Introduction
The prognosis of patients with glioblastoma
multiforme (GBM) and anaplastic astrocytoma remains poor, despite
the current treatment modalities (1).
Generally, radiation and chemotherapy are initiated following
surgical treatment to improve the curative effects (2). Temozolomide (TMZ), a chemotherapeutic
drug, is used as the drug of choice for treating malignant gliomas
in clinical practice. The cytotoxicity of TMZ is associated with
its ability to methylate guanine at the O6 position
(3). Although TMZ is used as a
second- and first-line treatment for astrocytoma and GBM,
respectively, its effect is barely satisfactory as it extends the
median survival of patients by only 2 months (4–6). Cells
containing elevated levels of O6-methylguanine DNA
methyltransferase repair the aberrant methylation induced by TMZ,
and consequently reverse the cytotoxic effect. In addition, DNA
mismatch repair deficiency makes cells tolerant to methylation and
to the cytotoxic effects of TMZ (7).
Therefore, the development of novel chemotherapeutic drugs for the
treatment of gliomas is essential.
Evading apoptosis is a major hallmark of
tumorigenesis and chemoresistance, particularly in high-grade
glioblastomas and astrocytomas (8).
Recently, particular attention has been paid to the crosstalk
between apoptosis and autophagy. The term ‘autophagy,’ which
originates from a Greek term meaning self (auto)-eating (phagy),
was first coined and defined by De Duve in 1963 (9). Autophagy is the degradation process of
cytoplasmic constituents in the lysosome-vacuole and is a
fundamental mechanism, well conserved among eukaryotes (10). Autophagy serves a vital role in
development, protein and organelle quality control, senescence,
neurodegeneration and tumorigenesis. In 1999, Liang et al
(11) first reported the role of
Beclin1, one of the most important constituents for autophagosome
formation, in the induction of autophagy and inhibition of
tumorigenesis. In 2000, Kabeya et al (12) identified two forms of microtubule
associated protein 1 light chain 3α (LC3), termed LC3-I and LC3-II,
respectively. LC3-II serves an important role in the formation of
the autophagosome membrane. Subsequent investigations in mammals
used Beclin1 and LC3 as autophagic markers. In addition, Beclin1
and LC3 are reliable markers of brain tumors.
Autophagy is associated with drug resistance in
numerous types of tumor cells. Notably, although autophagy impedes
the therapeutic effects of anti-cancer drugs in some cases
(13), it potentiates responses to
conventional therapies in gliomas (14,15). In
addition, expression levels of the autophagic proteins Beclin1 and
LC3-II are much lower in higher-grade astrocytomas compared with
lower-grade astrocytomas and normal brain tissue, and prognosis is
positively associated with the level of autophagy (16,17). This
evidence suggests that a decrease in autophagic activity may be
involved in the progression of astrocytic or glioma tumors.
Therefore, restoration of autophagy may inhibit tumor progression
and may prove promising as a future therapeutic strategy.
Evodiamine (Evo) is a quinazolinocarboline alkaloid
isolated from the fruit of Evodiae fructus, a traditional
Chinese herb. E. fructus has been widely used for the
treatment of gastrointestinal disorders, headache and postpartum
hemorrhage (18). Evo has been
reported to have various therapeutic benefits associated with the
treatment of cancer, inflammation, obesity, cardiovascular diseases
and pain (19–22). Studies investigating the anticancer
activity of Evo have demonstrated that it inhibits the growth and
metastasis of various cancer cells by regulating the cell cycle,
apoptosis and autophagy (21,23). Evo induces intracellular calcium-JNK
signaling-mediated autophagy and calcium-mitochondria-mediated
apoptosis in glioma cells (20).
However, the potential of Evo is hindered due to its limited
efficiency and drug-like properties, including aqueous solubility
and rapid plasma clearance (24). The
development of Evo analogs with optimized drug properties is
crucial for the treatment of malignant gliomas. WZY-321 is a novel
Evo analog with improved drug properties. WZY-321 has demonstrated
promising cytotoxic effects in cancer cells (25). The present study evaluated the
regulation of autophagy and the therapeutic potential of WZY-321 in
gliomas.
Materials and methods
Reagents
The Cell Counting Kit-8 (CCK-8) kit and
3-methyladenine (3-MA) were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS) and penicillin-streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The Annexin V-fluorescein isothiocyanate (FITC) apoptosis
staining kit and antibodies used in the study were purchased from
Thermo Fisher Scientific, Inc. The lysis buffer (RABLYSIS1) and
propidium iodide (PI; cat. no. P4170) were purchased from
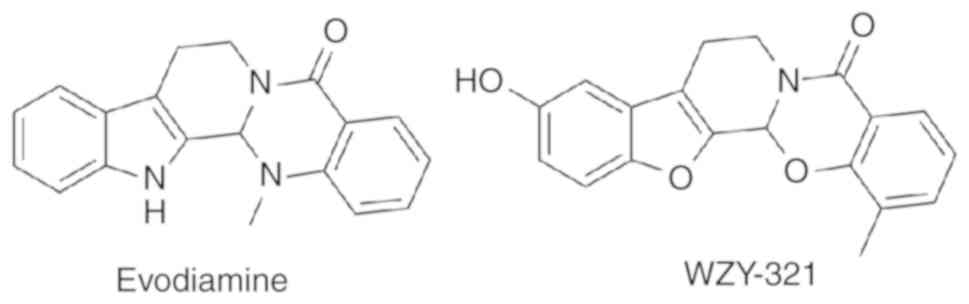
Sigma-Aldrich (Merck KGaA). WZY-321, a novel analog of Evo
(Fig. 1), was designed via a scaffold
hopping strategy. A series of newly-designed Evo analogs, including
WZY-321, were synthesized in the laboratory of Dr Shengtao Xu at
the China Pharmaceutical University (Nanjing, China) (25). The chemical structure of WZY-321 was
characterized by 1H nuclear magnetic resonance (NMR),
13C NMR, and mass spectroscopy. The purity of the
compound was determined by analytical high performance liquid
chromatography and the biologically evaluated compound was
determined to be 98% pure.
