Introduction
Liver cancer is a common type of gastrointestinal
cancer, whose incidence is only second to that of stomach cancer
(1). In China, there are numerous
patients with hepatitis, among whom hepatitis B is more common, and
hepatitis B is often transferred into hepatitis B-cirrhosis-liver
cancer (1). However, the clinical
treatment of liver cancer is not ideal, which primarily results
from the recurrence and metastasis of hepatocellular carcinoma
(HCC) (1,2). Surgery remains the most effective
treatment of liver cancer, but surgical resection is aimed only at
the visible tumor, and it cannot remove the invisible small tumor
foci or micro-metastases. Therefore, seeking an effective treatment
method to inhibit tumor recurrence has become a hot research
topic.
The Janus kinase (JAK)/signal transducer and
activator of transcription (STAT) signaling pathway overactivated
in a number of tumors is a common signal transduction pathway in
tumors (3). It is has been identified
that the excessive activation of STAT is associated with the
recurrence and metastasis of various tumors, including breast
cancer, lung cancer, colon cancer, stomach cancer, glioma, melanoma
and other skin squamous cell carcinomas (4). The JAK/STAT signaling pathway is able to
regulate cell proliferation, apoptosis and angiogenesis (5). STAT has been identified to be
excessively expressed in HCC tissues, and it is positively
correlated with the clinical staging and grading of HCC (5).
It has been identified recently that the expression
levels of microRNAs in various tumors are abnormal to various
degrees, and they change upward or downward, which indicates
microRNAs have a certain association with the development of tumors
(5). The association between
microRNAs and cancer is an important focus of research worldwide
and has led to an increasing number of novel results and hypotheses
(6). The aim of the present study was
to investigate the function of microRNA-543 on liver cancer cell
proliferation and apoptosis, and also to identify whether the
JAK2/STAT3 signaling pathway was a direct target of
microRNA-543.
Materials and methods
Ethics statement and samples
The present study was approved by the institutional
ethics and scientific committee of The China Japan Union Hospital
of Jilin University (Changchun, China). All patients provided
written informed consent for the use of their tissues. All HCC
tumor tissues and matched adjacent normal tissues (n=56; male;
between 57 and 79 years of age) were collected at the Department of
Gastrointestinal Medicine, The China Japan Union Hospital of Jilin
University between March 2015 and May 2015. Tissue samples were
collected and stored at −80°C until further use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared from tissues samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
A 100 ng amount of total RNA was reverse-transcribed into cDNA
using a TaqMan™ MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Subsequently, qPCR was performed using
SYBR Select Master mix for CFX (Invitrogen; Thermo Fisher
Scientific, Inc.) to quantify microRNA-543 expression. microRNA-543
forward, 5′-TGGCAAAGGAGCAGATTAGTAGG-3′, and reverse,
5′-CTGCCACAAGCCACTAGAGGATAAGA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. microRNA expression was measured
using the comparative cycle quantification (Cq;
2−ΔΔCq) method (7).
Cell culture
HCC MHCC97 and Hep3B cells were obtained from the
Experiment Center of Jilin University and maintained in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell transfection
Negative control (5′-CCCCCCCCCCCCCC-3′),
microRNA-543 inhibitor
(5′-ATATATGCTCTGGAAATGATTCAGCAGTACGAAAGCC-3′) and microRNA-543
activator (5′-GGCTTTCGTACTGCTGAATCATTTCCAGAGCATATAT-3′) were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Plasmids were transfected into MHCC97 and Hep3B cells using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) to a final concentration of 20 nmol/l.
Cell proliferation assay
MHCC97 and Hep3B cells transfection were seeded on
96-well plates at an initial density of 3×103
cells/well. A 100 µl volume of sterile MTT (0.5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to the
cells for 4 h following 0, 24 and 48 h of incubation at 37°C. The
culture medium was removed and 150 µl dimethylsulfoxide was added
for 30 min to dissolve the formazan product. Optical density was
determined at 490 nm.
Western blot analysis
Transfected MHCC97 and Hep3B cells were seeded on
6-well plates at initial density of 1×106 cells/well.
