Introduction
Colorectal cancer (CRC) was the third most common
carcinoma of the human digestive system worldwide in 2014 (1). Influenced by genetic, environment and
life style factors, the risk of CRC has increased annually,
becoming one of the most common types of cancer with a high
mortality rate reported in China (2014) (1). The current treatments of CRC are
primarily surgery and chemotherapy (2). A previous study indicated that estrogen
serves a potential role in the development and prognosis of CRC
(3).
With the development of modern industry, numerous
endocrine-disrupting chemicals (EDCs), including nonylphenol (NP)
and bisphenol A, have been identified in the environment (4–7). EDCs may
have an estrogen-associated or androgen-associated effect by
binding to hormone receptors and promoting the development of
hormone-dependent tumors (8). NP is
an EDC, which could influence the development of estrogen-dependent
cancer types, including breast and prostate cancer (9,10). Our
previous study indicated that NP could activate
extracellular-signal-regulated kinase (ERK)1/2 to induce the
proliferation of CRCs cells (11).
However, the underlying molecular mechanism of NP on the
development of CRCs remains unclear.
Protein kinase C (PKC), which exists in various
cells and tissues, is a type of multifunctional serine and
threonine kinase. This protein could mediate the proliferation and
differentiation of cells, and has been reported to be involved in
the regulation of the cell cycle and apoptosis, promoting the
development and metastasis of tumors (12). PKCζ is a member of the PKC family,
which influences proliferation and transfer of various types of
cancer cells (13–15). Inhibiting the expression of PKCζ may
reduce the invasion ability of CRC, breast cancer and glioma
(16). To the best of our knowledge,
no evidence exists on whether NP could mediate the development of
CRC by regulating the expression of PKCζ.
To further examine the effect of NP on the
proliferation of CRC cells and the expression and activity of PKCζ,
different concentrations of NP were used to treat the COLO205 CRC
cells, and to knock down the expression of PKCζ by PKCζ small
interfering (si)RNA. The in vitro effects of NP were further
examined with different concentrations of NP on the cell cycle and
apoptosis of COLO205 cells, and the altered expression of PKCζ was
analyzed.
Materials and methods
Cell culture and treatment
Human CRC COLO205 cells were obtained from the
Chinese Academy of Sciences Institute of Cell Resource Center
(Shanghai, China). The cells were cultured in RPMI-1640 (GE
Healthcare Life Sciences, Little Chalfont, UK) supplemented with
10% fetal bovine serum (FBS; Procell Life Science & Technology
Co., Ltd., Wuhan, China), 100 IU/ml penicillin and 100 mg/ml
streptomycin at 37°C in an atmosphere containing 5% CO2.
NP with analytical standard purities was purchased from Shanghai
Aladdin Biochemical Technology Co., Ltd. (Shanghai, China), and was
dissolved in absolute ethyl alcohol to 50 mM.
siRNA design and cell
transfection
The siRNA oligo was synthesized by Sangon Biotech
Co., Ltd., (Shanghai, China). Sequences were as follows: si-PKCζ,
5′-GGAGGACCTTAAGCCAGTT-3′ and siRNA negative control (NC)
5′-AGACTGTGAATCTAGATCAAG-3′.
For transfection, COLO205 cells were cultured for 12
h in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 0.1 mg/ml streptomycin, and the fragment of siRNA
(50 nM) was transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. After 72 h of
transfection, cells were washed by PBS and then lysed with 1X
radioimmunoprecipitation (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) supplemented with 0.2 mM
phenylmethylsulphonyl fluoride protease inhibitor (cat. no. 36978;
Thermo Fisher Scientific, Inc.) for 30 min on ice for western blot
analysis.
MTT analysis
Cell viability was detected using the CellTiter
96® Non-Radioactive Cell Proliferation assay (MTT; cat.
no. G4000; Promega Corporation, Madison, WI, USA). COLO205 cells
(1×104 cells/ml) were seeded in 96-well plates for 24 h
prior to treatment, with normal saline and cell culture mediums
serving as the control, and three wells were prepared for each of
the following groups: 0; 1×10−6; 1×10−7 and
1×10−8 mol/l NP alone (17,18);
1×10−6 mol/l NP with si-PKCζ; and 1×10−6
mol/l NP with NC. Following treatment for 0, 24, 48 and 72 h, a
total of 15 µl provided dye solution was added to each well, and
the 96-well plate was incubated at 37°C for 4 h, subsequent to
adding 100 µl Solubilization/Stop Solution from the kit. Viability
was recorded at a wavelength of 570 nm on a microplate reader
(Multiskan; Thermo Fisher Scientific, Inc.). The assay was repeated
in triplicate.
Flow cytometry analysis of cell cycle
and apoptosis
The effect of NP and si-PKCζ on cell cycle
progression was determined by flow cytometry. After 24 h of
treatment, the cells were digested and fixed with 70% ethanol for
24 h at 4°C. Following fixation, cells were stained with 50 µg/ml
propidium iodide (PI) solution and 100 µg/ml RNase A in PBS for 30
min in the dark on ice and then subjected to cell cycle analysis.
The apoptotic rate was measured using Annexin V/PI double staining
(Annexin V-FITC Apoptosis Detection kit; cat. no. C1062; Beyotime
Institute of Biotechnology). A total of 300 µl binding buffer was
used for cell resuspension 1×106, and 5 µl Annexin
V-fluorescein isothiocyanate was added to the cell suspension for
10 min in the dark at room temperature. A total of 5 µl PI was
subsequently added to the cell suspension for 5 min in the dark on
ice. The samples were analyzed with a FACSCalibur flow cytometer
and the FACSCalibur system (both BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
Total protein was extracted from COLO205 cells
following treatment using RIPA buffer, and the concentrations were
determined by bicinchoninic acid (Thermo Fisher Scientific, Inc.).