Cell culture
SHG-44 glioma cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). The human
glioma cell line SWO-38 was established by the Department of
Neurosurgery at the Jiangning Hospital (Nanjing, China) (26). The cells were cultured at 37°C in DMEM
supplemented with 10% FBS, 200 mM L-glutamine, 100 U-ml penicillin,
100 µg-ml streptomycin, 100 mM sodium pyruvate and 1% non-essential
amino acids. Cells were incubated at 37°C in a humidified incubator
containing 5% CO2. The medium was changed every 2 days
and the cells were passaged every 3 days. The density of the seeded
cells in different biological replicates was the same.
Assessment of cell viability
SHG-44 and SWO-38 cells (5×103 cells-100
µl) were seeded into each well of a 96-well plate and pre-incubated
for 24 h in a humidified incubator (37°C; 5% CO2). Total
volumes of 10 µl of various concentrations of WZY-321 (0, 5, 10, 30
and 50 µM) were added to the cells and incubated for 24 or 48 h.
Following incubation, 10 µl CCK-8 solution was added to each well
and incubated for 1–4 h. Cell viability was proportional to the
optical density and was quantitatively measured by
spectrophotometry (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 450 nm. The viability of cells in the control group incubated
for 48 h was defined as 100% viable.
Measurement of apoptosis
SHG-44 cells (1×105 cells-well) were
cultured in complete medium in 6-well plates for 24 h, and treated
in triplicate with different concentrations of WZY-321 (10, 30 and
50 µM) for 24 h. The control cells were treated with vehicle [1%
dimethyl sulfoxide (DMSO) in complete medium]. Cell morphology was
observed under optical microscope (magnification, ×20). The cells
were subsequently harvested, washed, and stained with PI and
FITC-Annexin V in the dark at 25°C for 15 min using the Annexin
V-FITC apoptosis staining kit, according to the manufacturer's
protocols. The percentage of apoptotic cells was determined by flow
cytometry using an FC500 cytometer (Beckman Coulter, Inc., Brea,
CA, USA). Annexin V-FITC binding was analyzed by flow cytometry
(excitation=488 nm; emission=350 nm) using the FITC signal detector
(usually FL1), and PI staining by the phycoerythrin emission signal
detector (usually FL2), using FlowJo 8.8 software (Tree Star, Inc.,
Ashland, OR, USA).
Cell cycle study
Progression through the cell cycle was assessed by
flow cytometry DNA determination with PI. SHG-44 cells were seeded
in 6-well plates (1×105 cells-well) and incubated at
37°C for 24 h. Cells were incubated with WZY-321 at certain
concentrations (0, 10, 30 and 50 µM). Cells treated with the
solvent (DMSO) were included. Following 24 h of treatment, cells
were fixed with 70% ethanol at −20°C for 12 h, treated with RNase,
and stained with PI at 37°C for 30 min. Cellular DNA content for
the cell cycle distribution analysis was measured using a flow
cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
The percentages of cells in different phases of cell cycle were
analyzed by ModFit 4.1 software (Verity Software House, Topsham,
ME, USA).
Western blot analysis
SHG-44 cells were incubated at 37°C in the presence
or absence of WZY-321 at a concentration of 0, 10, 30 and 50 µM for
24 h. 3-MA was used as an autophagy inhibitor and cells were
pretreated with 2.5 mM 3-MA at 37°C for 12 h prior to treatment
with WZY-321. Following incubation, cells were collected,
centrifuged at 13,000 x g at 4°C for 15 min and washed twice
with ice cold PBS. The pellets were re-suspended in lysis buffer.
The cells were lysed on ice for 20 min and the lysates were
centrifuged at 13,000 × g at 4°C for 15 min. The protein
concentration in the supernatant was determined using bicinchoninic
acid protein assay reagents. Equal amounts of protein (20 µg-well)
were separated via SDS-PAGE on a 10% gel and transferred to a
polyvinylidene fluoride Hybond-P membrane. Membranes were blocked
with 5% non-fat milk for 1 h at room temperature and incubated with
primary antibodies against: Beclin1 (cat. no. sc-48341); LC3 (cat.
no. sc-271625); apoptosis regulator Bcl-2 (Bcl-2; cat. no. sc-509);
cyclin-dependent kinase 1 (cdc2; cat no. sc-54); cyclin D1 (cat.
