Introduction
The incidence of thyroid carcinoma has markedly
increased worldwide in the past 4 decades (1,2). Papillary
thyroid carcinoma (PTC) represents the most common type of
well-differentiated thyroid cancer, accounting for 80–85% of
thyroid malignancies (3). Patients
with PTC have a generally favorable prognosis (2); however, a 30-year follow-up study
demonstrated that recurrence rates and cancer mortality are high in
PTC patients with local tumor invasion and lymph node metastasis
(LNM) (4). Although the predictive
value of several biomarkers for PTC prognosis, including
microRNA-451 (5), B-Raf
proto-oncogene, serine/threonine kinase mutation (6) and EH domain-containing 2 (7) have been investigated, their efficacy has
yet to be validated. To the best of our knowledge, reliable
biomarkers able to predict the invasiveness of PTC and prognosis of
patients are still lacking.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent extracellular proteases that maintain and remodel
tissue architecture. The degradation of basement membranes and of
extracellular matrix (ECM) is an essential step in tumor invasion
and migration; therefore, MMPs serve an essential role in cancer
metastasis (8,9). MMP-9, also known as 92 kDa
gelatinase/type IV collagenase, is a prominent MMP that is
responsible for the migratory and invasive abilities of diverse
types of cancer (10–16). MMPs are closely involved in the
stimulation of angiogenesis, which is essential for tumor growth
and progression (17). In addition,
MMP-9 facilitates the release of tissue-bound fibroblast growth
factor (FGF) (18,19) and vascular endothelial growth factor
(VEGF), which contribute to tumor growth. Consequently, MMP-9 may
be considered a novel biomarker and a potential therapeutic target
in human cancer.
Considering its contribution to cancer initiation,
tumor growth, angiogenesis and migration, the diagnostic and
prognosis capacities of MMP-9 have been assessed in various types
of cancer (16,20,21).
Furthermore, the overexpression of MMP-9 in colorectal cancer
tissue is associated with tumor invasion, LNM and advanced
tumor-node-metastasis (TNM) stage (20). In addition, increased expression of
MMP-9 is observed in patients with glioma, which is associated with
advanced glioma grades and negative survival rates (21). Researchers have speculated that MMP-9
overexpression promotes circulating tumor cells to shed into the
bloodstream by breaking the basement membrane or digesting ECM
(16).
As determined by immunohistochemistry (IHC), MMP-9
is involved in the progression and aggressiveness of PTC, although
some inconsistencies regarding its predictive capacity have been
reported (22–24). The present study aimed to investigate
the expression of MMP-9 in tumors from patients with PTC in
comparison with samples obtained from patients with benign thyroid
nodules (BTNs). The association between MMP expression and the
clinicopathological characteristics of patients with PTC was also
analyzed. Eventually, the diagnostic and prognostic value of
immunohistochemical MMP-9 expression for PTC was investigated.
Patients and methods
Study population
A group of patients with PTC was retrospectively
reviewed at the First Hospital of Jilin University between January
1, 2012 and June 31, 2014. Furthermore, age- and sex-matched
subjects with BTN were recruited as controls. The diagnosis of PTC
and BTN was made on a pathological basis. BTN was defined as
follows: A lump inside the thyroid, an up and down movement when
swallowing, and a biopsy confirming the benign nature of the lump.
BTNs included nodular goiter and thyroid adenoma. The inclusion
criteria for PTC or BTNs were as follows: i) biopsy-confirmed PTC
or BTN; ii) age ranged from 18–70 years old. The exclusion criteria
for PTC and BTNs were as follows: i) Anticancer therapy prior to
admission, including radiotherapy, chemotherapy and thyroidectomy;
ii) severe systemic disorders, including heart failure and
malignancies; iii) incomplete or missing test results. The present
study was approved by the Ethics Committee of the First Hospital of
Jilin University, and all participants provided informed consent
prior to participation.