A total of 40 µg total protein was separated by SDS-PAGE (10%
spacer gel and 5% separation gel). Proteins were transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA
USA), and membranes were subsequently blocked with 5% skim milk
powder (BD Biosciences) for 60 min at room temperature. The
membranes were subsequently incubated with the following primary
antibodies: Cyclin D1 (dilution, 1:800; cat. no. ab134175); cyclin
E (dilution, 1:800; cat. no. ab33911), B-cell lymphoma 2 (Bcl-2)
associated agonist of cell death (Bad; dilution, 1:500; cat. no.
ab62465), Bcl-2 (dilution, 1:1,000; cat. no. ab32124),
cyclin-dependent kinase inhibitor (p27; dilution 1:1,000; cat. no.
ab32034), PKCζ (dilution 1:1,500; cat. no. ab59364), phosphorylated
(p)-PKCζ (dilution 1:1,500; cat. no. ab62372) (all from Abcam,
Cambridge, MA, USA), ERK1/2 (dilution 1:1,000; cat. no. 4695; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-ERK1/2 (dilution
1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.) and GAPDH
(dilution 1:1,000; cat. no. ab8245; Abcam) overnight at 4°C. The
membranes were subsequently probed with goat anti-rabbit
horseradish peroxidase-labeled secondary antibody (dilution
1:10,000; cat. no. ab6721; Abcam) for 1 h at room temperature.
Target proteins were detected with Clarity™ Western
enhanced chemiluminescence substrate (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocols. The
optical density was analyzed by AlphaEaseFC software (version 5.0,
ProteinSimple, San Jose, CA, USA). GAPDH was used as the internal
control. All experiments were conducted in triplicate.
Statistical analysis
Each experiment was repeated in triplicate.
Differences between different groups were evaluated by one-way
analysis of variance, followed by Duncan's multiple range post-hoc
test using GraphPad prism 6.0 (GraphPad Software, Inc., La Jolla,
CA, USA). Difference between two groups were analyzed by a
Student's t-test using Microsoft Excel 2017 (Microsoft Corporation,
Redmond, WA, USA). Results are presented as means ± standard
deviation and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of NP and si-PKCζ on the
proliferation of COLO205 cells
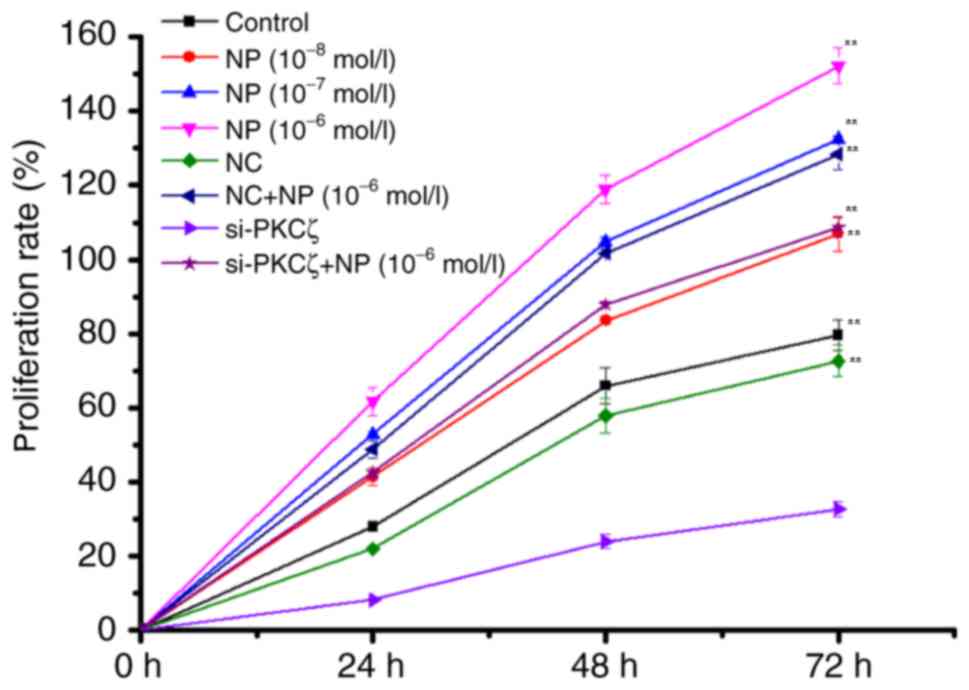
The results of MTT indicated that NP
(1×10−6−1×10−8 mol/l) could significantly
induce the proliferation of COLO205 cells (F=48.66; P<0.01) in a
time-and dose-dependent manner, compared with the control group
(Fig. 1). Compared with the NC group,
the proliferation of COLO205 cells demonstrated was significantly
reduced by si-PKCζ transfection in a time-dependent manner
(P<0.01); however, recovery of proliferation was indicated
following NP treatment (Fig. 1).