no. sc-70899); poly (ADP-ribose) polymerase (cat. no. sc-136208);
and β-actin (cat. no. sc-58673) or GAPDH (cat. no. sc-293335)
(1:1,000 dilution; all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) by gentle rotation overnight at 4°C. β-actin and GAPDH were
used as loading controls. The membranes were subsequently washed
and incubated with horseradish peroxidase (HRP)-conjugated
secondary antibody (1:20,000 dilution; cat. no. sc-2489; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature and visualized
using enhanced chemiluminescent reagent (EMD Millipore, Billerica,
MA, USA). The densitometric analysis was performed using Image J
1.48 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean calculated from three independent experiments. Statistical
analysis was performed using Student's t-test (for two groups) or
one-way analysis of variance followed by Duncan's multiple-range
test (for three or more groups) Analysis was performed using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
WZY-321 induces cytostatic effects in
SHG-44 and SWO-38 glioma cells
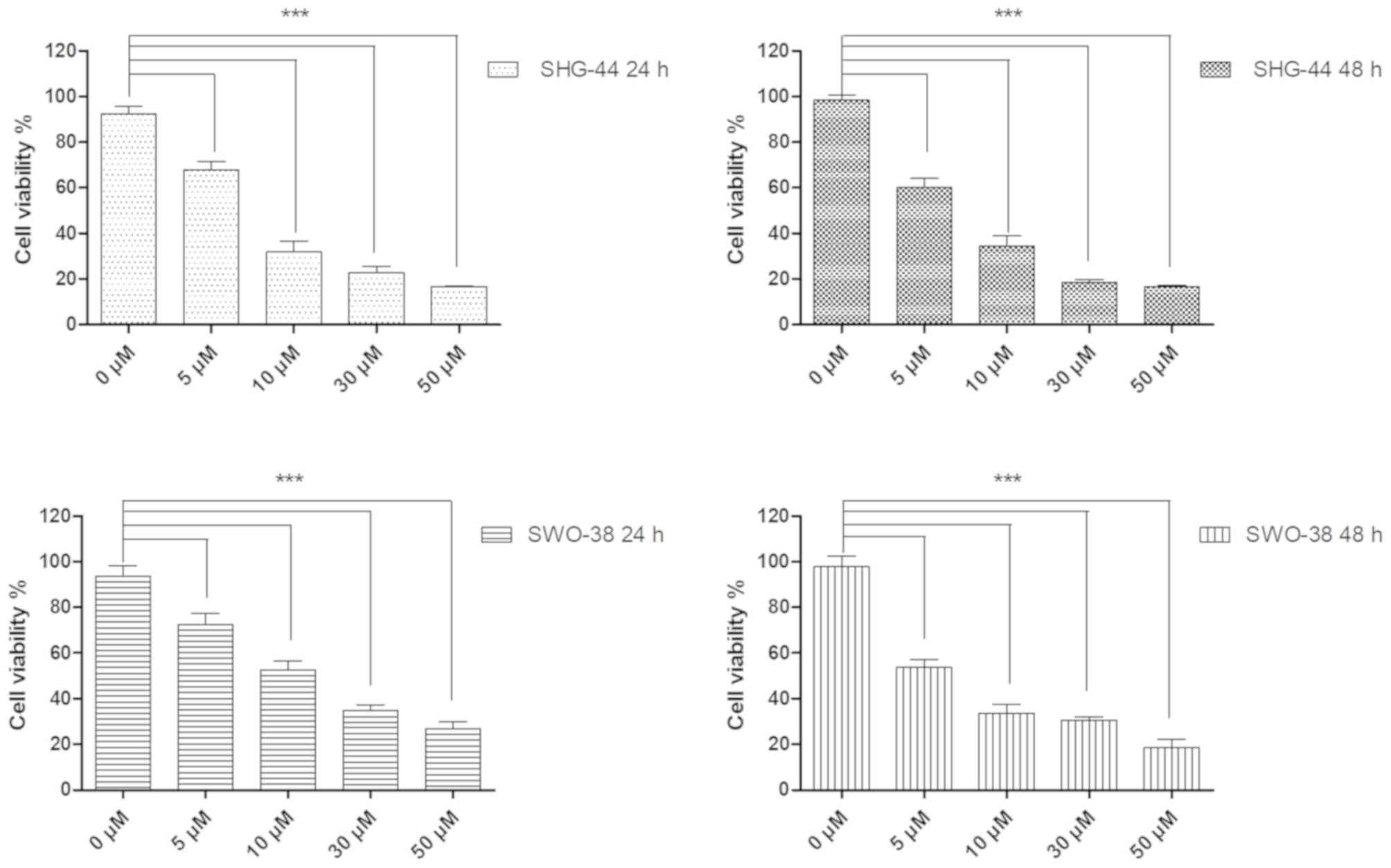
SHG-44 and SWO-38 glioma (26) cells were pretreated with various
concentrations of WZY-321 (5, 10, 30 or 50 µM) for 24 or 48 h. The
number of viable cells was determined using the CCK-8 assay. As
presented in Fig. 2, WZY-321
significantly reduced the viability of SHG-44 and SWO-38 cells in a
dose-dependent manner. Treatment with 10 µM WZY-321 for 48 h
reduced the cell count to ~30% compared with the control group. In
addition, incubation with WZY-321 for 24 h significantly decreased
the viability of SHG-44 glioma cells. Prolonged incubation of cells
with WZY-321 for up to 48 h displayed little benefit. SWO-38 cells
were less sensitive to WZY-321 compared with SHG-44 cells. The
histological subtypes of SHG-44 and SWO-38 cells may explain this
sensitivity (27). Therefore, in the
subsequent studies, SHG-44 glioma cells were incubated with WZY-321
for 24 h prior to processing the samples for subsequent
pharmacological tests.