Proteins were extracted using radioimmunoprecipitation lysis buffer
(Thermo Fisher Scientific, Inc.) and the protein content was
determined using a Bicinchoninic Acid Protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Equal amounts of
proteins (50 µg) were subjected to 10% SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes. Following
blocking with 5% non-fat milk Tris-buffered saline containing 0.1%
Tween-20 (TBST) for 1 h at 37°C, the membranes were washed with
TBST for 1 h at room temperature, and incubated with antibodies
against phospho- (p-)JAK2 (66245; 1:1,000), p-STAT3 (52075;
1:1,000), c-Myc (9402; 1:1,000), and B-cell lymphoma 2 (Bcl-2;
15071; 1:1,000) and GAPDH (5174; 1:5,000; all from Cell Signaling
Technology, Inc., Danvers, MA, USA) and GAPDH overnight at 4°C. The
membrane was washed with TBST and incubated with a horseradish
peroxidase-conjugated anti-rabbit IgG antibody (7074; 1:5,000; Cell
Signaling Technology, Inc.) for 1 h at 37°C. The membrane was
detected with BeyoECL Star (Beyotime Institute of Biotechnology)
reagent and images were captured using Image Lab 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. Kaplan-Meier survival analysis and log-rank
test were used to assess overall survival (OS) rates. Statistical
evaluation was performed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MicroRNA-543 expression and OS
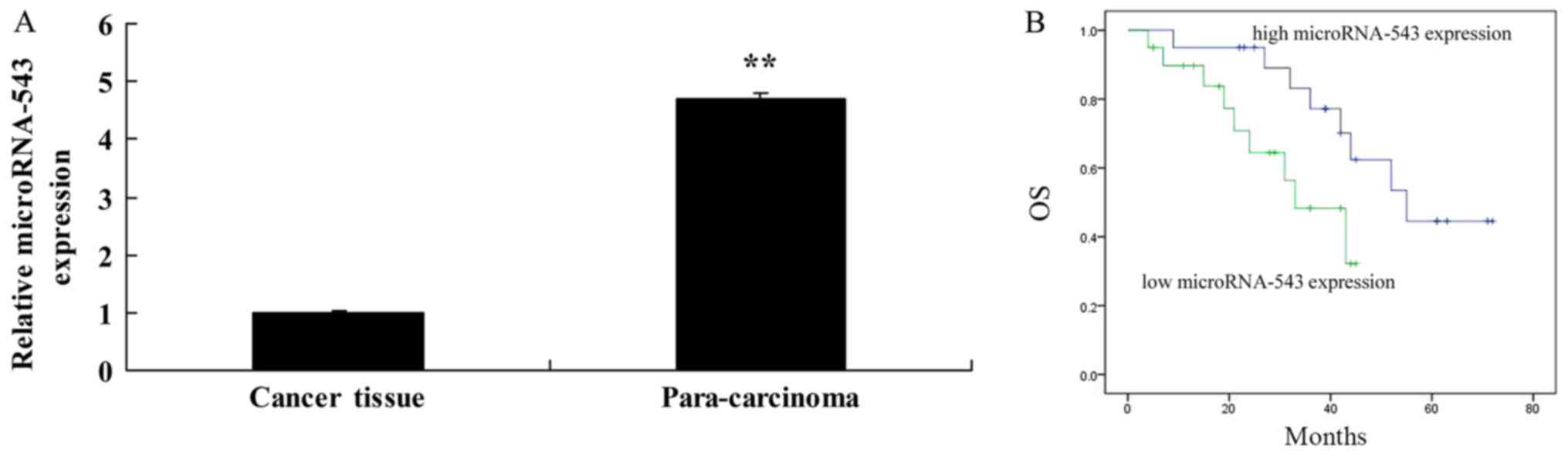
Using RT-qPCR, it was identified that microRNA-543
levels were significantly decreased in the HCC tissues in
comparison with the adjacent tissues (Fig. 1A). It was also identified that OS
rates of patients with high microRNA-543 expression were increased,
compared with that of patients with low microRNA-543 expression
(Fig. 1B).
