Introduction
Oral squamous cell carcinoma (OSCC) represents
almost 90% of head and neck cancers (1). OSCC is the sixth most common type of
malignant tumor; an estimated 50 million new cases occur annually
worldwide, with data reported that the overall 5-year survival rate
of OSCC has remained <50% over the past decade (2). Although there have been a large number
of studies at the genetic and transcriptional levels in squamous
cell carcinoma, the molecular mechanisms of carcinogenesis have not
been completely elucidated. Therefore, identification of the target
molecules that control the biological characteristics of OSCC would
be of great clinical significance.
Deubiquitinating enzymes (DUBs) are a protease
superfamily, which serve a role in the ubiquitin-proteasome system
by cleaving ubiquitin chains from substrate proteins (3). Ubiquitin-specific protease 22 (USP22) is
a deubiquitinating hydrolase that belongs to the DUB family
(4). The USP22 gene is located
on chromosome 17, and consists of 1578 base pairs that encode a
protein measuring 525 amino acids long (4,5). USP22 is
weakly expressed in several human tissues, including the liver,
skeletal muscle and heart, and it is also strongly expressed in
various types of cancer, which typically have a poor prognosis,
including salivary duct carcinoma, colorectal cancer, head and neck
cancer, breast cancer, and hepatocellular carcinoma (HCC) (6–12). USP22
is also considered to be a cancer stem cell marker that serves a
role in the development and progression of carcinomas (4,5). USP22 can
function as a subunit of the human Spt-Ada-Gcn5-acetyltransferase
complex, and is involved in the transcription of target genes
(5). A previous study demonstrated
that USP22 could inhibit the transcription of the cyclin-dependent
kinase inhibitor 1A (p21) gene by deubiquitinating the
transcriptional regulator fructose-1,6-bisphosphatase (FBP1)
(5). Furthermore, USP22 small
interfering (si)RNA decreased the expression of Cyclin B and
Survivin, and increased the expression of p21 in HCC (7). However, the precise mechanism by which
USP22 affects these proteins remains unknown.
Survivin is a member of the inhibitor of apoptosis
protein family and is also part of the chromosomal passenger
complex (CPC), together with Aurora B kinase, inner centromere
protein (INCENP) and Borealin (13).
The CPC functions as an important modulator of mitosis and
cytokinesis, which are known to serve a crucial role in cancer cell
proliferation, resulting in more aggressive, malignant types of
cancer (13,14). Several studies have suggested that
Survivin is overexpressed in a variety of human cancers; however,
is barely detectable in the majority of differentiated tissues
(15). Additionally, it has been
reported that ubiquitin carboxyl-terminal hydrolase FAF-X, a
deubiquitinating enzyme, regulates chromosome alignment and
segregation by regulating the dynamic association of Aurora B and
Survivin to centromeres (16).
Furthermore, different cullin-based ubiquitin ligase 3 adaptors
regulate Aurora B during mitosis, potentially by ubiquitinating
different pools of Aurora B at distinct subcellular localizations
(17). Ubiquitination regulates
dynamic protein-protein interactions and chromosome segregation
independently of protein degradation (16). Furthermore, USP22 is associated with
Survivin expression in HCC (7).
Therefore, we hypothesize that the expression of Survivin and
Aurora-B may be regulated by USP22 through deubiquitination.
In the present study, USP22 expression and its
correlation with Aurora-B, Survivin and the clinicopathological
features in OSCC were examined. The functional associations between
USP22, Aurora-B and Survivin in OSCC were also investigated.
Materials and methods
Patients and tissue samples
A total of 90 patients (66 males and 24 females),
who underwent complete surgical resection between January 2009 and
June 2015 at the Affiliated Hospital of Guilin Medical University
(Guilin, China) were enrolled in the present study. The inclusion
criteria were: i) A diagnosis of OSCC confirmed by pathology; ii)
Undergone surgery for the disease. All samples were obtained
following approval by the Ethics Committee of Guilin Medical
University (Guilin, China). The ages of the patients ranged from 26
to 80 years (mean, 56.5 years). Histologically, 70 cases were well
or moderately differentiated, 20 were poorly differentiated OSCC.
Clinicopathological data, including sex, age, tumor size, lymph
node metastasis and histological differentiation were recorded, the
classification by the World Health Organization (WHO) were used for
the degree of differentiation (18,19).
Tumors from each patient were 10% formalin-fixed in room
temperature for 24 h and cut into 4–5 µm sections. All the subjects
provided written informed consent.
Immunohistochemical staining
The tissue sections were incubated with a primary
monoclonal anti-USP22 antibody (cat. no ab71732; 1:100; Abcam,
Cambridge, MA, USA), monoclonal anti-Aurora-B antibody (cat. no
AMI-1; 1:200; Transduction Laboratories, San Diego, CA, USA), the
anti-Ki-67 monoclonal antibody (cat. no MIB-1; 1:200, Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), was used to examine
antigen Ki-67 expression and the polyclonal anti-survivin antibody
(1:1,000; Novus Biologicals, Littleton, CO, USA; cat. no.