The body mass index (BMI) of each patient was
calculated as the weight in kilograms divided by height in meters
squared (kg/m2). All patients with PCT were graded at
the time of diagnosis, according to the 7th edition of the American
Joint Committee on Cancer TNM staging system (25). Early-stage disease was named as TNM
stage I or II, whereas advanced-stage disease was named as stage
III or IV.
Thyroid surgery and follow-up
Bilateral or unilateral central-compartment neck
dissection was routinely performed in patients with PTC, followed
by total or partial thyroidectomy. In addition, patients underwent
lateral neck lymph node dissection when the lateral cervical lymph
nodes were suspected to be metastatic, based on explicit clinical
and/or imaging findings. The same team of surgeons performed all
surgical procedures.
Patients with PTC were followed up every 3 months
until December 2017. All patients were monitored for postoperative
PTC relapses by ultrasound, computed tomography or chest X-ray, and
in combination with biochemical measurements, including serum
thyroglobulin (Tg) and Tg antibody (TgAb). Disease status was
defined as any evidence of disease, including persistently
detectable TgAb, Tg ≥1 ng/ml, and structurally persistent disease
or metastasis. Structurally persistent disease was defined as the
occurrence of locoregional or distant metastasis, regardless of the
Tg level. A recurrence event was defined as the structural evidence
of a disease identified following a period of no evidence of
disease. Disease-free status was classified as no evidence of
disease, as aforementioned. Disease-free survival (DFS) time was
defined as the period from initial therapy to any evidence of
disease or last follow-up. Survival time without structurally
persistent disease/recurrent disease (SPRD) was calculated as the
interval from initial treatment to the occurrence of structurally
persistent disease, recurrent disease or last follow-up.
Pathological subtypes by hematoxylin
and eosin staining (H&E)
Pathological subtypes of enrolled subjects with PTC
were investigated by H&E staining for 20 min at room
temperature. The results were visualized by Olympus BX51 microscope
(Olympus Corporation, Tokyo, Japan).
Determination of tissue MMP-9
expression by IHC
Thyroid neoplastic tissues were collected from
patients with PTC or BTN during thyroidectomy. Tissue specimens
were fixed in 4% paraformaldehyde in TBS at 4°C for 18 h.
Paraffin-embedded tissue specimens were sectioned at a thickness of
2.0 µm, followed by deparaffinization in xylene and dehydration in
a graded series of ethanol solutions. Antigen retrieval was
performed by heating the samples (in rice cooker for 20 min at
95°C) in citrate buffer (pH 6.0), and endogenous peroxidase was
blocked with 3% hydrogen peroxide for 10 min at room temperature.
Afterwards, the sections were incubated with rabbit polyclonal
MMP-9 antibody (cat. no. ab38898; Abcam, Cambridge, MA, USA) at a
dilution of 1:250 at 4°C overnight. After washing with PBS, the
sections were incubated with horseradish peroxidase-labeled goat
anti-rabbit immunoglobulin (cat. no. ab6721; Abcam) at a dilution
of 1:1,000 at 37°C for 30 min, and the reaction was visualized
using 3,3′-diaminobenzidine. Eventually, the specimens were
counterstained with Mayer's hematoxylin at room temperature for 1
min. The images were photographed using an Olympus BX51 microscope
with an Olympus DP-21 digital camera image system (Olympus
Corporation). The primary antibody was replaced with non-immune
rabbit serum (cat. no. AR0010; Boster, Wuhan, Hubei, China) which
served as the negative control.
All samples were blindly inspected by two
independent pathologists. Positive immunostaining was visualized as
brown granules contained in the cytoplasm. The immunostaining of
MMP-9 was scored on the scale of semi-quantitative assessment by
evaluating the intensity and percentage of positively stained
cells. The intensity of MMP-9 cytoplasmic staining was scored as
follows: 0, none; 1, weak; 2, moderate; and 3, strong. The
percentage scores were assigned, as follows: 1, ≤25%; 2, 26–50%; 3,
51–75%; and 4, >75%. These scores were multiplied to arrive at a
final score ranging between 0 and 12.