Effects of NP and si-PKCζ on the cell
cycle
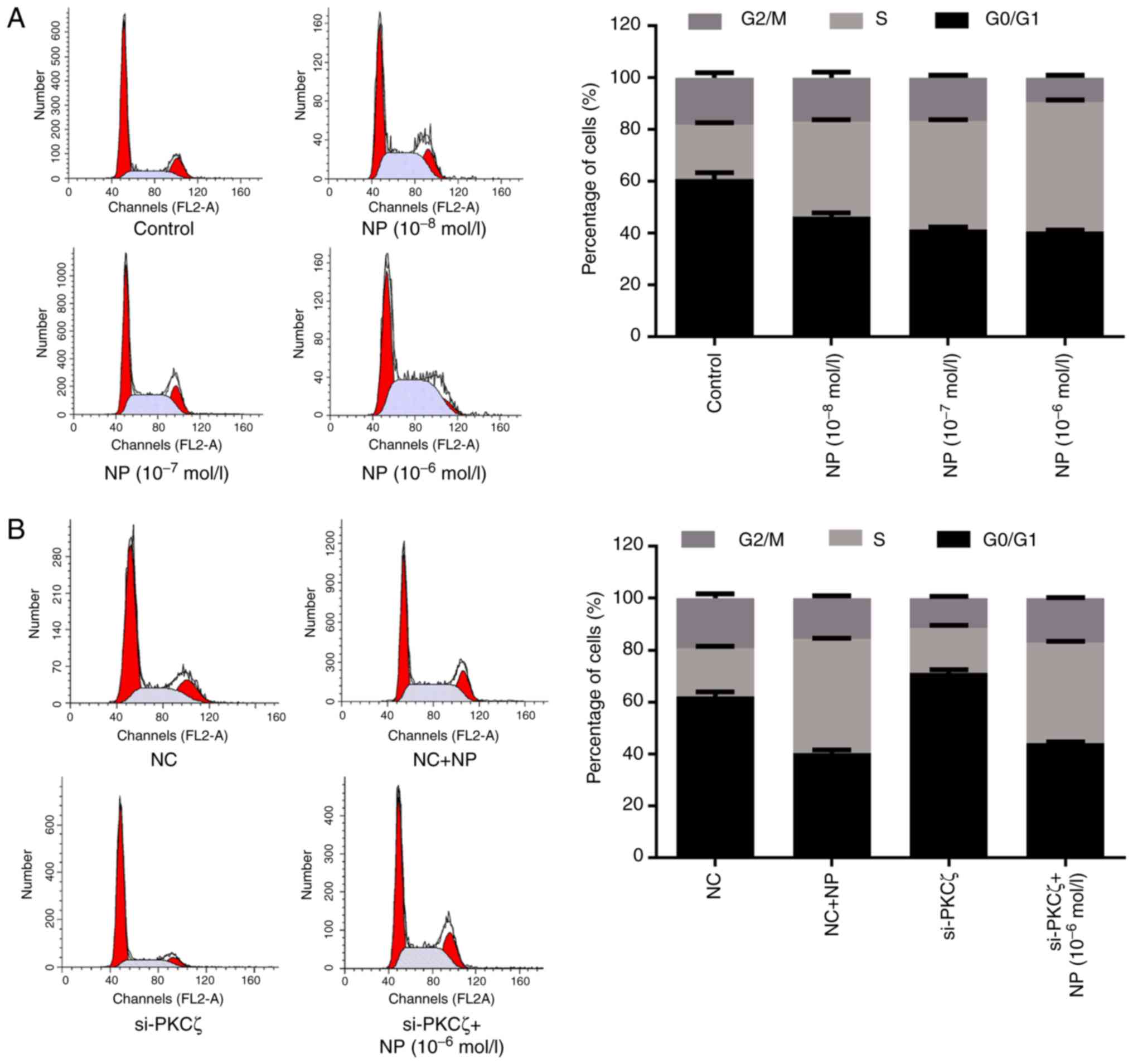
Flow cytometry was used to investigate the influence
of NP and PKCζ on the cell cycle. Compared with the control group,
the ratio of G0/G1 phase cells was significantly reduced by NP
treatment (t=9.25, 13.17 and 14.74 for 1×10−8,
1×10−7 and 1×10−6 mol/l NP, respectively; all
P<0.01; Fig. 2A), and
significantly increased by the suppression of PKCζ expression
(t=11.29; P<0.01), however, this elevation was suppressed by NP
treatment (t=33.35; P<0.01) (Fig.
2B). Furthermore, NP treatment increased the ratio of S phase
cells, compared with the control group (t=22.67, 37.02 and 47.41
for 1×10−8, 1×10−7 and 1×10−6
mol/l NP, respectively; all P<0.01; Fig. 2A). Following si-PKCζ transfection, the
ratio of S phase cells was significantly decreased compared with
the NC group (t=1.83; P<0.05), however, it was significantly
increased following NP treatment (t=51.31; P<0.01) (Fig. 2B).
Effects of NP and si-PKCζ on cell
apoptosis
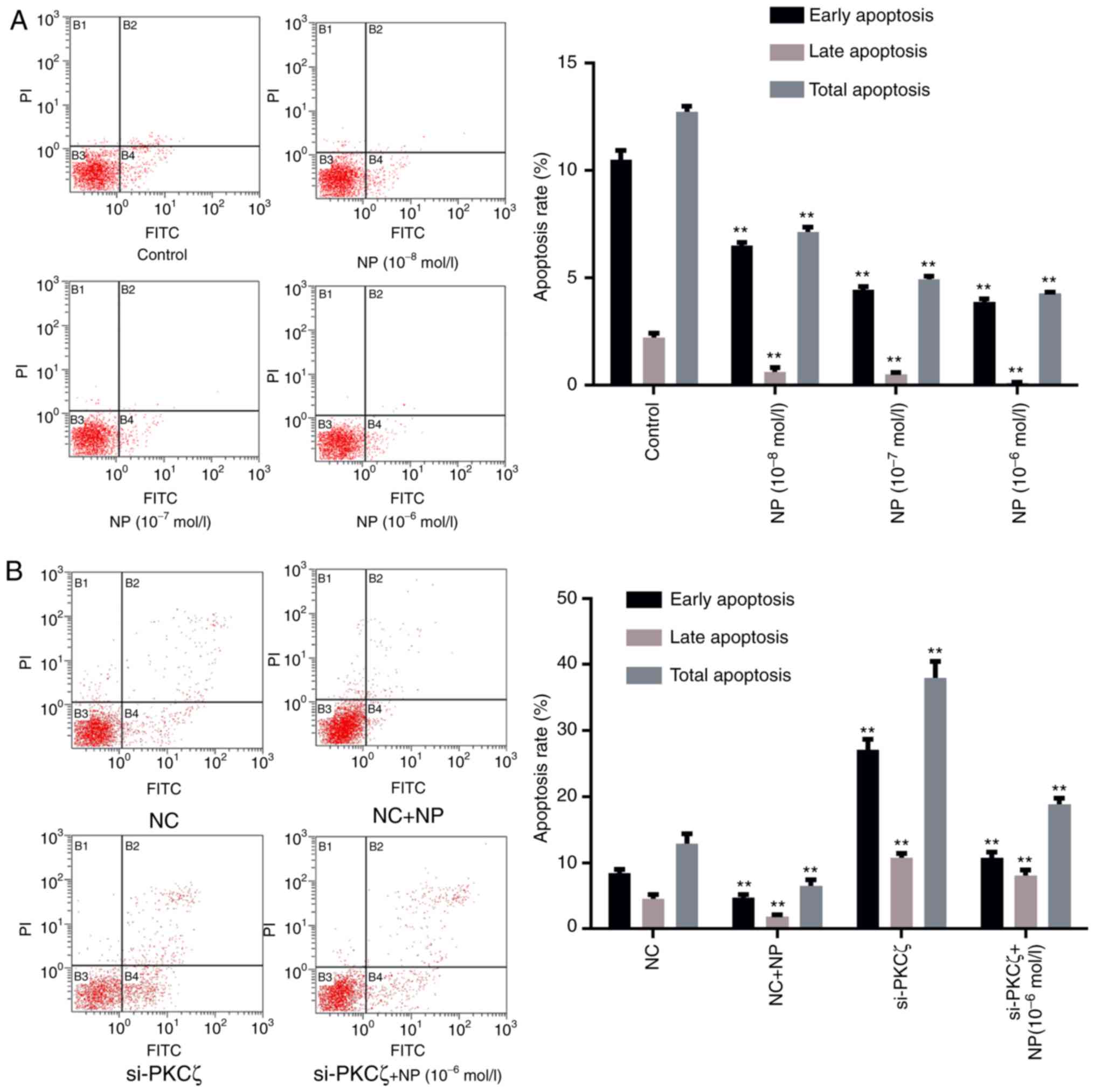
To evaluate the influence of NP and PKCζ on cell