NB500-201). The sections were incubated with the primary antibodies
at 4°C overnight following antigen retrieval for 5 min at boiling
temperature (100°C) twice by microwave treatment in a citrate
buffer solution (pH 6.0). Detection was performed using the
avidin-biotin peroxidase complex method using a Labelled
Strepavidin-biotin 2 (LSAB2) system-HRP (cat. no K0609; Dako;
Agilent Technologies, Inc.), according to the manufacturer's
protocol. The labeling index percentage of USP22, Aurora-B,
survivin or Ki-67 was determined by examining ≥1,000 tumor cells in
random 3 high-powered fields (×200 magnification; light microscope;
Olympus BX53F). The expression levels of Aurora-B and survivin were
divided into low expression (<20% positive cells) and high
expression (≥20% positive cells) groups. The expression levels of
USP22 and Ki-67 were divided into low expression (<50% positive
cells) and high expression (≥50% positive cells).
Cell culture
The human OSCC cell line Ca9-22 was purchased from
the Japanese Collection of Research Bioresources Cell Bank (Osaka,
Japan). The cells were cultured in RPMI-1640 (Gibco, Thermo Fisher
Scientific Inc.) containing penicillin (10,000 µ/ml) and
streptomycin (10,000 µg/ml) (cat. no P1400; Beijing Solarbio
Science & Technology Co. Ltd., Beijing, China) with 10% fetal
bovine serum (FBS; Samer Feishier Technology Co., Ltd.) and
maintained at 37°C in an atmosphere of 5% CO2. For the
growth assay, 5×103 cells were plated onto 24-well
plates, and the cells were counted at days 0, 2, 4 and 6.
Western blot analysis
The Ca9-22 cells were lysed in ice-cold RIPA buffer
(cat. no. R0020; Beijing Solarbio Science & Technology Co.
Ltd.) with protease inhibitor for 30 min and centrifuged at 12,000
× g for 20 min at 4°C. The protein concentration was determined by
the Bradford method (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
as the standard. Each protein lysate (40 µg) were separated on 10%
SDS-PAGE gels and transferred to a polyvinyl difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was
blocked with 5% skim milk (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) [PBS was diluted to obtain 5% milk seal solution (5 g/100
ml)] for 40 min at room temperature, and then incubated with the
primary (4°C overnight) and secondary antibodies (secondary goat
anti-rabbit (cat. no TA130015; 1:000) or anti-mouse antibody (cat.
no TA100015; 1:000); OriGene Technologies, Inc., Rockville, MD,
USA) for 1 h at room temperature. The primary antibodies were USP22
(1:1,000; Abcam; cat. no. ab71732), Survivin (cat. no. NB500-201;
Novus Biologicals; 1:2,000), Aurora-B (cat. no AMI-1; 1:000;
Transduction Laboratories), Cyclin B (cat. no. 610219), p21 (cat.
no. clone 70) (1:1,000; Transduction Laboratories) and β-actin
(1:2,000, OriGene Technologies, Inc.; cat. no TA-09).
siRNA transfection
Ca9-22 cells were transfected with
Lipofectamine® 3000 Transfection reagent (Thermo Fisher
Scientific, Inc.) with 150 pmol USP22 siRNA
(5′-GCAGCUUCAAGGUGGACAATT-3′) and negative control siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) (Guangzhou Ribobio Co., Ltd.,
Guangzhou, China) in 1 ml OPTI-MEM. At 48 h following transfection,
the cells were used for subsequent experiments.
Statistical analysis
The data were analyzed were using the SPSS 17.0
software package (SPPS, Inc., Chicago, IL, USA). Measurement data
are presented as mean ± standard deviation. The χ2-test
was used for the comparison of enumeration data. A paired student's
t-test was used to compare the number of cells data between the
siRNA-USP22 and the siRNA control groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of USP22, Aurora-B,
Survivin and Ki-67 in OSCC tissues
Firstly, USP22 expression was compared with
Aurora-B, Survivin and Ki-67 expression in 20 normal tissues and 90
OSCC by performing immunohistochemistry. In normal colonic mucosa,
USP22, Aurora-B, Survivin and Ki-67 were only distributed in the
basal layers and exhibited weak staining. However, USP22, Aurora-B,
Survivin and Ki-67 were strongly expressed in OSCC. Notably, the 4
proteins were more frequently expressed in poorly differentiated
OSCC tissues, compared with the Well/Moderate-differentiated OSCC
tissues (Fig. 1A, Tables I and II). USP22, Ki-67 and Aurora-B were
primarily localized in the nuclei, while Survivin was localized to
the cytoplasm and the nuclei (Fig.