Statistical analysis
SPSS software (version 18.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Data were presented as
the means ± standard deviation when data were normally distributed
and the results were compared using independent student's t-test.
Data expressed as median and interquartile ranges (IQRs) (25th
percentile and 75th percentile) were compared using a Mann-Whitney
nonparametric test. Categorical data were expressed as the number
of patients and percentages, and the proportions were compared
using χ2 test.
Receiver operating characteristic (ROC) curves were
designed to estimate the diagnostic and prognostic value of MMP-9
in tissues, in terms of sensitivity, specificity, positive
predictive value (PPV) and negative predictive value (NPV). The
optimal cutoff values of tissue MMP-9 were identified according to
the Youden index (26). The survival
curves were plotted using the Kaplan-Meier method, and compared
using log-rank test. Univariate Cox proportional hazard analysis
was conducted to determine the risks of disease status and SPRD.
Variables with significance (P<0.05) in univariate analyses were
entered into the multivariable phase. Odds ratios (OR) and their
95% confidence intervals (CIs) were calculated to assess the
independent contribution of each identified risk factor. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
subjects
In the present study, 112 patients with PTC and 42
control subjects with BTN were included and separated into two
groups. There was no significant difference in age between patients
with PTC (45.77±10.03 years) and controls (47.36±10.95 years old)
(P>0.05). In addition, there were no significant differences in
sex and proportion of subjects who were ≥45 years old between these
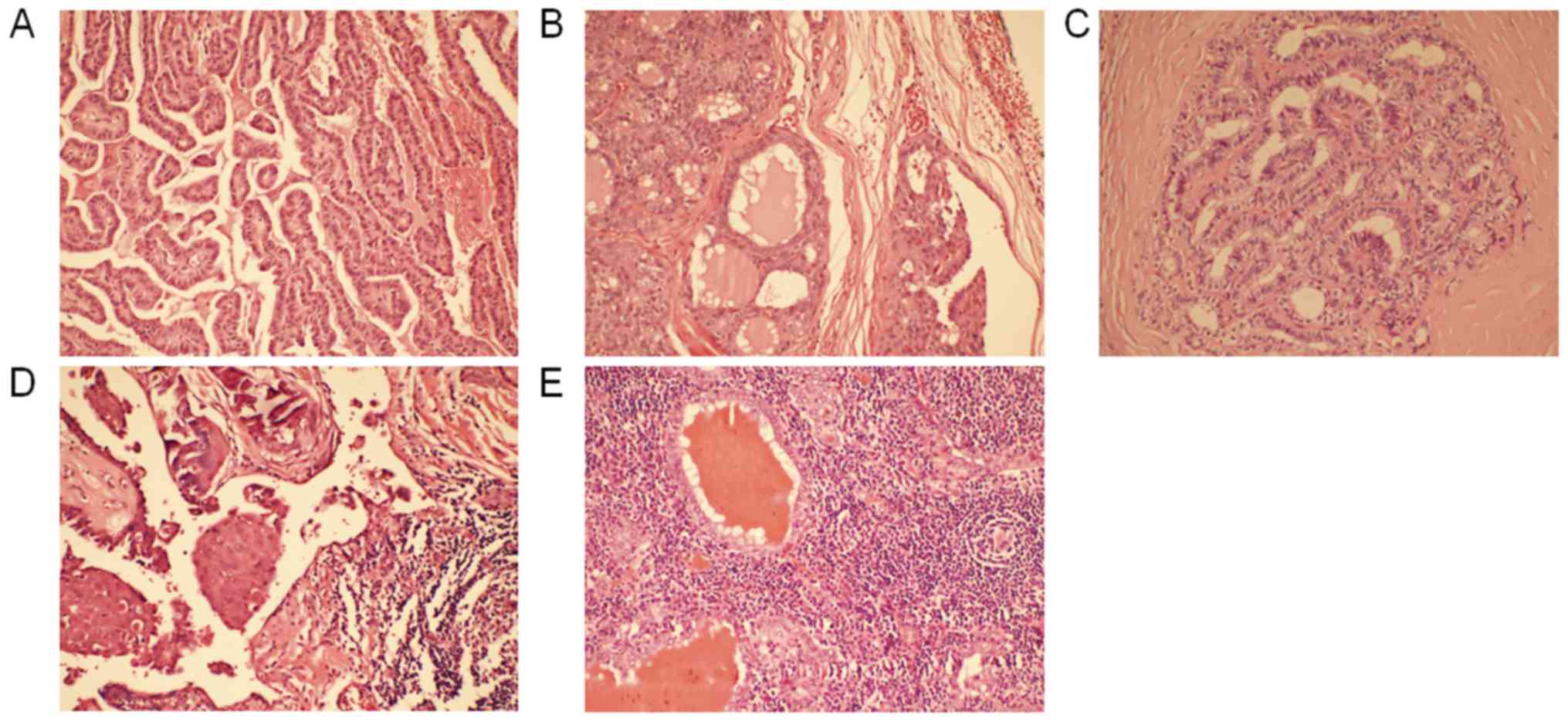
two groups (P>0.05). Numerous pathological subtypes of PTC were
identified, including 96 cases of classical PTC, nine follicular
variants of PTC, three tall cell variants, three diffuse sclerosing
variants and one Warthin variant (Fig.
1A-E). The clinicopathological characteristics of these
subjects are presented in Table
I.
 | Table I.Clinicopathological characteristics
of patients, n (%). |
Table I.
Clinicopathological characteristics
of patients, n (%).
| Characteristic | PTC (n=112) | BTN (n=42) | P-valuea |
|---|
| Age |
|
| 0.187 |
| <45
years old | 56 (50.0) | 16 (38.1) |
|
| ≥45