apoptosis, flow cytometry was utilized to identify any changes in
the apoptotic rate of cells following NP treatment or si-PKCζ
transfection. The results indicated that the ratio of viable to
non-viable apoptotic cells in the NP treatment group was lower than
that in the control group (P<0.01; Fig. 3A). Suppression of PKCζ expression
significantly increased the ratio of viable apoptotic and
non-viable apoptotic cells (P<0.01 vs. NC group), however, the
ratio of apoptotic cells decreased following NP treatment (Fig. 3B).
Expression change of cell
cycle-associated proteins
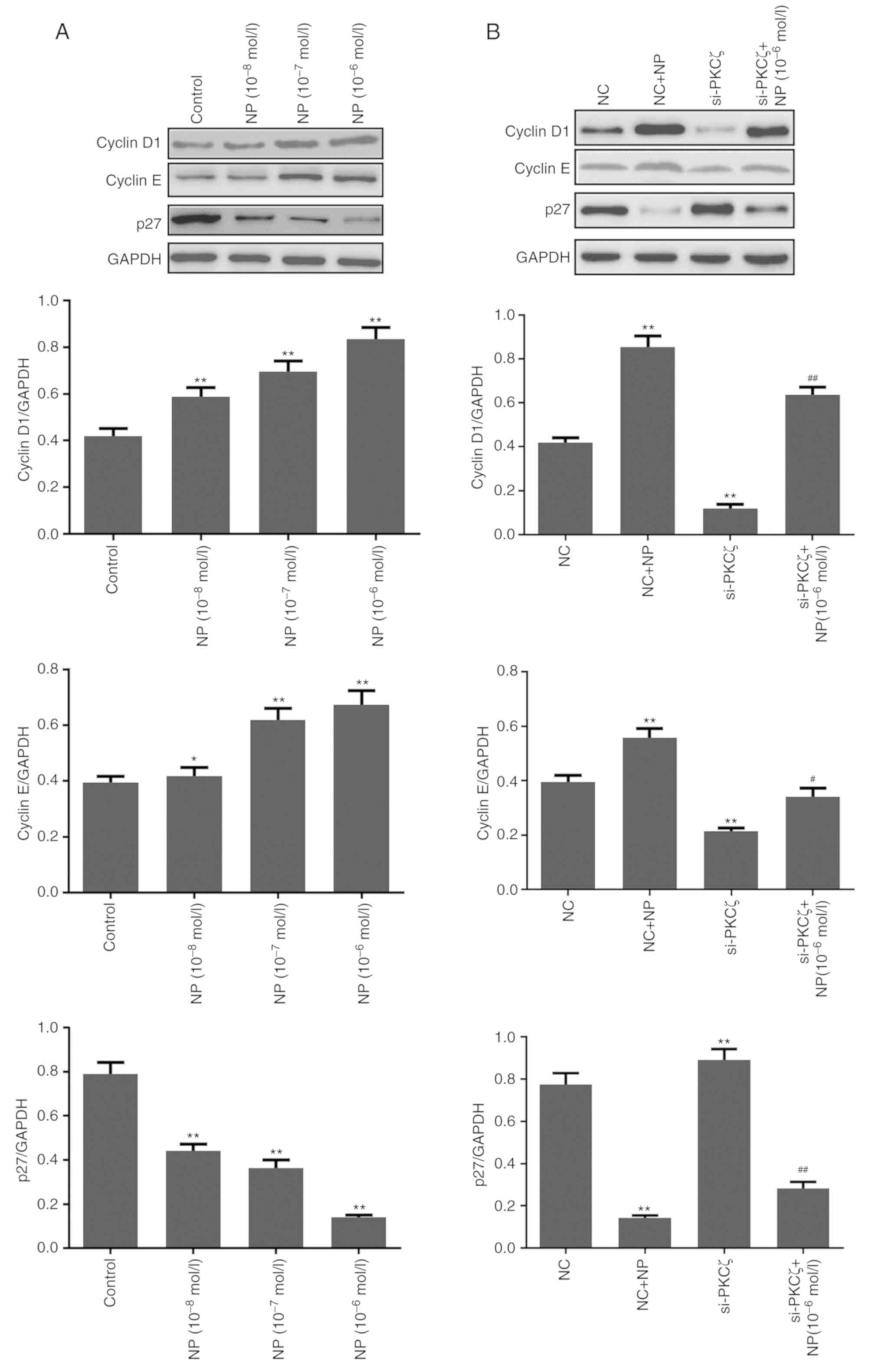
Compared to the control group, NP treatment
significantly upregulated the expression of cyclin D1 and cyclin E,
and downregulated the expression of p27 (all P<0.05; Fig. 4A). Following siRNA transfection, the
expression of cyclin D1 and cyclin E was significantly reduced by
PKCζ suppression, and the expression of p27 was increased (all
P<0.01 vs. NC group). Following NP treatment in the
si-PKCζ-transfected cells, the expression of cyclin D1 and E
increased, whereas the expression of p27 decreased (Fig. 4B).