1A). The number of high USP22, Survivin, Aurora-B and Ki-67
expression cases were 46/90 (51.11%), 51/90 (56.67%), 37/90
(41.11%) and 25/90 (27.78%), respectively. The number of low USP22,
Survivin, Aurora-B and Ki-67 expression cases were 44/90 (48.89%),
46/90 (43.33%), 53/90 (58.89%) and 65/90 (72.22%), respectively
(Fig. 1B).
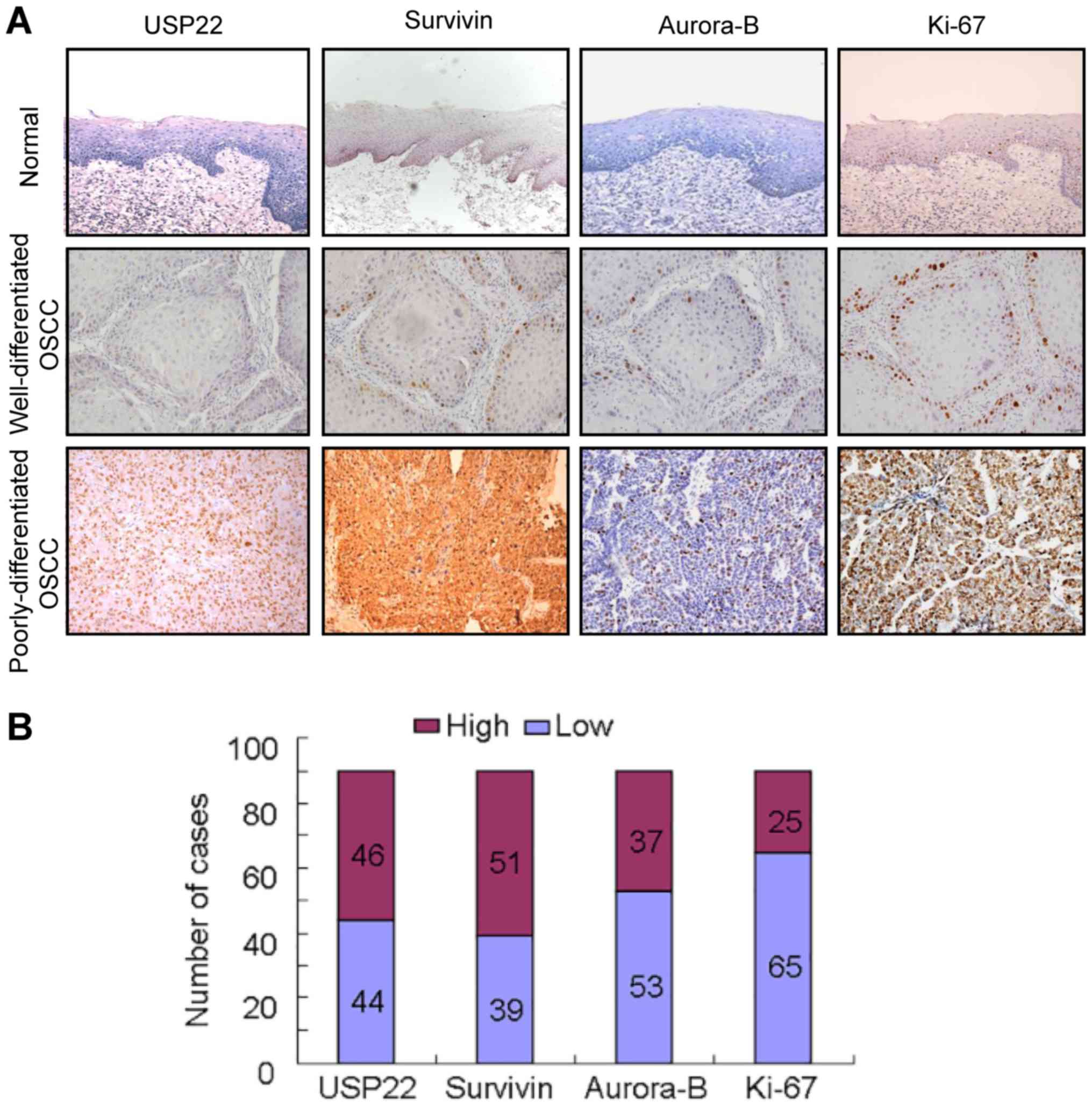 | Figure 1.USP22 expression and its association
with Survivin, Aurora-B and Ki-67 in OSCC. (A) Expression of USP22,
Survivin, Aurora-B and Ki-67 was examined by immunohistochemistry
in normal oral mucosa and OSCC tissues. In normal oral mucosa,
USP22 were only distributed in the basal and parabasal layers and
exhibited weak staining. In well-differentiated OSCC cases,
USP22-positive cells were observed predominantly in the periphery
of the tumor nests, while in poorly differentiated OSCC cases,
USP22-positive cells were present throughout the tumor nests. The
expression patterns of Survivin, Aurora-B and Ki-67 appear similar
to USP22 in the same cases. (B) The quantity of high or low
expression of USP22, Survivin, Aurora-B and Ki-67 in 90 OSCC cases.