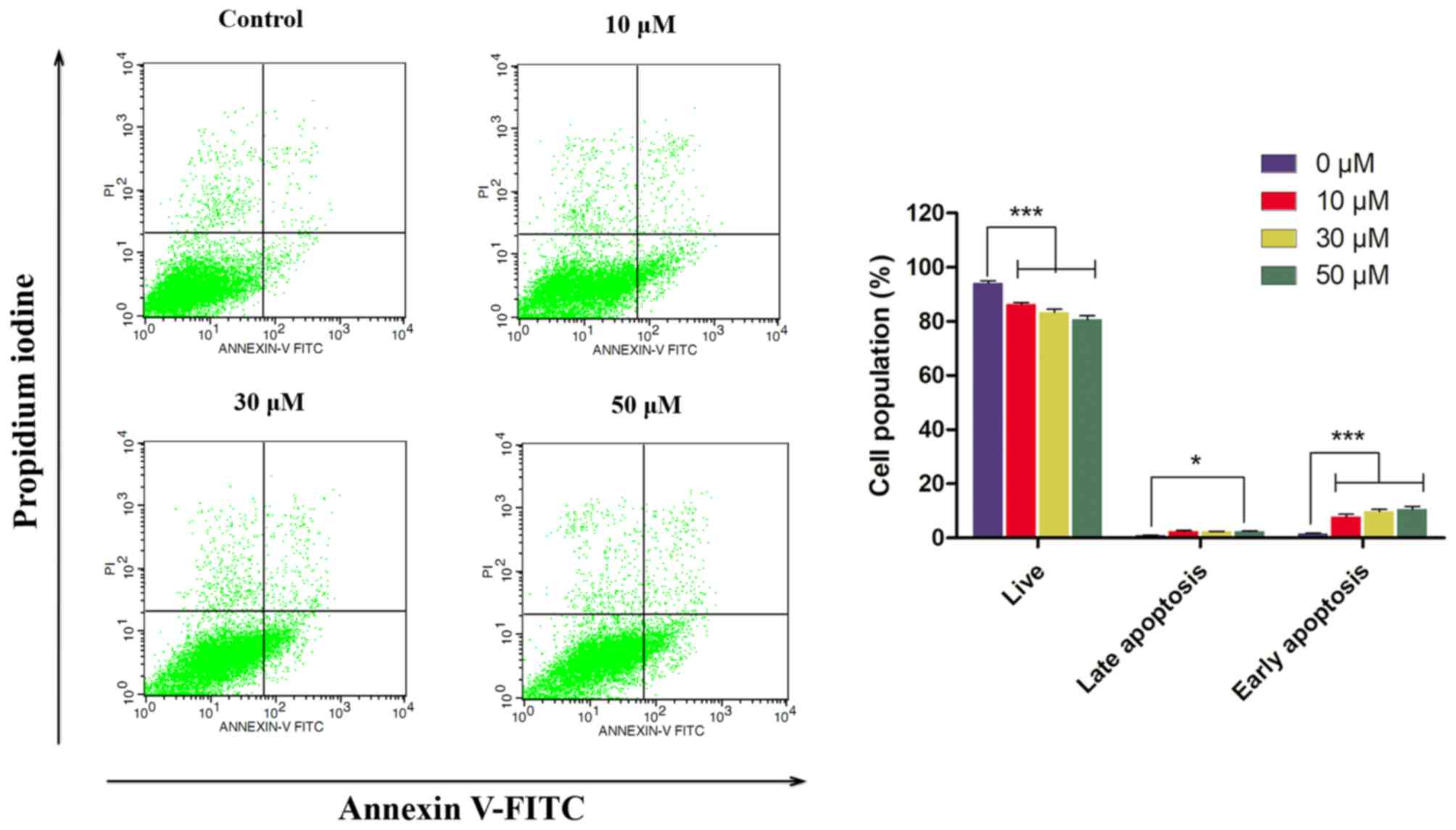
WZY-321 triggers apoptosis in SHG-44
glioma cells
SHG-44 cells treated with WZY-321 for 24 h displayed
marked alterations in cell morphology, exhibiting chromatin
condensation, which suggested the cytostatic effect of WZY-321 may
be associated with cell apoptosis (Data not shown). To confirm
whether WZY-321 is able to induce apoptosis in SHG-44 cells,
vehicle- or WZY-321-treated SHG-44 cells were stained with Annexin
V-PI for flow cytometry analysis. Externalization of
phosphatidylserine (PS) from the inner leaflet to the outer leaflet
of the plasma membrane is a distinct phenomenon observed in early
apoptotic cells. Annexin V has high affinity for PS, and
fluorochrome-labeled Annexin V may be used for the detection of
apoptotic cells. By contrast, PI is used to detect necrotic cells
due to its ability to permeate the damaged cell membrane. In the
present study, flow cytometric analysis identified four groups of
cells: i) Viable cells (Annexin V-, PI-); ii) early apoptotic cells
(Annexin V+, PI-); iii) late apoptotic cells (Annexin V+, PI+); and
iv) necrotic cells (Annexin V-, PI+). The percentage of apoptotic
SHG-44 cells detected by Annexin V-FITC and PI double staining
following incubation with various concentrations of WZY-321 (0, 10,
30 or 50 µM) for 24 h is presented in Fig. 3. The number of early-stage apoptotic
SHG-44 cells was increased in the 50 µM WYZ-321-treated group
(11.3%) compared with that in the control group (1.6%), in a
dose-dependent manner. The upper left quadrants representing the
necrotic cells were comparable in each group. However, the increase
in the number of advanced stage apoptotic cells following treatment
with WZY-321 was not significant. These results demonstrated that
WZY-321 may dose-dependently induce apoptosis in SHG-44 cancer
cells.
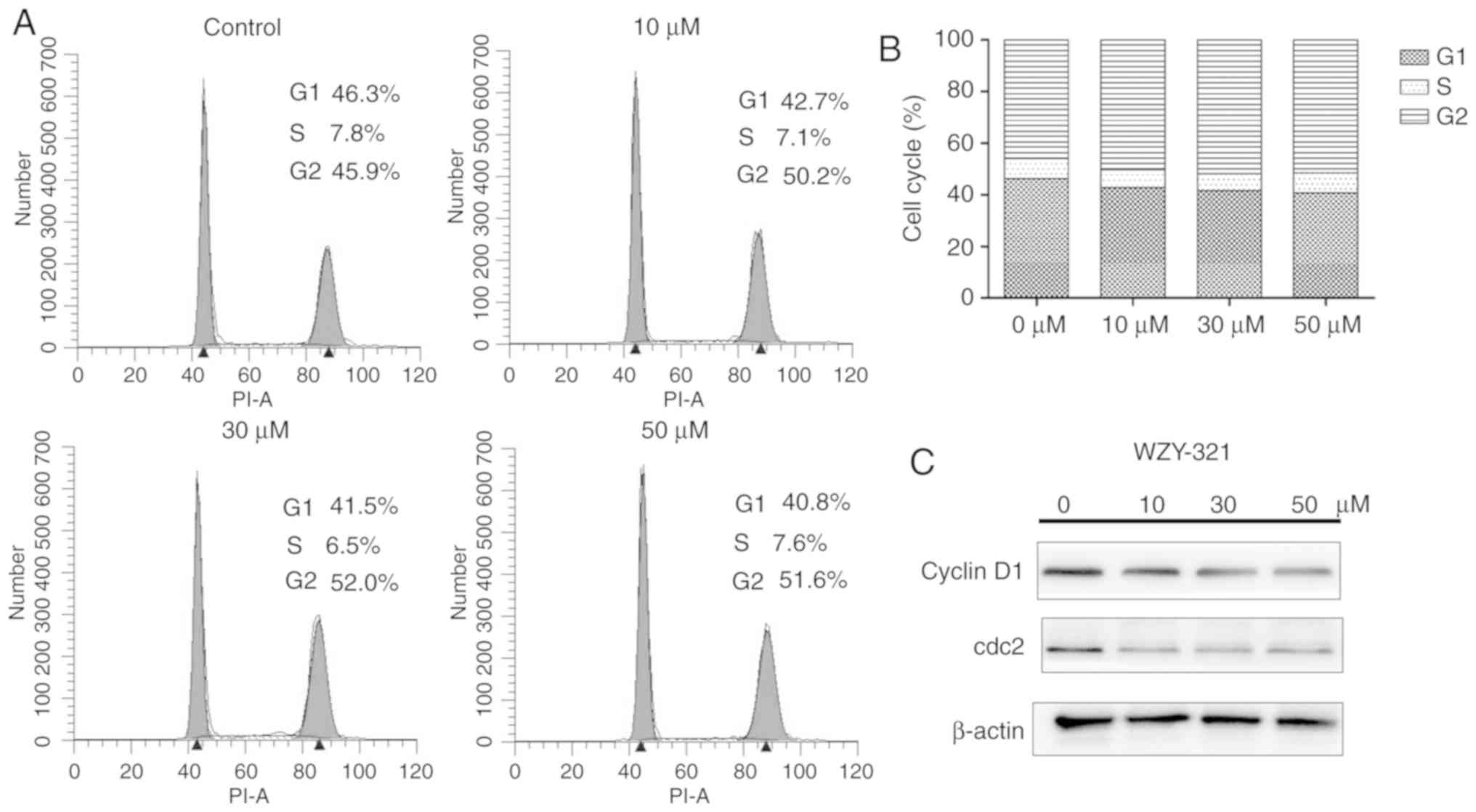
WZY-321 induces cell cycle arrest at
the G2-M phase
One of the mechanisms by which chemical antitumor
agents inhibit cell proliferation is through the induction of cell
cycle arrest (28). To determine this
mechanism involved in the suppression of cell growth by WZY-321,
the DNA content of the cell nuclei was detected by flow cytometry.
SHG-44 cells were treated with varying concentrations of WZY-321