Inhibition of microRNA-543 expression
increases cell proliferation and inhibits cell apoptosis of liver
cancer cells
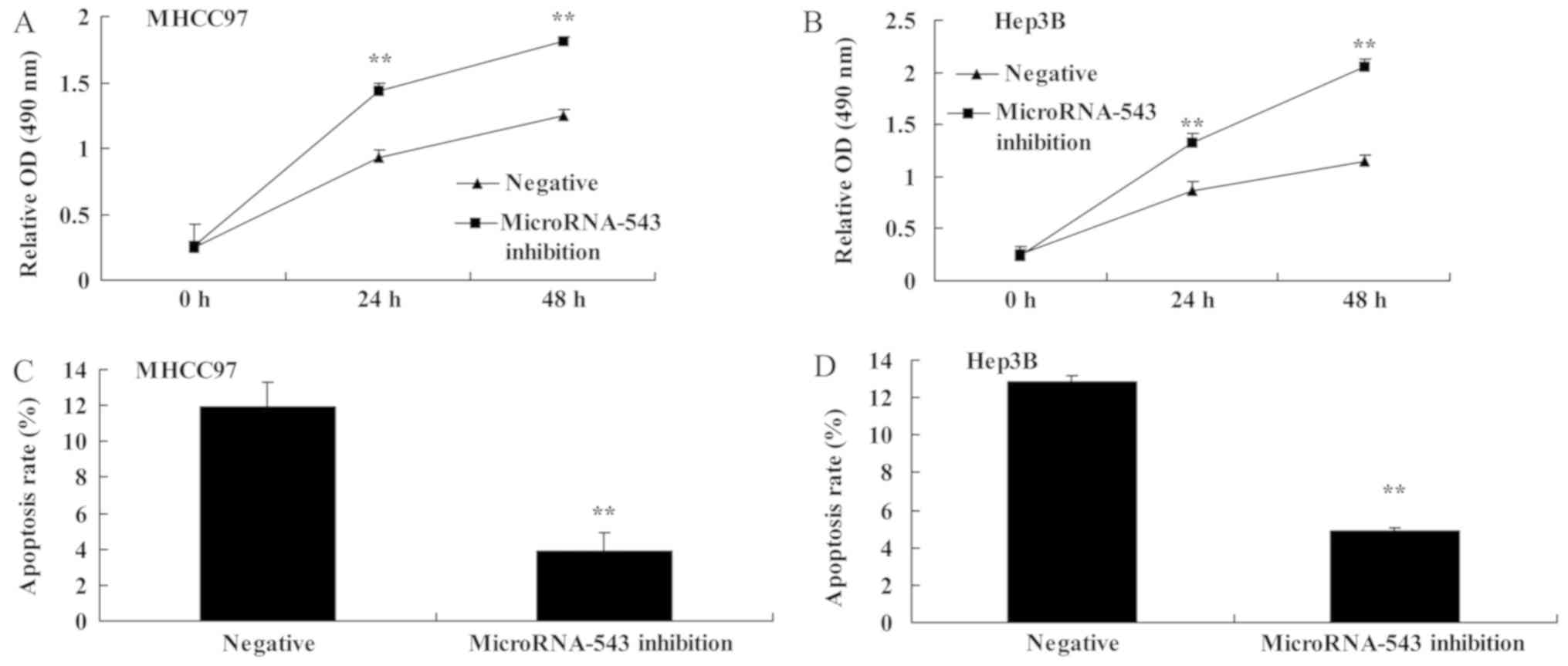
To investigate whether the inhibition of
microRNA-543 expression had an effect on HCC cell proliferation and
apoptosis, MHCC97 and Hep3B HCC cells were transfected with
microRNA-543 inhibitor. The proliferation of MHCC97 and Hep3B cells
transfected with microRNA-543 inhibitor was significantly increased
compared with that of the negative control group (Fig. 2A and B). The apoptosis rate of MHCC97
and Hep3B cells transfected with microRNA-543 inhibitor was
significantly decreased compared with that of the negative control
group (Fig. 2C and D).
Inhibition of microRNA-543 expression
activates the protein expression of p-JAK2, p-STAT3, c-Myc and
Bcl-2 in liver cancer cells
The effect of inhibiting microRNA-543 on the
expression of various proteins in HCC cells was investigated. As
presented in Fig. 3, the inhibition
of microRNA-543 expression significantly increased p-JAK2, p-STAT3,
c-Myc and Bcl-2 protein expression in MHCC97 and Hep3B cells,
compared with the negative control group.