years old | 56 (50.0) | 26 (61.9) |
|
| Sex |
|
| 0.821 |
|
Female | 95 (84.8) | 35 (83.3) |
|
|
Male | 17 (15.2) | 7
(16.7) |
|
| Tumor size |
|
|
|
| ≤2
cm | 81 (72.3) |
|
|
| >2
cm | 31 (27.7) |
|
|
| Capsule
invasion |
|
|
|
| No | 56 (50.0) |
|
|
|
Yes | 56 (50.0) |
|
|
| Multifocality |
|
|
|
|
Unifocal | 69 (61.6) |
|
|
|
Multifocal | 43 (38.4) |
|
|
| Nodal status |
|
|
|
| N0 | 49 (43.8) |
|
|
|
N1a | 42 (37.5) |
|
|
|
N1b | 21 (18.7) |
|
|
| Extrathyroidal
invasion |
|
|
|
|
Negative | 78 (69.6) |
|
|
|
Microscopic | 25 (22.3) |
|
|
|
Macroscopic | 9 (8.0) |
|
|
| Vascular
invasion |
|
|
|
| No | 107 (95.5) |
|
|
|
Yes | 5 (4.5) |
|
|
| Distant
metastasis |
|
|
|
| No | 109 (97.3) |
|
|
|
Yes | 3 (2.7) |
|
|
| TNM stage |
|
|
|
|
I+II | 77 (68.8) |
|
|
|
III+IV | 35 (31.3) |
|
|
Diagnostic accuracy of MMP-9 for
distinguishing PTC
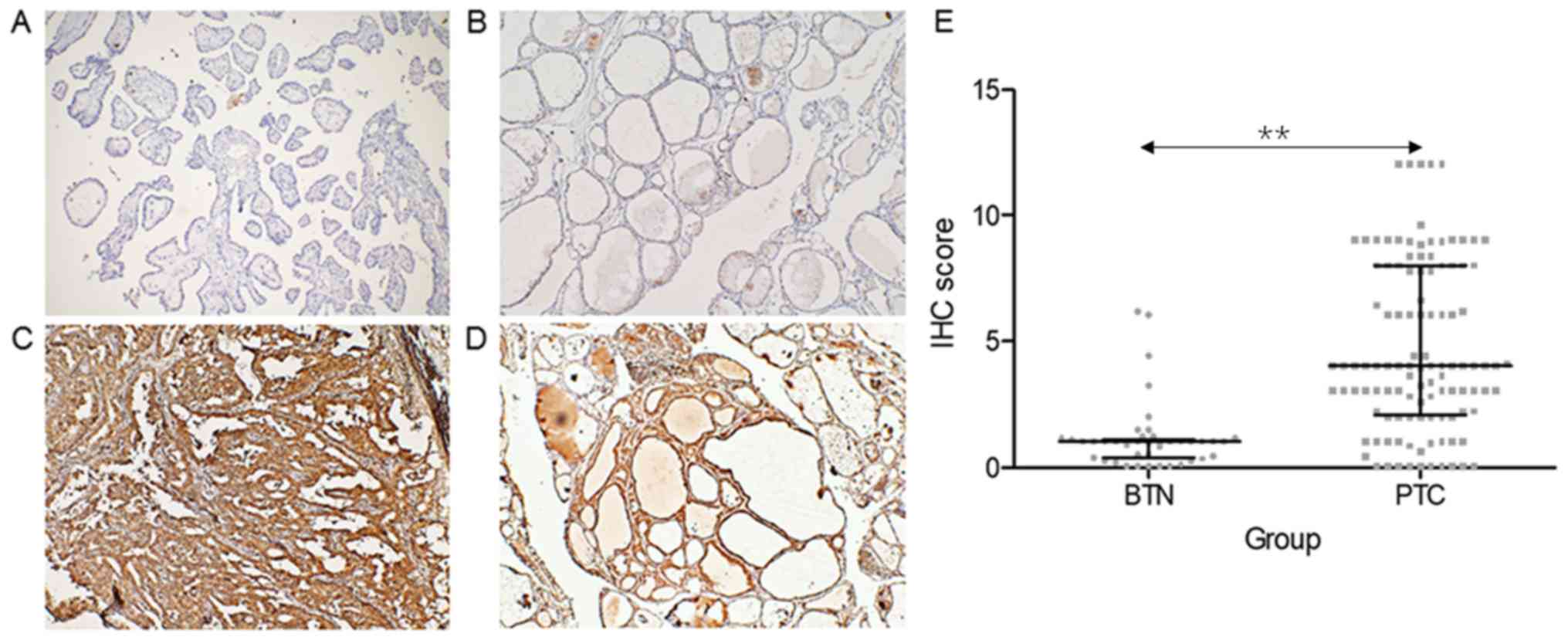
MMP-9 was overexpressed in samples obtained from
patients with PTC, when compared with control subjects with BTN
(Fig. 2A-D). Furthermore, patients
with PTC had a significantly higher IHC score of MMP-9 (median,
4.0; IQR, 2.0–8.0), when compared with control subjects (median,
1.0; IQR, 0.0–1.0) (P<0.001, Fig.
2E).