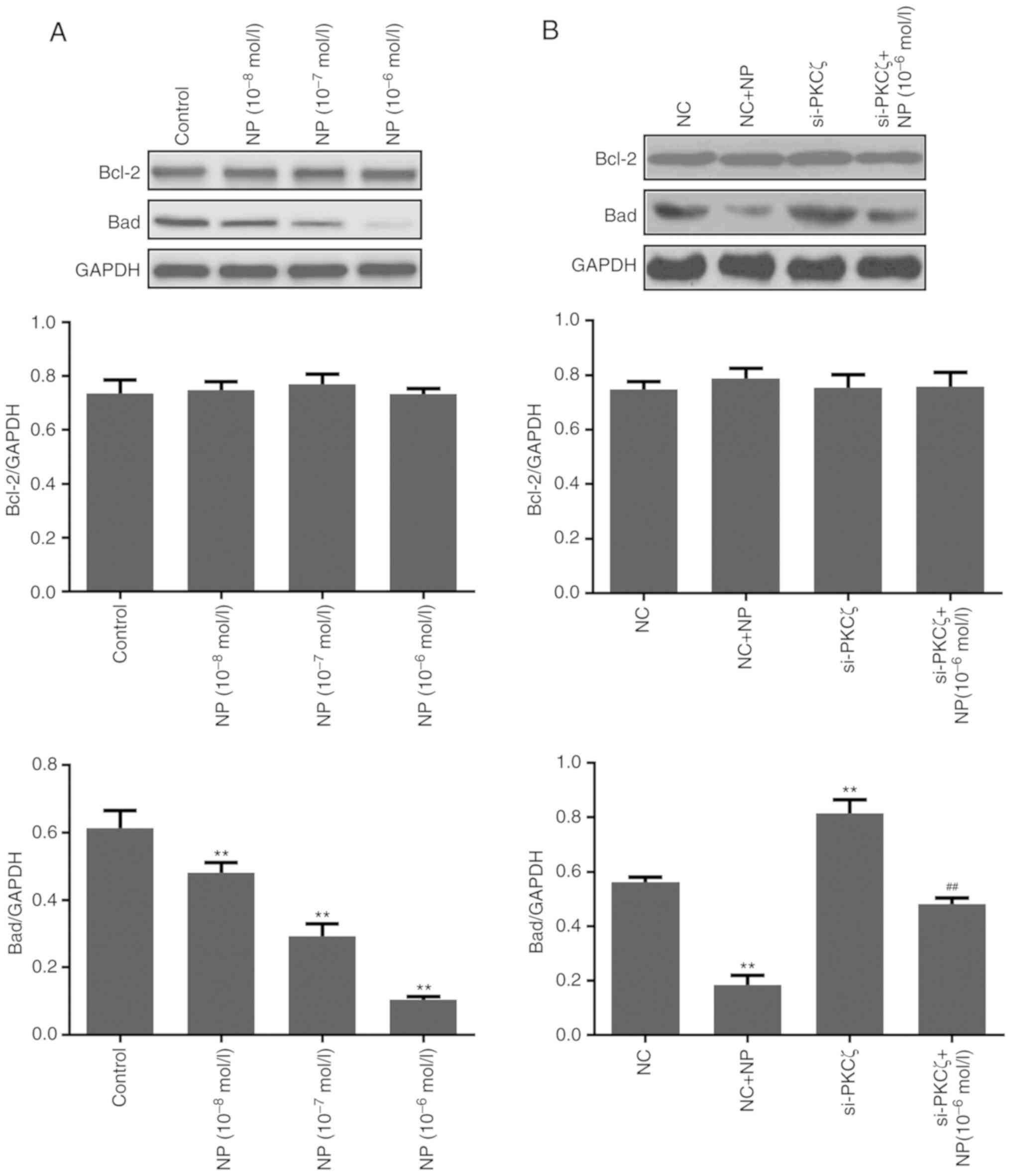
Expression change of
apoptosis-associated protein
Additionally, the expression of apoptosis-associated
proteins Bcl-2 and Bad was examined (Fig.
5A). The results of the western blot analysis indicated that NP
treatment had no significant effect on the expression of Bcl-2, but
significantly reduced the expression of Bad (P<0.01 for all
concentrations of NP vs. control). The expression of Bad was
significantly increased following si-PKCζ transfection (P<0.01
vs. NC group) and significantly reduced by subsequent NP treatment
(P<0.01 vs. si-PKCζ group) (Fig.
5B).