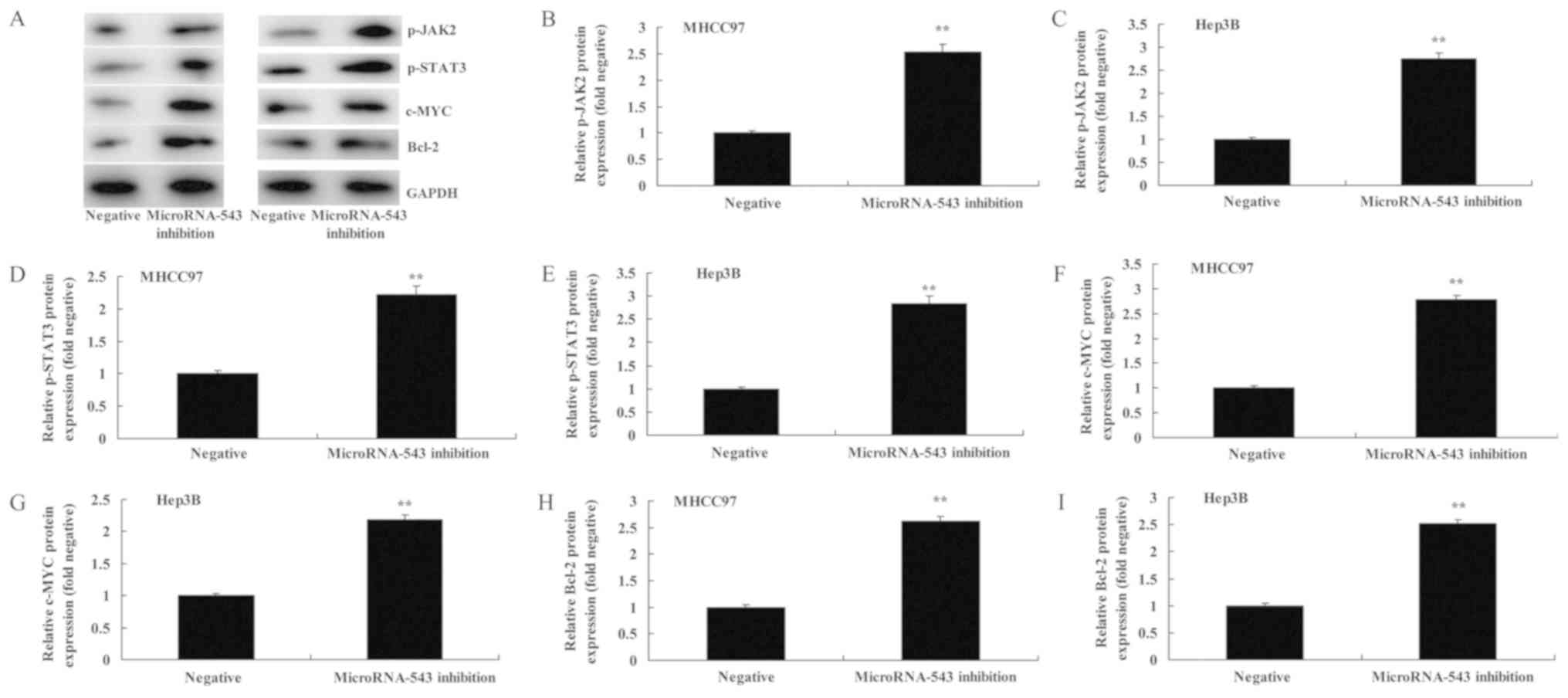 | Figure 3.Inhibition of microRNA-543 expression
increases the protein expression of p-JAK2, p-STAT3, c-Myc and
Bcl-2 in liver cancer cells. (A) Western blot analysis of p-JAK2,
p-STAT3, c-Myc and Bcl-2 proteins in (left) MHCC97 and (right)
Hep3B cells. Quantification of p-JAK2 in (B) MHCC97 and (C) Hep3B
cells, p-STAT3 in (D) MHCC97 and (E) Hep3B cells, c-Myc in (F)
MHCC97 and (G) Hep3B cells, and Bcl-2 in (H) MHCC97 and (I) Hep3B
cells. **P<0.01 vs. negative group. p-, phospho-; JAK2, Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; Bcl-2, B-cell lymphoma 2. |
Activation of microRNA-543 expression
decreases cell proliferation and induces cell apoptosis of liver
cancer cell
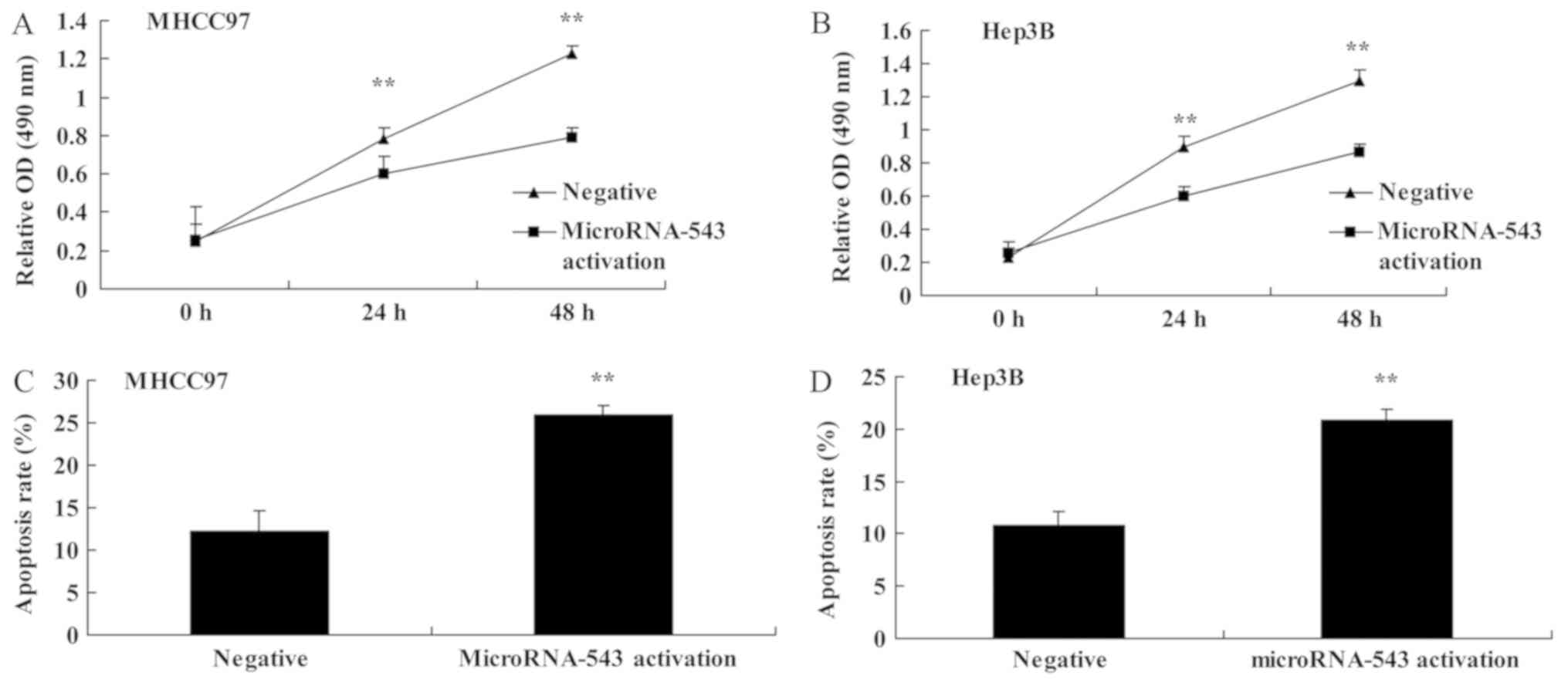
To further investigate the function of microRNA-543
in HCC progression, the expression of microRNA-543 was activated in
MHCC97 and Hep3B cells. Activation of microRNA-543 expression
significantly decreased cell proliferation (Fig. 4A and B) and induced apoptosis
(Fig. 4C and D) of MHCC97 and Hep3B
cells, compared with the negative control group.
Activation of microRNA-543 expression
decreases the protein expression of p-JAK2, p-STAT3, c-Myc and
Bcl-2 in liver cancer cells
Western blot analysis was used to analyze the