The quantity of high USP22, Survivin, Aurora-B and Ki-67 expression
cases were 46/90, 51/90, 37/90 and 25/90 respectively. The quantity
of low USP22, Survivin, Aurora-B and Ki-67 expression cases were
44/90, 46/90, 53/90 and 65/90 respectively. USP22,
ubiquitin-specific protease 22; Ki-67, antigen Ki-67; OSCC, oral
squamous cell carcinoma. |
 | Table I.USP22 expression and its association
with clinicopathological features in oral squamous cell
carcinoma. |
Table I.
USP22 expression and its association
with clinicopathological features in oral squamous cell
carcinoma.
|
| USP22 expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | Low | High | P-value |
|---|
| Tissue type |
|
|
|
|
Normal | 20 | 0 |
|
| OSCC | 44 | 46 |
|
| Age, years |
|
|
|
|
≥50 | 34 | 39 | 0.363 |
|
<50 | 10 | 7 |
|
| Sex |
|
|
|
|
Male | 30 | 36 | 0.280 |
|
Female | 14 | 10 |
|
| Tumor size, mm |
|
|
|
|
≥15 | 20 | 18 | 0.544 |
|
<15 | 24 | 28 |
|
| Histological
differentiation |
|
|
|
|
Poor | 3 | 17 | <0.001 |
|
Well/Moderate | 41 | 29 |
|
| Lymph node
metastasis |
|
|
|
|
Negative | 41 | 31 | 0.002 |
|
Positive | 3 | 15 |
|
 | Table II.Survivin and Aurora-B expression and
their associations with clinicopathological features in oral
squamous cell carcinoma. |
Table II.
Survivin and Aurora-B expression and
their associations with clinicopathological features in oral
squamous cell carcinoma.
|
| Survivin
expression |
| Aurora-B
expression |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
features | Low | High | P-value | Low | High | P-value |
|---|
| Tissue type |
|
|
|
|
|
|
|
Normal | 20 | 0 |
| 20 | 0 |
|
|
OSCC | 39 | 51 |
| 53 | 37 |
|
| Age, years |
|
|
|
|
|
|
|
≥50 | 33 | 40 | 0.458 | 45 | 28 | 0.271 |
|
<50 | 6 | 11 |
| 8 | 9 |
|
| Sex |
|
|
|
|
|
|
|
Male | 17 | 43 | 0.0011 | 34 | 32 | 0.018 |
|
Female | 16 | 8 |
| 19 | 5 |
|
| Tumor size, mm |
|
|
|
|
|
|
|
≥15 | 16 | 22 | 0.841 | 20 | 18 | 0.302 |
|
<15 | 23 | 29 |
| 33 | 19 |
|
| Histological
differentiation |
|
|
|
|
|
|
|
Poor | 2 | 18 | 0.0006 | 4 | 16 | <0.001 |
|
Well/Moderate | 37 | 33 |
| 49 | 21 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
Negative | 36 | 35 | 0.0064 | 47 | 24 | 0.007 |
|
Positive | 3 | 16 |
| 6 | 13 |
|
Association between USP22, Aurora-B,
Survivin and the clinicopathological features of OSCC
Next, the associations between the
clinicopathological features of OSCCC and USP22, Aurora-B, Survivin
expression and the clinicopathological features of OSCC were
examined. High expression of USP22 was associated with lymph node
metastasis (P<0.01) and histological grade (P<0.01), however,
not with age, sex and tumor size in OSCC (Table I). The expression of Aurora-B and
Survivin were associated with sex (P<0.05 and P<0.01,
respectively), lymph node metastasis (P<0.001) and histological
differentiation (P<0.01); however, not with tumor size and age
in OSCC (Table II).
Association between USP22, Aurora-B,
Survivin and Ki-67 expression in OSCC
In order to understand the role of USP22, the
present study examined the association between USP22, Aurora-B,
Survivin and Ki-67 in OSCC. Among the 90 OSCC cases, 46 cases
exhibited the high USP22 expression, and 44 displayed a low USP22
expression in OSCC tissues (Table
III). Of the 46 cases of high USP22 expression, the number of
high Survivin, Aurora-B and Ki-67 expression cases were 33/46
(71.74%), 27/46 (58.70%) and 20/46 (43.48%), respectively (Table III). Of the 44 cases with a low
USP22 expression, the number of low Survivin, Aurora-B and Ki-67
expression cases were 26/44 (59.09%), 34/44 (77.27%) and 39/44
(88.64%), respectively (Table III).
USP22 expression was positively associated with Aurora-B, Survivin
and Ki-67 (P<0.01, Table III).