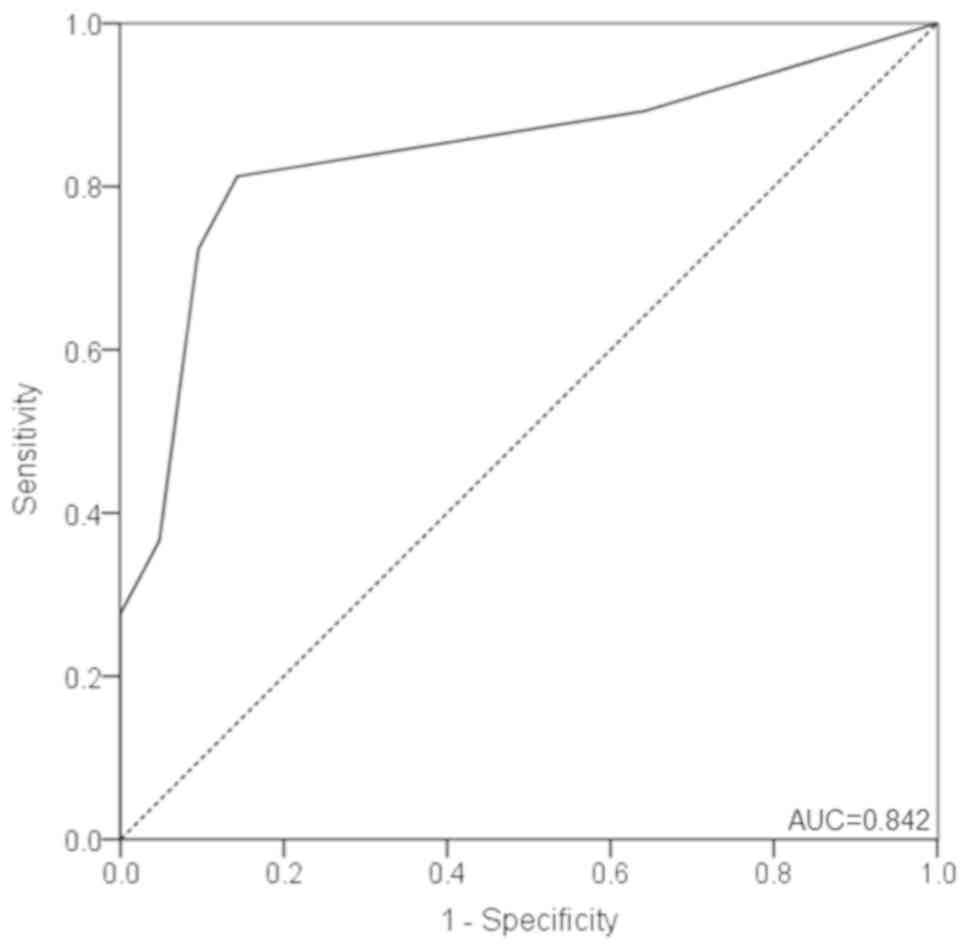
The ROC curve for distinguishing PTC from BTN is
presented in Fig. 3. MMP-9 scores
yielded an area under the curve (AUC) of 0.842 (95% CI,
0.776–0.908), with a sensitivity and specificity of 81.3 and 85.7%,
respectively, at a cutoff value of 2.0 points (Fig. 3). Furthermore, the PPV was 93.8%,
whereas the NPV was 63.2%. In addition, the positive likelihood
ratio was 5.690, whereas the negative likelihood ratio was
0.218.
MMP-9 IHC expression in PTC with
different invasive characteristics
Mann-Whitney test analysis was conducted to explore
the association between MMP-9 and the invasive characteristics of
PTC. The IHC expression of MMP-9 was greater if patients had
central LNM (CLNM), lateral LNM (LLNM) or an advanced TNM stage
(III+IV) (all P<0.05; Table II).
There was no difference in IHC MMP-9 expression in the presence or
absence of capsule invasion, multifocality, extrathyroidal
invasion, vascular invasion and distant metastasis (Table II).
 | Table II.MMP-9 immunohistochemistry expression
differences in the groups stratified according to
clinicopathological characteristics. |
Table II.
MMP-9 immunohistochemistry expression
differences in the groups stratified according to
clinicopathological characteristics.
| Variable | MMP-9 scorea | P-valueb |
|---|
| Tumor size (>2
cm vs. ≤2 cm) | 6.0 (3.0–9.0) vs.
4.0 (2.0–6.0) | 0.053 |
| Capsule invasion
(yes vs. no) | 4.0 (2.0–9.0) vs.
3.0 (2.0–6.0) | 0.090 |
| Multifocality (yes
vs. no) | 4.0 (1.0–8.0) vs.