protein expression of p-JAK2, p-STAT3, c-Myc and Bcl-2 protein
expression in MHCC97 and Hep3B cells transfected with microRNA-543
activator. It was identified that p-JAK2, p-STAT3, c-Myc and Bcl-2
protein expression in MHCC97 and Hep3B cells transfected with
microRNA-543 activator was significantly inhibited, compared with
the negative control group (Fig.
5).
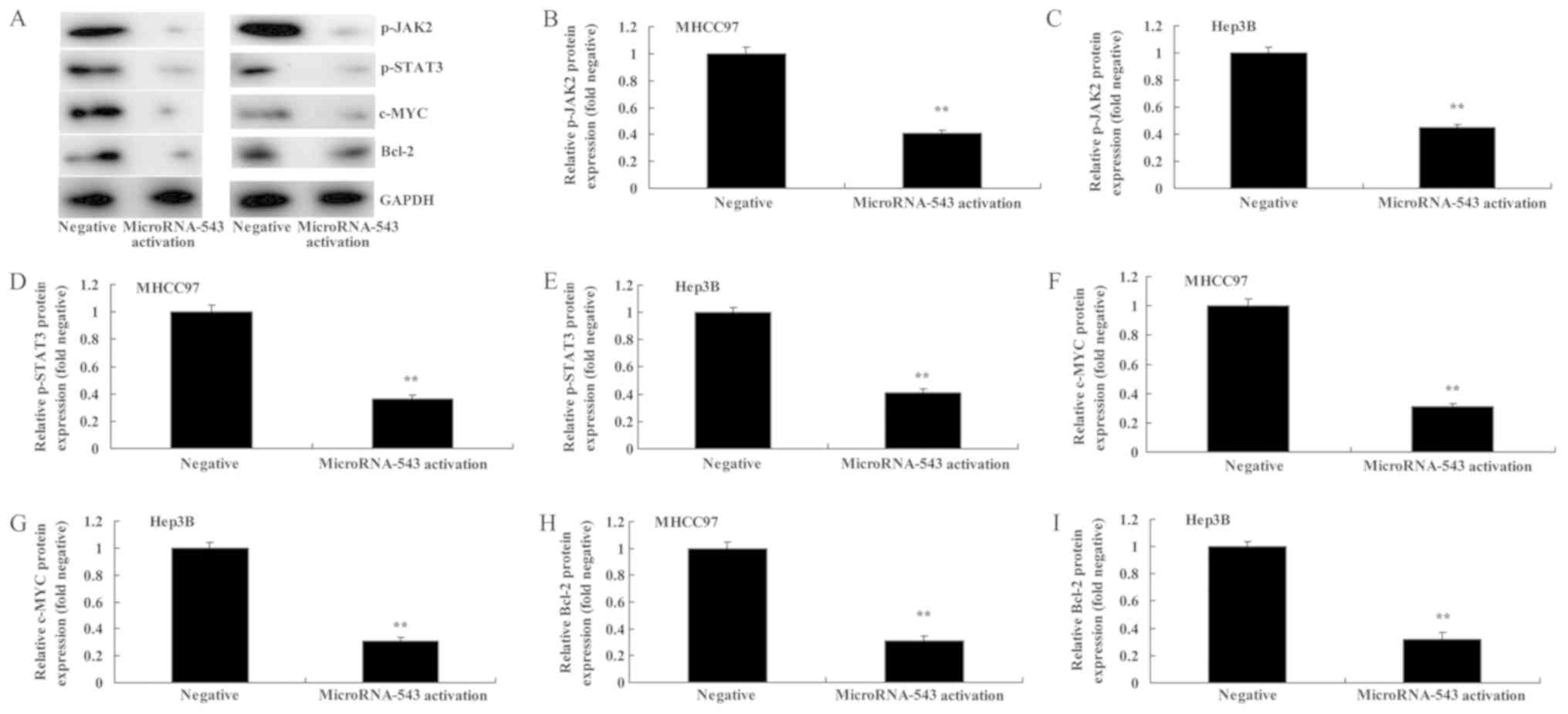 | Figure 5.Activation of microRNA-543 expression
decreases the protein expression of p-JAK2, p-STAT3, c-Myc and
Bcl-2 in liver cancer cells. (A) Western blot analysis of p-JAK2,
p-STAT3, c-Myc and Bcl-2 proteins in (left) MHCC97 and (right)
Hep3B cells. Quantification of p-JAK2 in (B) MHCC97 and (C) Hep3B
cells, p-STAT3 in (D) MHCC97 and (E) Hep3B cells, c-Myc in (F)
MHCC97 and (G) Hep3B cells, and Bcl-2 in (H) MHCC97 and (I) Hep3B
cells. **P<0.01 vs. negative group. p-, phospho-; JAK2, Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; Bcl-2, B-cell lymphoma 2. |
Discussion
Liver cancer is the most common malignant liver
disease in adults (8). Although the
success of its therapy is improving continuously, and there are a
variety of therapies, including surgical resection, liver
transplantation, radiotherapy and chemotherapy, none has
significantly extended the mean 5-year survival of patients with
liver cancer (9,10). One of the most important reasons for
the high mortality and poor prognosis of patients with liver cancer
is that the underlying molecular mechanism is poorly understood.
However, with the development of modern cell molecular biology, it
has been identified that the formation of liver cancer is typically
accompanied by a series of abnormal molecules and signaling
pathways. Therefore, investigating the underlying molecular
mechanism and treat the characteristics of molecules as antitumor
targets may open up a new avenue for the clinical prevention and
treatment of liver cancer (8). The
results of the present study indicated that the activation of
microRNA-543 expression decreases proliferation and induces
apoptosis of liver cancer cells, which suggests that microRNA-543
may function as a tumor suppressor in HCC.
The JAK/STAT signaling pathway is a well-known
signal transduction pathway, which is typically overexpressed in a
number of tumors (11). It has been
reported that excessive activation of STAT is markedly associated
with the invasion and metastasis of breast cancer, lung cancer,
colon cancer, stomach cancer, glioma, melanoma, squamous cell
carcinoma and other tumors (12). The
JAK/STAT signaling pathway may affect, directly or indirectly, cell
proliferation, apoptosis, invasion, metastasis and angiogenesis.