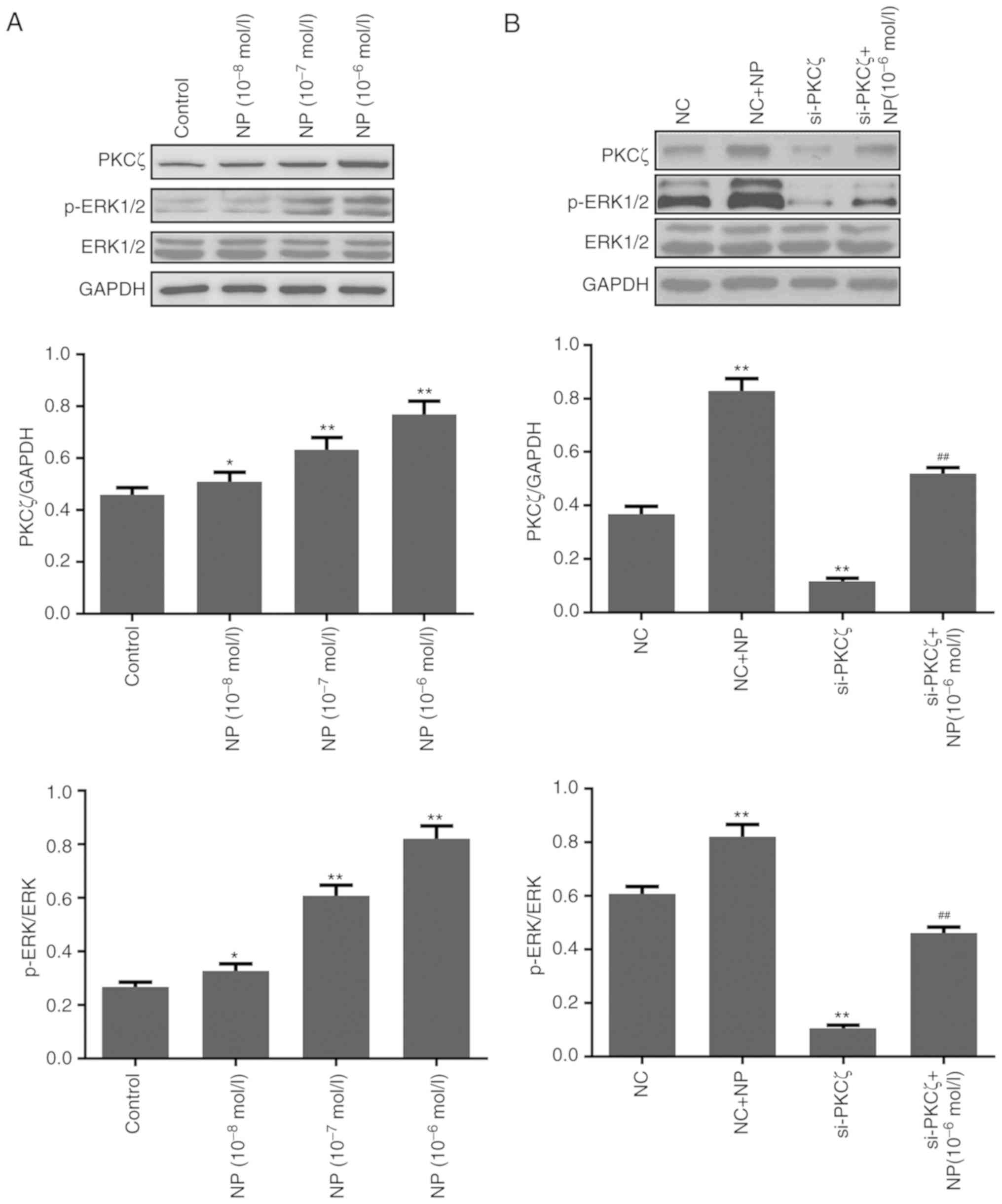
Expression change of PKCζ and
ERK1/2
The results of the western blot analysis indicated
that the expression of PKCζ and the phosphorylation of ERK1/2 were
significantly increased by NP (P<0.05 for 1×10−8;
P<0.01 for 1×10−7−1×10−6; Fig. 6A). Following si-PKCζ transfection, the
expression of PKCζ and the phosphorylation of ERK1/2 were
significantly reduced (both P<0.01 vs. NC), however, this was
significantly recovered following NP treatment (both P<0.01 vs.
siPKCζ alone) (Fig. 6B). The
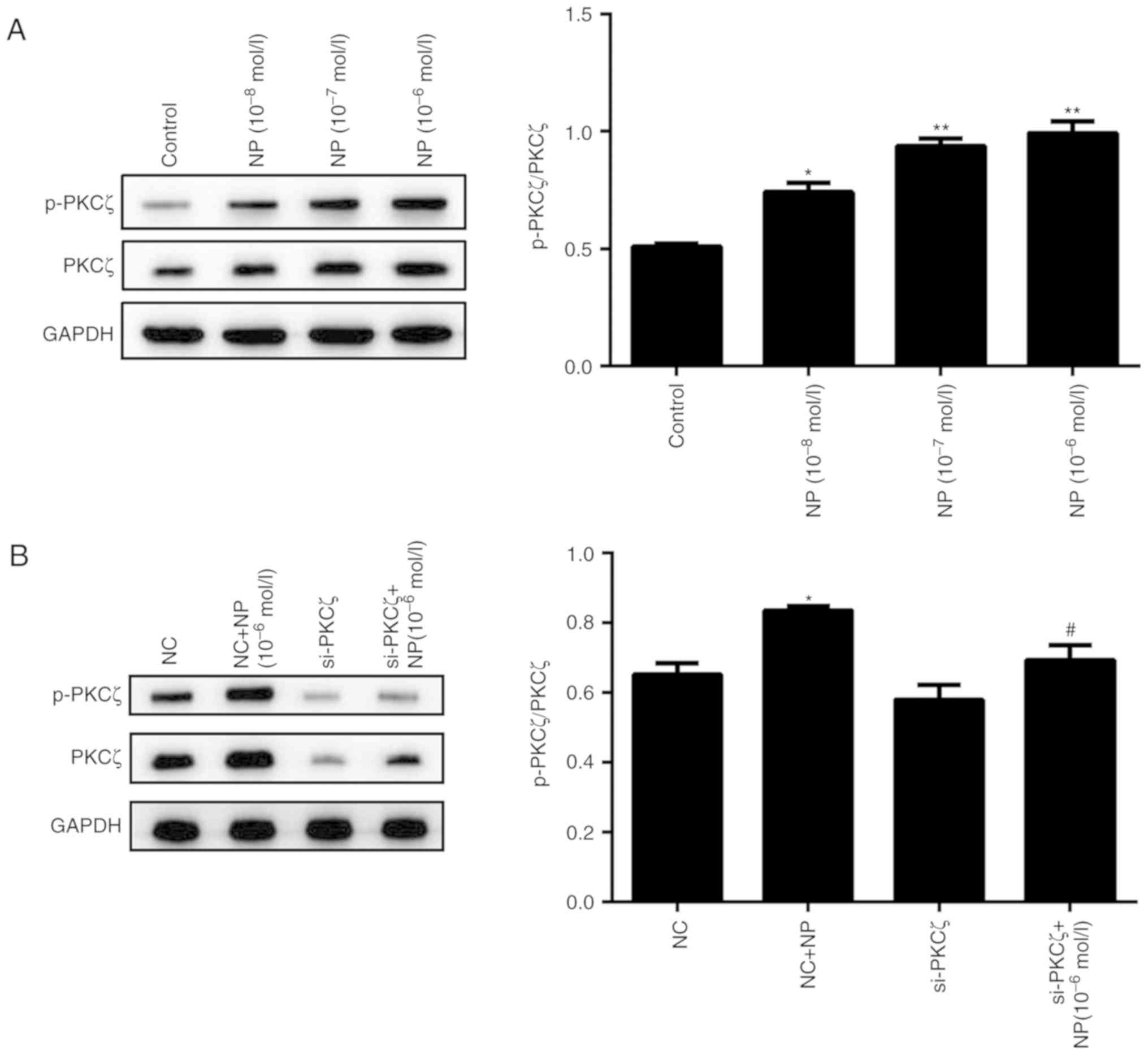
phosphorylation of PKCζ was also influenced by NP treatment.
Following NP treatment, the phosphorylation of PKCζ was
significantly increased for all concentrations of NP, compared with
the control (P<0.01; P<0.05; Fig.
7A). The phosphorylation level of PKCζ did not change
significantly following si-PKCζ transfection, however, subsequent
NP treatment significantly increased the phosphorylation (P<0.01
vs. si-PKCζ alone; Fig. 7B).
Discussion
Globally, CRC is the third leading cause of
cancer-associated morbidity and is the fourth leading cause of
cancer-associated mortality (2014) (1). In China, the incidence of CRCs presents
an annual rising trend (1), bearing a
serious threat to human health. The risk of human CRC is associated
with environment, dietary and genetics factors (19). A previous study indicated that CRC was
an estrogen-dependent tumor type, and the level of estrogen in
patients was directly associated with the development and prognosis
of CRC (2). NP is an EDC, which has
the ability to induce endocrine disruption, reproduction disorders
and the development of various types of cancer (20–23).
In the present study, NP was indicated to
significantly induce the proliferation of COLO205 cells by
promoting cells from the G1 phase into the S phase. Cyclin D1 and E
are two proteins that have been demonstrated to induce cells
transforming from the G1 phase to the S phase, and p21 and p27 are
two kinases, which have been reported to inhibit the cell cycle
transformation (24–26). In the present study, it was indicated
that NP could upregulate the expression of cyclin D1 and E, and
downregulate the expression of p27.
Abnormalities in the cell apoptosis mechanism can
induce cell proliferation, which may result in the development of
tumors (27). Bcl-2 is an important
anti-apoptosis protein, which is considered as an oncogene. Bad is
a type of pro-apoptosis protein, which binds to Bcl-2 to prevent
apoptosis (28). The dynamic balance