In addition, the clinicopathological findings were compared with
the co-expression of USP22, Aurora-B and Survivin in OSCC. OSCC
cases with a high expression of USP22, Aurora-B and Survivin
exhibited increased lymph node metastasis (P<0.01) and poor
differentiation (P=0.00006) when compared with OSCC cases with low
expression of USP22, Aurora-B and Survivin (Table IV). These data indicate that USP22
promotes the development of OSCC together with Aurora-B and
Survivin. These data indicate that USP22 promotes the development
of OSCC together with Survivin and Aurora-B.
 | Table III.Association between USP22 and
survivin, between USP22 and Aurora-B expression and between USP22
and Ki-67 in oral squamous cell carcinoma. |
Table III.
Association between USP22 and
survivin, between USP22 and Aurora-B expression and between USP22
and Ki-67 in oral squamous cell carcinoma.
|
| USP22
expression |
|
|
|---|
|
|
|
|
|
|---|
| Protein
expression | Low (n=44) | High (n=46) | Total | P-value |
|---|
| Survivin |
|
|
|
|
|
Low | 26 | 13 | 39 | 0.003 |
|
High | 18 | 33 | 51 |
|
| Aurora-B |
|
|
|
|
|
Low | 34 | 19 | 53 | 0.001 |
|
High | 10 | 27 | 37 |
|
| Ki-67 |
|
|
|
|
|
Low | 39 | 26 | 65 | 0.001 |
|
High | 5 | 20 | 25 |
|
 | Table IV.USP22 and survivin, and USP22 and
Aurora-B expression and their associations with clinicopathological
features in oral squamous cell carcinoma. |
Table IV.
USP22 and survivin, and USP22 and
Aurora-B expression and their associations with clinicopathological
features in oral squamous cell carcinoma.
|
|
USP22/survivin/Aurora-B expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | All high
(n=26) | Other (n=38) | All low (n=26) | P-value |
|---|
| Age, years |
|
≥50 | 21 | 31 | 21 | 0.995 |
|
<50 | 5 | 7 | 5 |
|
| Sex |
|
Male | 22 | 30 | 14 | 0.025 |
|
Female | 4 | 8 | 12 |
|
| Tumor size, mm |
|
≥15 | 9 | 20 | 9 | 0.232 |
|
<15 | 17 | 18 | 17 |
|
| Histological
differentiation |
|
Poor | 13 | 7 | 0 | <0.001 |
|
Well/Moderate | 13 | 31 | 26 |
|
| Lymph node
metastasis |
|
Negative | 16 | 29 | 26 | 0.003 |
|
Positive | 10 | 9 | 0 |
|
Suppression of cell growth by USP22
knockdown in OSCC cells
To understand the role of USP22 in the development
of OSCC, USP22 was silenced by siRNA in OSCC cells. It was
identified that USP22 siRNA decreased the expression of USP22
protein (Fig. 2A) and also inhibited
cell growth in OSCC cells (Fig. 2B).
Moreover, USP22 siRNA increased the p21 protein levels and
decreased the Cyclin B protein levels in OSCC cells (Fig. 2A). Thus, we have found a possible
relationship between USP22, Aurora-B and Survivin in OSCC tissues
(Fig. 1 and Table III). Notably, USP22 siRNA reduced