(0, 10, 30 and 50 µM). As presented in Fig. 4A and B, the number of cells which
arrested at the G2-M phase increased from 45.9 to 51.6% in a
dose-dependent manner, along with a concomitant decrease in the
percentage of cells in the S and G1 phases. Western blot analysis
was performed to determine the expression levels of cell cycle
regulatory proteins. As displayed in Fig.
4C, treatment of SHG-44 cells with WZY-321 resulted in a
decrease in the protein expression levels of cdc2 and cyclin D1, in
a dose-dependent manner. These results suggest an association
between WZY-321-induced apoptosis and the accelerated entry of
cells into G2-M arrest.
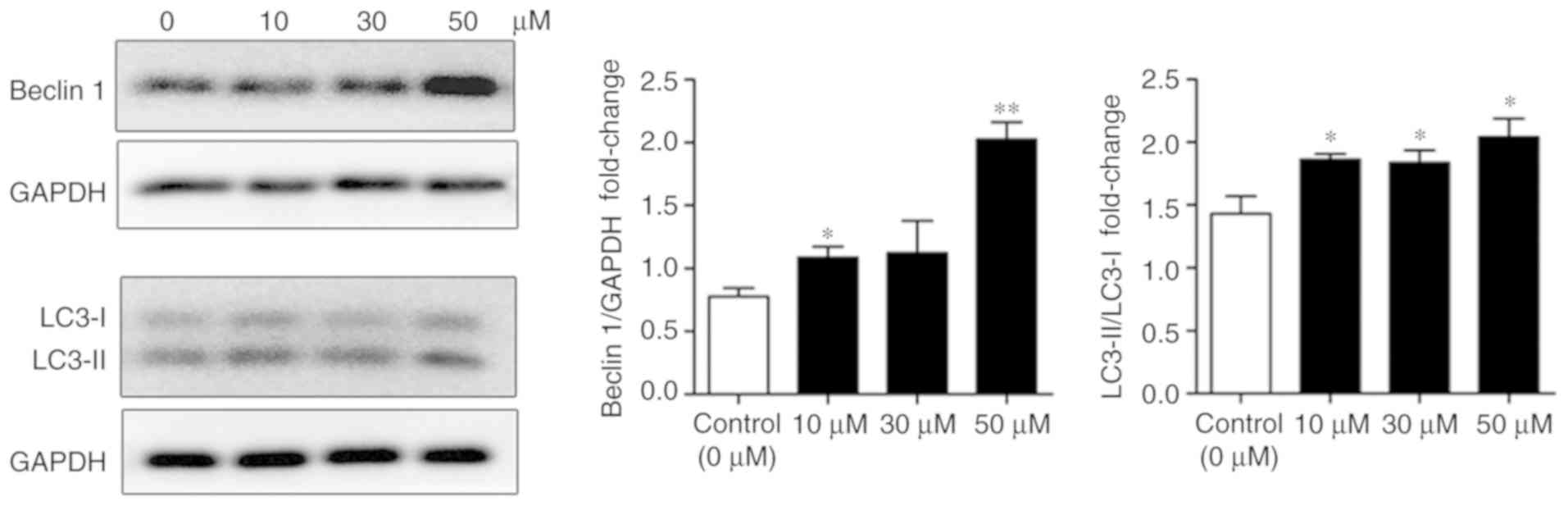
WZY-321 enhances autophagy in SHG-44
glioma cells
The cytotoxicity of certain anti-tumor agents is
induced by autophagy (29–31). To assess the association of autophagy
with the pro-apoptotic effects of WZY-321, a number of autophagic
markers were analyzed. Beclin1, a novel Bcl-2-interacting
coiled-coil protein has structural similarity to yeast
autophagy-related protein 6. The proven importance of Beclin1 in
tumor suppression triggered research into the role of autophagy in
tumor suppression. LC3 is the mammalian homolog of yeast
autophagy-related gene 8. LC3-II is derived from LC3-I following
cleavage from the C-terminal and serves an important role in the
formation of autophagosomes, as it localizes to the outer and inner
membranes. Beclin1 and LC3 are frequently-used markers for the
evaluation of tumorigenesis in brain tumors. In the present study,
SHG-44 glioma cells were incubated with varying concentrations of
WZY-321 for 24 h and the expression levels of Beclin1 and LC3 were
evaluated. As presented in Fig. 5,
incubation with various concentrations of WZY-321 increased the
expression levels of Beclin1 and LC3-II. Accordingly, statistical
analysis of the immunoblotting confirmed the above findings
(Fig. 5). Notably, while expression
levels of Beclin1 and LC3-II in cells incubated with 30 µM WZY-321
were comparable with those treated with 10 µM WZY-321, levels in
the 50 µM group were slightly higher compared with the untreated
group for LC3-II-LC3-I (*P<0.05), and significantly higher for
Beclin-1 (**P<0.01), indicating a dose-dependent induction of
autophagy by WZY-321 in SHG-44 cells.