4.0 (3.0–8.0) | 0.499 |
| Central lymph node
metastasis (yes vs. no) | 7.0 (3.0–9.0) vs.
3.0 (2.0–4.0) | 0.002 |
| Lateral lymph node
metastasis (yes vs. no) | 8.0 (5.0–9.0) vs.
3.0 (2.0–4.0) |
<0.001 |
| Extrathyroidal
invasion (yes vs. no) | 5.0 (3.0–9.0) vs.
4.0 (2.0–6.0) | 0.065 |
| Vascular invasion
(yes vs. no) | 6.0 (1.0–9.0) vs.
4.0 (2.0–8.0) | 0.691 |
| Distant metastasis
(yes vs. no) | 3.0 (2.0–6.0) vs.
4.0 (2.0–8.0) | 0.721 |
| TNM stage (III+IV
vs. I+II) | 8.0 (3.0–9.0) vs.
3.0 (2.0–6.0) | 0.004 |
Prognostic accuracy of MMP-9 for
predicting disease status and SPRD
During the follow-up, patients with a disease status
had a significantly higher MMP-9 score (median: 8, IQR: 6.0–9.0)
than patients with a disease-free status (median: 3.0, IQR:
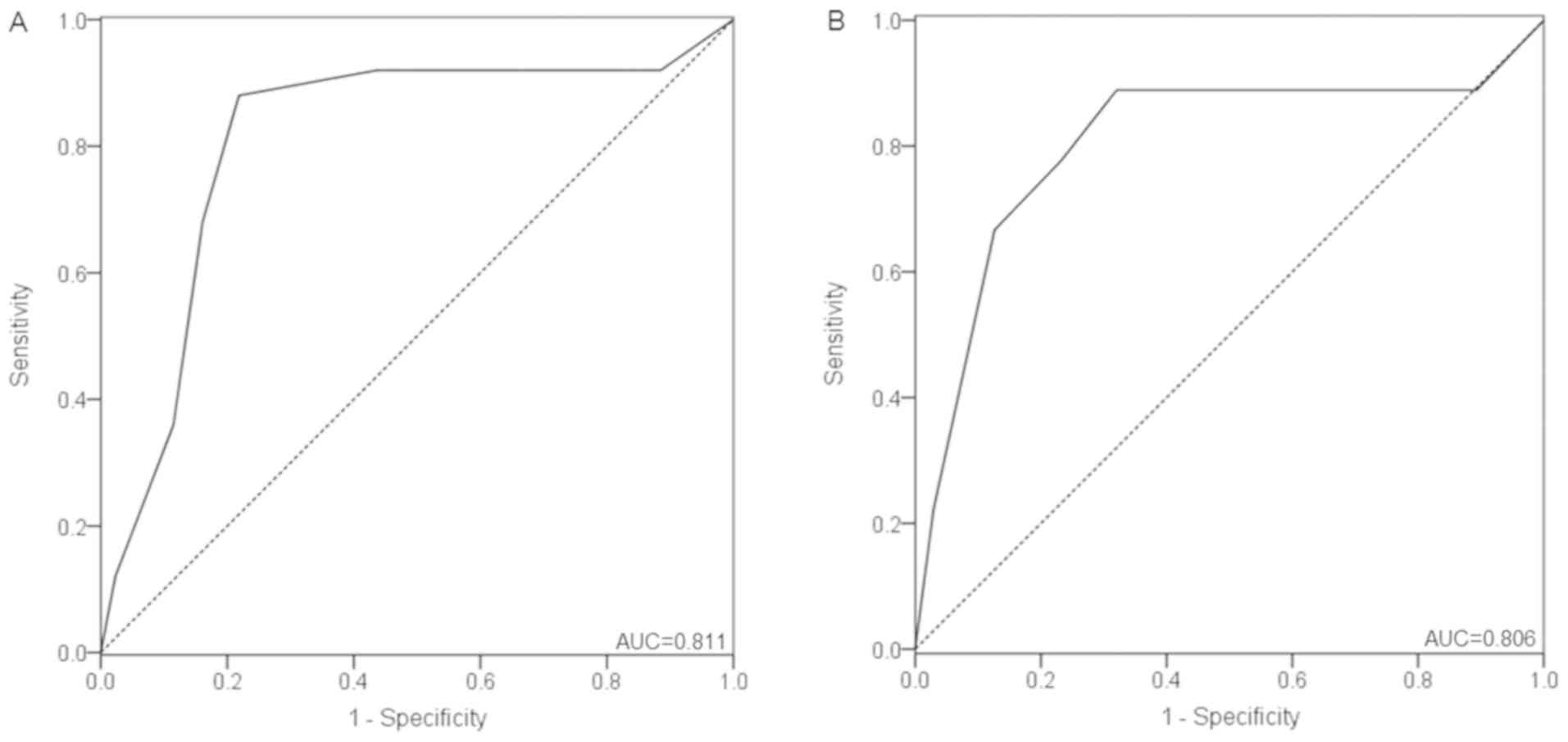
2.0–4.0; P<0.001). At a cutoff value of 6.0 points, MMP-9
yielded an AUC of 0.811 (95% CI, 0.706–0.917), a sensitivity of
88.0% and a specificity of 78.2% for predicting disease-free status
(Fig. 4A).
Patients with SPRD had a significantly higher MMP-9
score (median: 9.0; IQR: 7.0–10.5) than patients without SPRD
(median: 4.0; IQR: 2.0–6.0) (P=0.010). Furthermore, when MMP-9 was
employed to predict SPRD, an AUC of 0.806 (95% CI, 0.620–0.992) was
obtained at a cutoff value of 8.0 points, with a sensitivity and
specificity of 77.8 and 76.7%, respectively (Fig. 4B).
Risk factors of disease status and
SPRD
A univariate logistic regression analysis was
performed to identify potential risk factors for predicting disease