the protein levels of Aurora-B and Survivin in OSCC cells (Fig. 2B). These results suggest that the
expression of Aurora-B and Survivin may be regulated by USP22.
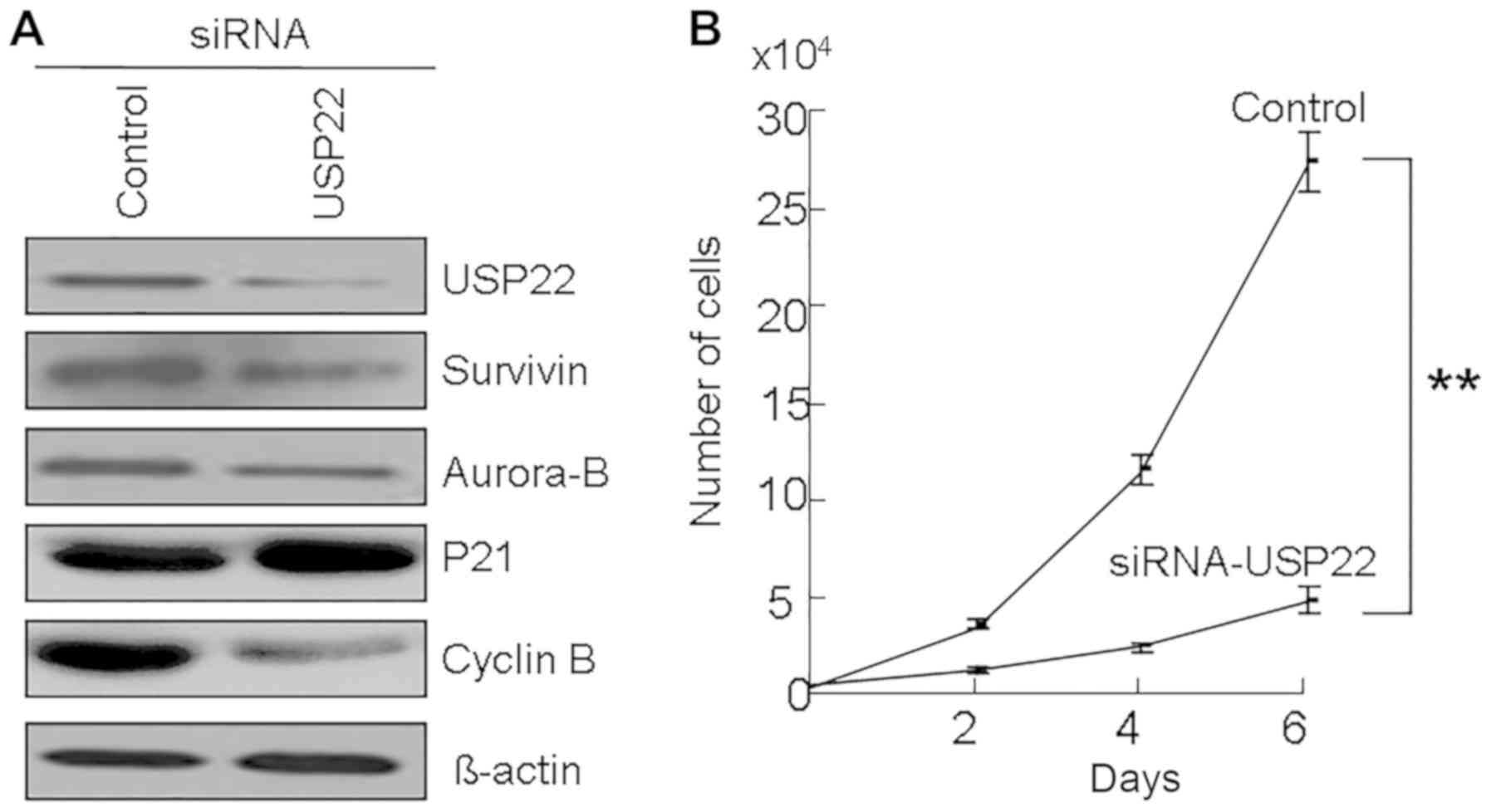 | Figure 2.The effects of USP22 knockdown in OSCC
cells. (A) USP22 siRNA were transfected into Ca9-22 cells. At 48 h
following transfection, cells were collected and the expression of
Survivin, Aurora-B, p21 and cyclin B was examined by western blot
analysis. β-actin was used as a control. (B) Cell growth of siRNA
treated Ca9-22 cells. At 48 h following USP22 siRNA treatment,
5,000 cells were plated on 24-well plates. At 24 h, the cell number
was counted as 0 day. The cell number was subsequently counted at
days 2, 4, and 6. **P<0.01. USP22, ubiquitin-specific protease
22; OSCC, oral squamous cell carcinoma; siRNA, small interfering
RNA; p21, cyclin-dependent kinase inhibitor 1A. |
Discussion
The putative cancer stem cell marker USP22 has been
demonstrated to be overexpressed in a number of types of cancer
(6–12). The present study identified that USP22
was highly expressed in OSCC, particularly in poorly differentiated
cancers, and that high USP22 expression was significantly
associated with the malignant behaviors of OSCC, including lymph
node metastasis and poor differentiation. Previous findings
demonstrated that a high expression of USP22 was associated with a
poor prognosis in various types of cancer, such as salivary duct
carcinoma, colorectal cancer, head and neck cancer, breast cancer,
and HCC (7–12). The results of the present study also
demonstrated that USP22 expression was positively associated with
the expression of the cell proliferation marker Ki-67.
Additionally, it was observed that cell growth was suppressed by
USP22 siRNA in OSCC cells. USP22 siRNA also increased the
expression of p21 and reduced the expression of Cyclin B in OSCC
cells. USP22 is a positive regulator of tumor growth and depletion
of USP22 leads to cell cycle arrest at the G1 phase (5). USP22, a member of the ubiquitin
hydrolase family, promotes the occurrence and development of tumors
by blocking ubiquitin-mediated protein degradation, including FBP1,
p21, p53, c-Myc and BMI-1 (the Polycomb repressor complex 1),
thereby enhancing the stability of certain cancer genes (4,17,20). Previous studies have demonstrated that
USP22 deubiquitinates histones H2A and H2B and regulates cell
growth, cell-cycle regulation and signal transduction (4,5,17). Furthermore, USP22 regulates cell
proliferation by deubiquitinating the transcriptional regulator,
FBP1 (5). USP22 may also alter the
expression level of multiple tumor-associated regulatory factors,
such as c-Myc, BMI-1, p53, p21 and Cyclin D (14,20).