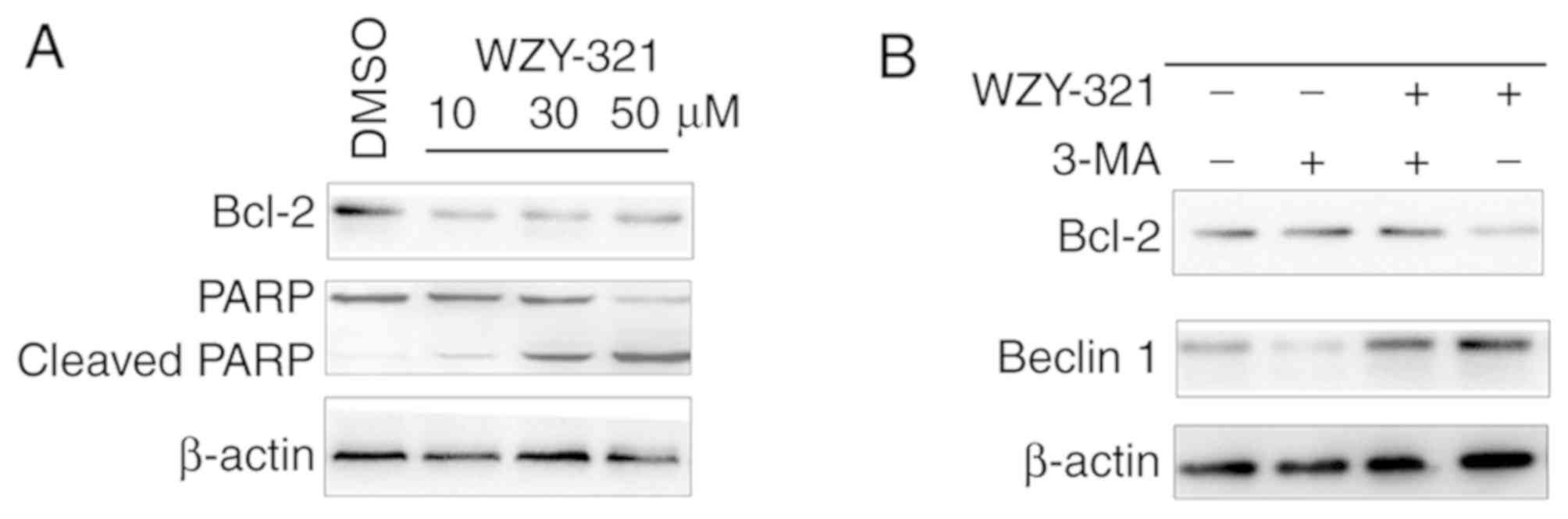
Treatment with an autophagy inhibitor
inhibits the apoptotic effects of WZY-321
A complex association exists between autophagy and
apoptosis. The activation of autophagy may potentiate cytotoxicity,
or impede autophagic cell death. In order to probe whether enhanced
autophagy contributed to the anti-cancer potential of WZY-321, or
impeded the therapeutic effects of WZY-321, 3-MA was used as an
autophagy inhibitor and the expression levels of Bcl-2 and Beclin1
were analyzed (Fig. 6). In
WZY-321-treated SHG-44 cells, a marked decrease was observed in the
protein expression of Bcl-2, confirming the apoptotic effect of
WZY-321. However, this apoptotic effect was blocked when cells were
pretreated with 3-MA prior to treatment with WZY-321. As presented
in Fig. 6B, the expression level of
Beclin1 was lower in 3-MA-pretreated SHG-44 cells treated with
WZY-321 compared with cells treated with WZY-321 alone. In
addition, the expression levels of Bcl-2 were higher in
3-MA-pretreated SHG-44 cells treated with WZY-321 compared with
cells treated with WZY-321 alone.
Discussion
In the present study, it was hypothesized that there
is an association between autophagy and the anti-tumorigenic
potential of WZY-321. Treatment with WZY-321 inhibited the growth
of glioma cells in a dose-dependent manner. This is the first time,
to the best of our knowledge, that the association of the
anti-tumorigenesis potential of WZY-321 with increased autophagic
levels in glioma has been demonstrated.
Recent studies have reported that Evo inhibits the
proliferation of a variety of cancer cells by inducing apoptosis
(20,32). However, the moderate anti-tumor
potency, sub-optimal physicochemical properties and patent
ineligibility of Evo have impeded its direct clinical application.