of these aforementioned proteins serve an important role in
maintaining a normal function of cells. The present study indicated
that NP slightly affected the expression of Bcl-2, however, NP
could significantly reduce the expression of Bad. Therefore, NP may
inhibit apoptosis by inhibiting the pro-apoptotic function of
Bad.
PKCζ is involved in the proliferation and transfer
of various tumor cells (13–15). Inhibiting the expression of PKCζ has
been reported to suppress the invasion ability of CRC, breast
cancer and glioma (16). A previous
study indicated that PKCζ promotes tumor cell proliferation and the
regulation of apoptosis by phosphorylation of STAT3 (29). The results of the present study
indicated that NP could induce the expression of PKCζ, in addition
to the phosphorylation of ERK1/2. However, suppression of PKCζ
significantly reduced the phosphorylation of ERK1/2. The
aforementioned results indicated that NP may induce the
proliferation of CRC cells by upregulating the expression of PKCζ
and increasing the phosphorylation of ERK1/2. Further investigation
is required in order to examine the underlying mechanism of PKCζ
overexpression by NP.
In conclusion, the present study identified the
effect of NP on the expression and activity of PKCζ using RNAi
technology. The results indicated that NP could induce the
proliferation of COLO205 cells by activating the ERK pathway
through PKCζ activation. The present study provides new direction
for CRC prevention and therapy. However, the underlying mechanisms
of NP on the development of CRCs requires further study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Scientific Foundation in the Science and Technology Department of
Guizhou Province, China (grant no. J20142185).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH and MW cultured the COLO205 cells, and performed
the NP treatment and siRNA transfection. JX examined the
proliferation, cell cycle and apoptosis of the COLO205 cells. MX
and XZ designed the experiment. XY performed the western blot
analysis and was a major contributor in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing financial
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fogel EL, Shahda S, Sandrasegaran K,
DeWitt J, Easler JJ, Agarwal DM, Eagleson M, Zyromski NJ, House MG,
Ellsworth S, et al: A multidisciplinary approach to pancreas cancer
in 2016: A review. Am J Gastroenterol. 112:537–554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stedman KE, Moore GE and Morgan RT:
Estrogen receptor proteins in diverse human tumors. Arch Surg.