CPC contains Survivin, Borealin, Aurora-B and
INCENP, and acts as a critical mitotic regulator that controls the
cell cycle and serves a crucial role in the expansion of tumor
cells (13,14). In the present study, the expression of
USP22 was identified to be positively associated with Aurora-B and
Survivin in OSCC. In addition, high USP22, Aurora-B, or Survivin
expression is associated with a poor prognosis in OSCC, especially
when all are highly expressed concomitantly. Survivin inhibits
apoptosis, regulates chromosome separation and cell division
(13–15), and is highly expressed in various
types of cancer, which typically have a poor prognosis, including
colorectal cancer, head and neck cancer, endometrial carcinoma and
hepatocellular carcinoma (7,21–26).
Aurora-B regulates cytokinesis and chromosome segregation together
with Survivin, Borealin and INCENP (7,8,23,24).
Aurora B is highly expressed in a number of types of cancer,
including head and neck, colon, liver and breast cancer, and is
associated with malignancy indicators, including the histological
differentiation and lymph node metastasis (23–26).
USP22, Aurora-B and Survivin are highly expressed in OSCC, and
serve an important role in the tumorigenesis of oral cancer most
likely by disrupting cell cycle progression (23,26,27).
USP22, Aurora-B and Survivin expression may be promising markers
for predicting the malignant behaviors of OSCC. These observations
are supported by previous data suggesting that high levels of
Survivin and Aurora-B were associated with more malignant
phenotypes and that they were independent prognostic indicators for
multifarious cancers (9,22–26).
Our previous research demonstrated that USP22 can
positively regulate the expression of Survivin in HCC (7); however, it is unclear whether USP22
regulates the expression of Aurora-B or Survivin in OSCC. In the
present study, the results demonstrated that the expression of
USP22 was positively associated with Aurora-B and Survivin in OSCC.
A previous study reported that BMI-1 induces repressive epigenetic
controlling of the Survivin promoter (16). USP22 also upregulates BMI-1 and may
upregulate Survivin via BMI-1 overexpression (20). Previous studies have indicated that
Aurora-B is degraded by the ubiquitin/proteasome pathway in the
final stage of cell division (28,29).
Indeed, the present study identified that USP22 siRNA decreased
Aurora-B and Survivin expression in OSCC cells. These data indicate
that the expression of Survivin and Aurora-B may be regulated by
USP22. However, the mechanism of USP22 regulating Survivin and
Aurora-B is still unclear and needs further investigations.
In conclusion, the results of the present study
suggest that USP22 may be involved in the progression of OSCC, in
cooperation with Aurora-B and Survivin. USP22, Aurora-B and
Survivin are markedly associated with the occurrence and
development of OSCC and are novel potential targets for the
diagnosis and treatment of OSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of Guangxi (grant nos. 2015GXNSF, AA139110 and
2017GxNSFDA198022); and The National Natural Science Foundation of
China (grant nos. 81460411 and 81660450).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL, JL, GQ and SZ designed the present research. TL,
YK and GQ analyzed and explained the patient data, and were major
contributors in writing the manuscript. QC and SJ performed the
histological examination of the samples. SM, WS, YK analyzed and
interpreted the patient data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All steps involved in the study involving human
participants were according to the ethical standards of the
institutional and national research committee and the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. The present study was conducted following approval by
the Ethics Committee of Guilin Medical University (Guilin, China).
Written informed consent was provided by all individual
participants included in the work.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wikner J, Gröbe A, Pantel K and Riethdorf
S: Squamous cell carcinoma of the oral cavity and circulating tumor
cells. World J Clin Oncol. 5:114–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mascitti M, Orsini G, Tosco V,
Monterubbianesi R, Balercia A, Putignano A, Procaccini M and
Santarelli A: An overview on current non-invasive diagnostic
devices in oral oncology. Front Physiol. 9:15102018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amerik AY and Hochstrasser M: Mechanism
and function of deubiquitinating enzymes. Biochim Biophys Acta.
1695:189–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XY, Pfeiffer HK, Thorne AW and
McMahon SB: USP22, an hSAGA subunit and potential cancer stem cell
marker, reverses the polycomb-catalyzed ubiquitylation of histone
H2A. Cell Cycle. 7:1522–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XY, Varthi M, Sykes SM, Phillips C,
Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL and McMahon SB: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jordan CT: Cancer stem cell biology: From
leukemia to solid tumors. Curr Opin Cell Biol. 16:708–712. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang B, Liang X, Tang F, Zhang J, Zeng S,
Jin S, Zhou L, Kudo Y and Qi G: Expression of USP22 and survivin is
an indicator of malignant behavior in hepatocellular carcinoma. Int
J Oncol. 47:2208–2216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM
and Baek KH: The expression patterns of deubiquitinating enzymes,
USP22 and Usp22. Gene Expr Patterns. 6:277–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glinsky GV, Berezovska O and Glinskii AB:
Microarray analysis identifies a death-from-cancer signature
predicting therapy failure in patients with multiple types of
cancer. J Clin Invest. 115:1503–1521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu YL, Yang YM, Xu H and Dong XS:
Aberrant expression of USP22 is associated with liver metastasis
and poor prognosis of colorectal cancer. J Surg Oncol. 103:283–289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, Zeng FQ, Gu ZH, Wang L, Wang ZY,
Jiang GS and Xiao XY: Quantitative analysis of the putative cancer
stem cell marker USP22 mRNA in the transitional cell carcinoma of
the bladder and the relationship between USP22 and the grading of
tumor. J Clin Urol. 24:140–144. 2009.
