Ther. 7:1802016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang C, Wang H, Zhou L, Jiang T, Xu Y and
Xia L: MicroRNA-212 inhibits the metastasis of nasopharyngeal
carcinoma by targeting SOX4. Oncol Rep. 38:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin Y, Zhao M, Xie Q, Zhang H, Wang Q and
Ma Q: MicroRNA-338-3p functions as tumor suppressor in breast
cancer by targeting SOX4. Int J Oncol. 47:1594–1602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen B, Liu J, Qu J, Song Y, Li Y and Pan
S: MicroRNA-25 suppresses proliferation, migration, and invasion of
osteosarcoma by targeting SOX4. Tumour Biol.
39:10104283177038412017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Zu L, Wang Y, Wang M, Chen P and
Zhou Q: miR-132 inhibits lung cancer cell migration and invasion by
targeting SOX4. J Thorac Dis. 7:1563–1569. 2015.PubMed/NCBI
|
|
19
|
Vishnubalaji R, Hamam R, Yue S, Al-Obeed
O, Kassem M, Liu FF, Aldahmash A and Alajez NM: MicroRNA-320
suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1.
Oncotarget. 7:35789–35802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Volinia S, Visone R, Galasso M, Rossi E
and Croce CM: Identification of microRNA activity by Targets'
Reverse EXpression. Bioinformatics. 26:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu F, Min J, Cao X, Liu L, Ge Z, Hu J and
Li X: MiR-363-3p inhibits the epithelial-to-mesenchymal transition
and suppresses metastasis in colorectal cancer by targeting Sox4.
Biochem Biophys Res Commun. 474:35–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smits M, Nilsson J, Mir SE, van der Stoop
PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J,
Krichevsky AM, et al: miR-101 is down-regulated in glioblastoma
resulting in EZH2-induced proliferation, migration, and
angiogenesis. Oncotarget. 1:710–720. 2010.PubMed/NCBI
|
|
24
|
Andersen CL, Christensen LL, Thorsen K,
Schepeler T, Sørensen FB, Verspaget HW, Simon R, Kruhøffer M,
Aaltonen LA, Laurberg S, et al: Dysregulation of the transcription
factors SOX4, CBFB and SMARCC1 correlates with outcome of
colorectal cancer. Br J Cancer. 100:511–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koumangoye RB, Andl T, Taubenslag KJ,
Zilberman ST, Taylor CJ, Loomans HA and Andl CD: SOX4 interacts
with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal
cancer cells. Mol Cancer. 14:242015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan H, Yang H, Wei H, Xiao W, Lou N, Qiu
B, Xu G, Song Z, Xiao H, Liu L, et al: Overexpression of SOX4
promotes cell migration and invasion of renal cell carcinoma by
inducing epithelial-mesenchymal transition. Int J Oncol.
51:336–346. 2017. View Article : Google Scholar : PubMed/NCBI
|