Structural modification of natural products may increase their
potential to be used as drugs, in addition to the likelihood of
obtaining patents. WZY-321 is a novel analog of Evo designed using
a scaffold-hopping-based structural modification strategy. In the
present study, the antiproliferative effect of WZY-321 on the
growth of SHG-44 and SWO-38 cells was assessed using the CCK-8
assay. The results indicated that the viability of SHG-44 cells
treated with WZY-321 for 24 h was significantly decreased. This
indicated the potent cytotoxicity of WZY-321, which merited further
investigation for the development of novel potential anti-tumor
agents. SHG-44 cells treated with WZY-321 for 24 h displayed marked
alterations in cell morphology, exhibiting chromatin condensation,
which indicated cell apoptosis. The apoptosis-associated protein
Bcl-2 was also detected in order to verify apoptosis. However,
there are limitations to the present study; cells were only exposed
to the drug for 24 or 48 h, whereas exposure for 72 h may have
enhanced the apoptotic potential of WZY-321. Furthermore, there is
absence of data relating to additional apoptosis-associated
proteins, including caspase 3, 7 and 9.
There is a complex association between autophagy and
apoptosis. In the treatment of GBM and anaplastic astrocytoma,
autophagy mediates cell survival against anti-cancer therapies,
while its excessive activation potentiates cytotoxicity and induces
autophagic cell death. The inhibition of autophagy has been
reported to synergize with the effect of erlotinib in inducing GBM
cell death (33). By contrast, in
malignant glioma cell lines, arsenic trioxide has been reported to
inhibit glioma growth through the induction of autophagic cell
death (34). Autophagy contributes to
gefitinib-induced growth inhibition in glioma cells (35). These seemingly controversial
observations result from the complex tumor microenvironment and
diverse therapeutic mechanisms of anti-cancer drugs (36), all of which adds complexity to the
study of autophagy-associated cancer therapies.
A previous report indicated the induction of
intracellular calcium-JNK signaling-mediated autophagy and
calcium-mitochondria-mediated apoptosis by Evo in glioma cells
(37). These reports confirm the
findings from the current study that the therapeutic potential of
WZY-321 in glioma cells was associated with autophagy. However,
these results require careful interpretation; the present results
indicated that reduced autophagy impeded the apoptotic effects of
WZY-321. However, the association between apoptosis and autophagy
in SHG-44 glioma cells treated with WYZ-321 requires further
investigation. Furthermore, the exact role of autophagy in the
anti-cancer action of WZY-321 requires further pharmacological
evaluation. In addition, topoisomerase 1 was previously identified
as one of the targets of Evo (38).
As an analog of Evo, future studies on the potential targets of
WZY-321 and its mechanisms of action are required.
In conclusion, the application of WZY-321, an analog
of Evo, in targeting glioma cell survival was identified. WZY-321
decreased the proliferation of SHG-44 glioma cells in a
dose-dependent manner by enhancing apoptosis and inducing cell
cycle arrest at the G2-M phase. Treatment with WYZ-321
significantly increased the expression of two autophagy-associated
proteins, LC3 and Beclin1, in a dose-dependent manner in SHG-44
glioma cells, and this may be associated with its therapeutic
potential. The results from the present study provide a possible
insight into the development of novel autophagy-based therapeutic
strategies for the treatment of glioblastoma.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81672499 and 81000963),
Jiangsu Province's Natural Science Foundation (grant nos.
BK20141256 and BK20161318) and the Yancheng Medical Science
Development Foundation (grant nos. YK2014011 and YK 2015001).
Availability of data and materials
The datasets used and-or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS, ML and YC conceived and designed the study; GS
and JG were responsible for the development and methodology of the
study; GS, CZ and HS acquired the data; GS and CZ analyzed the
data; GS wrote the manuscript; YC and ML supervised the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Curry RC, Dahiya S, Alva Venur V, Raizer
JJ and Ahluwalia MS: Bevacizumab in high-grade gliomas: Past,
present, and future. Expert Rev Anticancer Ther. 15:387–397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang KY: Specificity protein 1-modulated
superoxide dismutase 2 enhances temozolomide resistance in
glioblastoma, which is independent of O6-methylguanine-DNA
methyltransferase. Redox Biol. 13:655–664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumthekar P: A phase II trial of arsenic
trioxide and temozolomide in combination with radiation therapy for
patients with malignant gliomas. J Neurooncol. 133:589–594. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blakeley JO, Grossman SA, Mikkelsen T,
Rosenfeld MR, Peereboom D, Nabors LB, Chi AS, Emmons G, Garcia
Ribas I, Supko JG, et al: Phase I study of iniparib concurrent with
monthly or continuous temozolomide dosing schedules in patients
with newly diagnosed malignant gliomas. J Neurooncol. 125:123–131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong ET, Timmons J, Callahan A, O'Loughlin
L, Giarusso B and Alsop DC: Phase I study of low-dose metronomic
temozolomide for recurrent malignant gliomas. BMC Cancer.
16:9142016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part I:
Resistance mechanisms and strategies to overcome resistance of
glioblastoma to temozolomide. Drug Discov Today. 20:899–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakada M, Kita D, Watanabe T, Hayashi Y
and Hamada J: Mechanism of chemoresistance against tyrosine kinase
inhibitors in malignant glioma. Brain Tumor Pathol. 31:198–207.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klionsky DJ: Autophagy revisited: A
conversation with Christian de Duve. Autophagy. 4:740–743. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohsumi Y: Historical landmarks of
autophagy research. Cell Res. 24:9–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis bybeclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hart LS, Cunningham JT, Datta T, Dey S,
Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, et al: ER
stress-mediated autophagy promotes Myc-dependent transformation and
tumor growth. J Clin Invest. 122:4621–4634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoki H, Kondo Y, Aldape K, Yamamoto A,
Iwado E, Yokoyama T, Hollingsworth EF, Kobayashi R, Hess K,
Shinojima N, et al: Monitoring autophagy in glioblastoma with
antibody against isoform B of human microtubule-associated protein
1 light chain 3. Autophagy. 4:467–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palumbo S and Comincini S: Autophagy and
ionizing radiation in tumors: The ‘survive or not survive’ dilemma.
J Cell Physiol. 228:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang X, Bai HM, Chen L, Li B and Lu YC:
Reduced expression of LC3B-II and Beclin 1 in glioblastoma
multiforme indicates a downregulated autophagic capacity that
relates to the progression of astrocytic tumors. J Clin Nuronsci.