|
|
13
|
Carmena M, Wheelock M, Funabiki H and
Earnshaw WC: The chromosomal passenger complex (CPC): From easy
rider to the godfather of mitosis. Nat Rev Mol Cell Biol.
13:789–803. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van der Waal MS, Hengeveld RC, van der
Horst A and Lens SM: Cell division control by the chromosomal
passenger complex. Exp Cell Res. 318:1407–1420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vong QP, Cao K, Li HY, Iglesias PA and
Zheng Y: Chromosome alignment and segregation regulated by
ubiquitination of survivin. Science. 310:1499–1504. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, Lang G, Ito S, Bonnet J, Metzger
E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG,
Krasnov A, et al: A TFTC/STAGA module mediates histone H2A and H2B
deubiquitination, coactivates nuclear receptors, and counteracts
heterochromatin silencing. Mol Cell. 29:92–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Z, Hu S, Luo Q, Ye D, Hu D and Chen F:
Overexpression of SENP3 in oral squamous cell carcinoma and its
association with differentiation. Oncol Rep. 29:1701–1706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and genetics of head and neck tumours. IARC
Press. World Health Organization Classification of Tumours.
168–175. 2005.
|
|
20
|
Liu YL, Zheng J, Tang LJ, Han W, Wang JM,
Liu DW and Tian QB: The deubiquitinating enzyme activity of USP22
is necessary for regulating HeLa cell growth. Gene. 572:49–56.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maerki S, Olma MH, Staubli T, Steigemann
P, Gerlich DW, Quadroni M, Sumara I and Peter M: The Cul3-KLHL21 E3
ubiquitin ligase targets aurora B to midzone microtubules in
anaphase and is required for cytokinesis. J Cell Biol. 187:791–800.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi G, Tuncel H, Aoki E, Tanaka S, Oka S,
Kaneko I, Okamoto M, Tatsuka M, Nakai S and Shimamoto F:
Intracellular localization of survivin determines biological
behavior in colorectal cancer. Oncol Rep. 22:557–562.
2009.PubMed/NCBI
|
|
23
|
Qi G, Kudo Y, Ando T, Tsunematsu T,
Shimizu N, Siriwardena SB, Yoshida M, Keikhaee MR, Ogawa I and
Takata T: Nuclear Survivin expression is correlated with malignant
behaviors of head and neck cancer together with Aurora-B. Oral
Oncol. 46:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuncel H, Shimamoto F, Kaneko Guangying Qi
H, Aoki E, Jikihara H, Nakai S, Takata T and Tatsuka M: Nuclear
Aurora B and cytoplasmic Survivin expression is involved in lymph
node metastasis of colorectal cancer. Oncol Lett. 3:1109–1114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hori M, Miki T, Okamoto M, Yazama F,
Konishi H, Kaneko H, Shimamoto F, Ota T, Temme A and Tatsuka M: The
detergent-soluble cytoplasmic pool of survivin suppresses anoikis
and its expression is associated with metastatic disease of human
colon cancer. PLoS One. 8:e557102013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi G, Ogawa I, Kudo Y, Miyauchi M,
Siriwardena BS, Shimamoto F, Tatsuka M and Takata T: Aurora-B
expression and its correlation with cell proliferation and
metastasis in oral cancer. Virchows Arch. 450:297–302. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi G, Mi S, Jin S, Shao W, Liu J, Liu T,
Zeng S and Lu H: The role of the cancer stem cell marker USP22 in
tumors. Stem Cell Trans Invest. 3:e12192016.
|
|
28
|
Kurai M, Shiozawa T, Shih HC, Miyamoto T,
Feng YZ, Kashima H, Suzuki A and Konishi I: Expression of Aurora
kinases A and B in normal, hyperplastic, and malignant human
endometrium: Aurora B as a predictor for poor prognosis in
endometrial carcinoma. Hum Pathol. 36:1281–1288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen HG, Chinnappan D, Urano T and Ravid
K: Mechanism of Aurora-B degradation and its dependency on intact
KEN and A-boxes: Identification of an aneuploidy-promoting
property. Mol Cell Biol. 25:4977–4992. 2005. View Article : Google Scholar : PubMed/NCBI
|
