115:244–248. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang KA, Park SH, Yi BR and Choi KC: Gene
alterations of ovarian cancer cells expressing estrogen receptors
by estrogen and bisphenol a using microarray analysis. Lab Anim
Res. 27:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeung EB and Choi KC: Toxicological
mechanism of endocrine disrupting chemicals: Is estrogen receptor
involved? Toxicol Res. 26:237–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park MA, Hwang KA and Choi KC: Diverse
animal models to examine potential role(s) and mechanism of
endocrine disrupting chemicals on the tumor progression and
prevention: Do they have tumorigenic or anti-tumorigenic property?
Lab Anim Res. 27:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schug TT, Janesick A, Blumberg B and
Heindel JJ: Endocrine disrupting chemicals and disease
susceptibility. J Steroid Biochem Mol Biol. 127:204–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roy JR, Chakraborty S and Chakraborty TR:
Estrogen-like endocrine disrupting chemicals affecting puberty in
humans-a review. Med Sci Monit. 15:RA137–RA145. 2009.PubMed/NCBI
|
|
9
|
Kang NH, Hwang KA, Kim TH, Hyun SH, Jeung
EB and Choi KC: Induced growth of BG-1 ovarian cancer cells by
17β-estradiol or various endocrine disrupting chemicals was
reversed by resveratrol via downregulation of cell cycle
progression. Mol Med Rep. 6:151–156. 2012.PubMed/NCBI
|
|
10
|
Kim SH, Nam KH, Hwang KA and Choi KC:
Influence of hexabromocyclododecane and 4-nonylphenol on the
regulation of cell growth, apoptosis and migration in prostatic
cancer cells. Toxicol In Vitro. 32:240–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Huang H, Wang M, Zheng X, Xu J and
Xie M: Effect of nonylphenol on the regulation of cell growth in
colorectal cancer cells. Mol Med Rep. 16:2211–2216. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mellor H and Parker PJ: The extended
protein kinase C superfamily. Biochem J. 332:281–292. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao S, Bee A, Brewer D, Dodson A, Beesley
C, Ke Y, Ambroisine L, Fisher G, Møller H, Dickinson T, et al:
PRKC-ζ expression promotes the aggressive phenotype of human
prostate cancer cells and is a novel target for therapeutic
intervention. Genes Cancer. 1:444–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo H, Gu F, Li W, Zhang B, Niu R, Fu L,
Zhang N and Ma Y: Reduction of protein kinase C zeta inhibits
migration and invasion of human glioblastoma cells. J Neurochem.
109:203–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang S, Ouyang N, Lin L, Chen L, Wu W, Su
F, Yao Y and Yao H: HGF-induced PKCζ activation increases
functional CXCR4 expression in human breast cancer cells. PLoS One.
7:e291242012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luna-Ulloa LB, Hernández-Maqueda JG,
Santoyo-Ramos P, Castañeda-Patlán MC and Robles-Flores M: Protein
kinase C ζ is a positive modulator of canonical Wnt signaling
pathway in tumoral colon cell lines. Carcinogenesis. 32:1615–1624.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YS, Hwang KA, Hyun SH, Nam KH, Lee CK
and Choi KC: Bisphenol A and nonylphenol have the potential to
stimulate the migration of ovarian cancer cells by inducing
epithelial-mesenchymal transition via an estrogen receptor
dependent pathway. Chem Res Toxicol. 28:662–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park MA and Choi KC: Effects of
4-nonylphenol and bisphenol A on stimulation of cell growth via
disruption of the transforming growth factor-β signaling pathway in
ovarian cancer models. Chem Res Toxicol. 27:119–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang L, Shen H, Li X, Li Z, Liu Z, Xu J,
Ma S, Zhao X, Bai X, Li M, et al: MiR-125a-5p decreases after long
non-coding RNA HOTAIR knockdown to promote cancer cell apoptosis by
releasing caspase 2. Cell Death Dis. 7:e21372016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonefeld-Jørgensen EC, Long M, Hofmeister
MV and Vinggaard AM: Endocrine-disrupting potential of bisphenol A,
bisphenol A dimethacrylate, 4-n-nonylphenol and 4-n-octylphenol in
vitro: New data and a brief review. Environ Health Perspect. 115
Suppl 1:S69–S76. 2007. View
Article : Google Scholar
|
|
21
|
In SJ, Kim SH, Go RE, Hwang KA and Choi
KC: Benzophenone-1 and nonylphenol stimulated MCF-7 breast cancer
growth by regulating cell cycle and metastasis-related genes via an
estrogen receptor alpha-dependent pathway. J Toxicol Environ Health
A. 78:492–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SH, Hwang KA, Shim SM and Choi KC:
Growth and migration of LNCaP prostate cancer cells are promoted by
triclosan and benzophenone-1 via an androgen receptor signaling
pathway. Environ Toxicol Pharmacol. 39:568–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Sun Y, Song Y, Saito T and Kurasaki
M: Nonylphenol diethoxylate inhibits apoptosis induced in PC12
cells. Environ Toxicol. 31:1389–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stamatakos M, Palla V, Karaiskos I,
Xiromeritis K, Alexiou I, Pateras I and Kontzoglou K: Cell cyclins:
Triggering elements of cancer or not? World J Surg Oncol.
8:1112010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy A and Banerjee S: p27 and leukemia:
Cell cycle and beyond. J Cell Physiol. 230:504–509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sandhu C and Slingerland J: Deregulation
of the cell cycle in cancer. Cancer Detect Prev. 24:107–118.
2000.PubMed/NCBI
|
|
27
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
28
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Butler AM, Scotti Buzhardt ML, Li S, Smith
KE, Fields AP and Murray NR: Protein kinase C zeta regulates human
pancreatic cancer cell transformed growth and invasion through a
STAT3-dependent mechanism. PLoS One. 8:e720612013. View Article : Google Scholar : PubMed/NCBI
|