Furthermore, overexpression of STAT has been identified in HCC,
which is positively correlated with the clinical staging and
pathological grading of HCC (13). In
the JAK/STAT signaling pathway, the interaction of external
stimulatory factors and cell membranes leads to receptor
dimerization, which can activate JAK molecules in the cytoplasm
(14). The activated JAK is coupled
with cytokine receptors on the cell membrane, which phosphorylates
the tyrosine residues of the receptor, and the phosphorylation site
becomes the recognition site of the Src homology 2 domain in STAT
proteins. Subsequently, STAT binds to the receptor and is
phosphorylated by JAK (15). When the
affinity between phosphorylated STAT and their receptors decreases,
the phosphorylated STAT separates from its receptor and generates
the active form of dimer. It is subsequently transferred to the
cell nucleus and binds to specific DNA-response elements to result
in the expression of corresponding genes [e.g., vascular
endothelial growth factor (VEGF) and matrix metalloproteinases
(MMPs)], the synthesis of a variety of proteins (e.g., VEGF and
MMPs), and the performance of various biological behaviors
(15). The results of the present
study indicated that the activation of microRNA-543 limits the
protein expression of p-JAK2 and p-STAT3 in MHCC97 and Hep3B cells,
thus indicating that microRNA-543 regulates HCC cell proliferation
through the JAK2/STAT3 signaling pathway.
The cell cycle is a series of ordered cellular
activities which cause the division and duplication of its DNA to
create two daughter cells (16). The
entire process is divided into two periods: Silence period and
proliferative period. However, by contrast, cancer is disorderly
cell proliferation (17). The cell
cycle is associated with the regulation of a number of proteins. In
the cell transition phase from G2 to M, the
mitosis-promoting factor is the most important regulatory element
(17). c-Myc distribution results in
different types of liver cancer tissue, which means that there are
distinct differences between poorly differentiated adenocarcinoma
and highly or moderately differentiated adenocarcinoma, and
undifferentiated adenocarcinoma is different from differentiated
adenocarcinoma. Consequently, it can be concluded that positive
c-Myc expression is associated with the degree of tumor
differentiation. c-Myc is slightly increased in slow-growing and
highly differentiated adenocarcinoma, whereas it is significantly
increased in fast-growing and poorly differentiated adenocarcinoma
(16,18). In addition, in the present study, it
was identified that microRNA-543 activation represses c-Myc
expression of MHCC97 and Hep3B cells. Thus, c-Myc expression may be
a crucial signaling pathway through which microRNA-543 affects
HCC.
Tumorigenesis arises owing to the imbalance between
cell proliferation and apoptosis. It has been identified that there
is abnormal expression of various cancer genes, tumor suppressor
genes and apoptosis genes in HCC. Among them, Bcl-2 and c-Myc genes
are key representatives (19). The
proto-oncogene Bcl-2 was originally identified in the chromosomal
translocation point of B-cell lymphoma. The proto-oncogene Bcl-2,
as an anti-apoptotic gene, is predicated to be associated with
tumor resistance and anti-radiation sensitivity (20). Previous studies have indicated that
Bcl-2 and its protein product are expressed in benign and malignant
tissue of several non-hematopoietic systems, except lymphoma, such
as lung cancer, stomach cancer and breast cancer; and they affect
the occurrence and development of tumors (19,21).
Transgenic animal experiments revealed that Bcl-2 expression by
liver cells may be unaffected by Fas-mediated hepatocyte apoptosis,
which leads to tumorigenesis (21).
It was identified that microRNA-543 activation also significantly
decreases Bcl-2 protein expression in MHCC97 and Hep3B cells.
In conclusion, the results of the present study
indicated that microRNA-543 inhibits liver cancer growth and leads
to apoptosis via the JAK2/STAT3 signaling pathway. The results also
suggested that microRNA-543 may serve as a novel diagnostic and
prognostic biomarker for HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81571939).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJ designed the experiment. DX, DW, JW and WZ
performed the experiment. FJ and DX analyzed the data. FJ wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
ethics and scientific committee of The China Japan Union Hospital
of Jilin University. All patients provided written informed consent
for the use of their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rauf N, Tahir SS, Dilawar S, Ahmad I and
Parvez S: Serum selenium concentration in liver cirrhotic patients
suffering from hepatitis B and C in Pakistan. Biol Trace Elem Res.
145:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gabrielson A, Tesfaye AA, Marshall JL,
Pishvaian MJ, Smaglo B, Jha R, Dorsch-Vogel K, Wang H and He AR:
Phase II study of temozolomide and veliparib combination therapy
for sorafenib-refractory advanced hepatocellular carcinoma. Cancer
Chemother Pharmacol. 76:1073–1079. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senggunprai L, Kukongviriyapan V, Prawan A
and Kukongviriyapan U: Quercetin and EGCG exhibit chemopreventive
effects in cholangiocarcinoma cells via suppression of JAK/STAT
signaling pathway. Phytother Res. 28:841–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koike K, Takaki A, Tatsukawa M, Suzuki M,
Shiraha H, Iwasaki Y, Sakaguchi K and Shiratori Y: Combination of
5-FU and IFNalpha enhances IFN signaling pathway and caspase-8
activity, resulting in marked apoptosis in hepatoma cell lines. Int
J Oncol. 29:1253–1261. 2006.PubMed/NCBI
|
|
5
|
Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu
BM and Li J: Emerging role of miRNAs in regulating macrophage
activation and polarization in immune response and inflammation.
Immunology. 148:237–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piontek K and Selaru FM: MicroRNAs in the
biology and diagnosis of cholangiocarcinoma. Semin Liver Dis.
35:55–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge YS, Xu GL, Zhang CH, Jia WD, Li JS, Ma
JL and Yu JH: Efficacy and feasibility of radiofrequency ablation
for hepatocellular carcinoma patients. Hepatogastroenterology.
59:2540–2542. 2012.PubMed/NCBI
|
|
9
|
Safran H, Charpentier KP, Kaubisch A,
Mantripragada K, Dubel G, Perez K, Faricy-Anderson K, Miner T, Eng
Y, Victor J, et al: Lenalidomide for second-line treatment of
advanced hepatocellular cancer: A brown university oncology group
phase II study. Am J Clin Oncol. 38:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu AX, Holalkere NS, Muzikansky A, Horgan
K and Sahani DV: Early antiangiogenic activity of bevacizumab
evaluated by computed tomography perfusion scan in patients with
advanced hepatocellular carcinoma. Oncologist. 13:120–125. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lejeune D, Dumoutier L, Constantinescu S,
Kruijer W, Schuringa JJ and Renauld JC: Interleukin-22 (IL-22)
activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a
rat hepatoma cell line. Pathways that are shared with and distinct
from IL-10. J Biol Chem. 277:33676–33682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaper F, Siewert E, Gomez-Lechon MJ,
Gatsios P, Sachs M, Birchmeier W, Heinrich PC and Castell J:
Hepatocyte growth factor/scatter factor (HGF/SF) signals via the
STAT3/APRF transcription factor in human hepatoma cells and
hepatocytes. FEBS Lett. 405:99–103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inamura K, Matsuzaki Y, Uematsu N, Honda
A, Tanaka N and Uchida K: Rapid inhibition of MAPK signaling and
anti-proliferation effect via JAK/STAT signaling by
interferon-alpha in hepatocellular carcinoma cell lines. Biochim
Biophys Acta. 1745:401–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang FB, Wang L, Jia HC, Li D, Li HJ,
Zhang YG and Sun DX: B7-H3 promotes aggression and invasion of
hepatocellular carcinoma by targeting epithelial-to-mesenchymal
transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int.
15:452015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunecke D, Spanel R, Langer F, Nam SW and
Borlak J: MYC-regulated genes involved in liver cell dysplasia
identified in a transgenic model of liver cancer. J Pathol.
228:520–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Radisky DC, Yang D, Xu R, Radisky
ES, Bissell MJ and Bishop JM: MYC suppresses cancer metastasis by
direct transcriptional silencing of alphav and beta3 integrin
subunits. Nat Cell Biol. 14:567–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Jin R, Zhang X, Lv F, Liu L, Liu D,
Liu K, Li N and Chen D: Oncogenic activation of glypican-3 by c-Myc
in human hepatocellular carcinoma. Hepatology. 56:1380–1390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leskov I, Pallasch CP, Drake A, Iliopoulou
BP, Souza A, Shen CH, Schweighofer CD, Abruzzo L, Frenzel LP,
Wendtner CM, et al: Rapid generation of human B-cell lymphomas via
combined expression of Myc and Bcl2 and their use as a preclinical
model for biological therapies. Oncogene. 32:1066–1072. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XW and Xu B: Differential regulation
of P53, c-Myc, Bcl-2, Bax and AFP protein expression, and caspase
activity during 10-hydroxycamptothecin-induced apoptosis in Hep G2
cells. Anticancer Drugs. 11:747–756. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Medrzycki M, Bunting ST and
Bunting KD: Stat5-deficient hematopoiesis is permissive for
Myc-induced B-cell leukemogenesis. Oncotarget. 6:28961–28972.
2015.PubMed/NCBI
|