17:1515–1519. 2010. View Article : Google Scholar
|
|
17
|
Miracco C, Cosci E, Oliveri G, Luzi P,
Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M,
Malagnino V, et al: Protein and mRNA expression of autophagy gene
Beclin 1 in human brain tumours. Int J Oncol. 30:429–436.
2007.PubMed/NCBI
|
|
18
|
National Pharmacopoeia Committee:
Pharmacopoeia of People's Republic of China, Part 1. The Medicine
Science and Technology Press of China; Beijing: pp. 160–161.
2010
|
|
19
|
Fan X, Zhu JY, Sun Y, Luo L, Yan J, Yang
X, Yu J, Tang WQ, Ma W and Liang HP: Evodiamine inhibits
zymosan-induced inflammation in vitro and in vivo: Inactivation of
NF-κB by inhibiting IκBα phosphorylation. Inflammation.
40:1012–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu WS, Chien CC, Liu KH, Chen YC and Chiu
WT: Evodiamine prevents glioma growth, induces glioblastoma cell
apoptosis and cell cycle arrest through JNK activation. Am J Chin
Med. 45:879–899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu LH, Xie JY, Guo WW, Wu GY, Chen ZF, Yi
JY, Zhang L, Zhang ZJ and Li Z: Evodiamine activates AMPK and
promotes adiponectin multimerization in 3T3-L1 adipocytes. J Asian
Nat Prod Res. 16:1074–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao T, Zhang X, Zhao Y, Zhang L, Bai X,
Zhang J, Zhao X, Chen L, Wang L and Cui L: Pretreatment by
evodiamine is neuroprotective in cerebral ischemia: Up-regulated
pAkt, pGSK3β, down-regulated NF-κB expression, and ameliorated BBB
permeability. Neurochem Res. 39:1612–1620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan X, Hartley JM, Hartley JA, White KN,
Wang Z and Bligh SW: Evodiamine, a dual catalytic inhibitor of type
I and II topoisomerases, exhibits enhanced inhibition against
camptothecin resistant cells. Phytomedicine. 19:618–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun HZ, Fang ZZ, Cao YF, Sun XY and Hong
M: Investigation of the in vitro metabolism of evodiamine:
Characterization of metabolites and involved cytochrome p450
isoforms. Phytother Res. 27:705–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu JY, Xu ST and Qiu YY: Evodiamine a
novel class of derivatives, their preparation and use. Chinese
patent 201611019652. Filed November. 16:2016.
|
|
26
|
Situ R, Wang HH, Wang JH, He LP, Zhu XL
and Ou WF: Establishment of human brain malignant glioma cell line
(SWO-38) and observation of its biologic properties. Chin J Cancer.
6:235–238. 1987.(In Chinese).
|
|
27
|
Pisapia DJ: The updated world health
organization glioma classification: Cellular and molecular origins
of adult infiltrating gliomas. Arch Pathol Lab Med. 141:1633–1645.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Liang Q, Shen K, Ma L, An N, Deng
W, Fei Z and Liu J: A novel class I histone deacetylase inhibitor,
I-7ab, induces apoptosis and arrests cell cycle progression in
human colorectal cancer cells. Biomed Pharmacother. 71:70–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Yue GG, Chan AM, Tsui SK, Fung KP,
Sun H, Pu J and Lau CB: Eriocalyxin B, a novel autophagy inducer,
exerts anti-tumor activity through the suppression of
Akt-mTOR-p70S6K signaling pathway in breast cancer. Biochem
Pharmacol. 142:58–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng YC, Hueng DY, Huang HY, Chen JY and
Chen Y: Magnolol and honokiol exert a synergistic anti-tumor effect
through autophagy and apoptosis in human glioblastomas. Oncotarget.
7:29116–29130. 2016.PubMed/NCBI
|
|
31
|
Wang XJ, Chu NY, Wang QH, Liu C, Jiang CG,
Wang XY, Ikejima T and Cheng MS: Newly synthesized
bis-benzimidazole derivatives exerting anti-tumor activity through
induction of apoptosis and autophagy. Bioorg Med Chem Lett.
22:6297–6300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang F, Shi L, Liang T, Ji L, Zhang G,
Shen Y, Zhu F and Xu L: Anti-tumor effect of evodiamine by inducing
Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys
Res Commun. 485:54–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eimer S, Belaud-Rotureau MA, Airiau K,
Jeanneteau M, Laharanne E, Véron N, Vital A, Loiseau H, Merlio JP
and Belloc F: Autophagy inhibition cooperates with erlotinib to
induce glioblastoma cell death. Cancer Biol Ther. 11:1017–1027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanzawa T, Zhang L, Xiao L, Germano IM,
Kondo Y and Kondo S: Arsenic trioxide induces autophagic cell death
in malignant glioma cells by upregulation of mitochondrial cell
death protein BNIP3. Oncogene. 24:980–991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang CY, Kuan YH, Ou YC, Li JR, Wu CC,
Pan PH, Chen WY, Huang HY and Chen CJ: Autophagy contributes to
gefitinib-induced glioma cell growth inhibition. Exp Cell Res.
327:102–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grandér D and Panaretakis T: Autophagy:
Cancer therapy's friend or foe? Future Med Chem. 2:285–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih
YL, Hou SY, Chiu WT, Hsiao SH and Shih CM: Evodiamine, a plant
alkaloid, induces calcium-JNK-mediated autophagy and
calcium-mitochondria-mediated apoptosis in human glioblastoma
cells. Chem Biol Interact. 205:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong G, Sheng C, Wang S, Miao Z, Yao J and
Zhang W: Selection of evodiamine as a novel topoisomerase I
inhibitor by structure-based virtual screening and hit optimization
of evodiamine derivatives as antitumor agents. J Med Chem.
53:7521–7531. 2010. View Article : Google Scholar : PubMed/NCBI
|