status. An age of ≥45 years old, a tumor size of >2 cm, and the
presence of CLNM, LLNM, vascular invasion, advanced TNM stage and
MMP-9 ≥6 points were identified as risk factors for the development
of the disease status (all P<0.05). In the multivariate model, a
tumor size >2 cm (OR, 3.011; 95% CI, 1.119–8.097; P=0.029) and
MMP-9 ≥6 points (OR, 12.210, 95% CI, 3.404–43.798; P<0.001) were
both independent risk factors for disease status in patients with
PTC (Table III).
 | Table III.Risk factors of disease status and
SPRD in patients with PTC, as determined by Cox regression
model. |
Table III.
Risk factors of disease status and
SPRD in patients with PTC, as determined by Cox regression
model.
| A, Disease
status |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age (≥45 years old
vs. <45 years old) | 4.528
(1.698–12.072) | 0.003 | 2.269
(0.677–7.600) | 0.184 |
| Sex (male vs.
female) | 1.511
(0.567–4.027) | 0.409 |
|
|
| BMI (≥25 kg/m2 vs.
<25 kg/m2) |
1.731(0.765–3.918) | 0.188 |
|
|
| Tumor size (>2
cm vs. ≤2 cm) | 3.353
(1.528–7.358) | 0.003 | 3.011
(1.119–8.097) | 0.029 |
| Capsule invasion
(yes vs. no) | 1.583
(0.711–3.524) | 0.261 |
|
|
| Multifocality (yes
vs. no) | 1.364
(0.619–3.005) | 0.441 |
|
|
| CLNM (yes vs.
no) | 3.915
(1.688–9.082) | 0.001 | 1.990
(0.729–5.430) | 0.179 |
| LLNM (yes vs.
no) | 3.932
(1.780–8.688) | 0.001 | 0.730
(0.256–2.085) | 0.557 |
| Extrathyroidal
invasion (yes vs. no) | 2.041
(0.916–4.545) | 0.081 |
|
|
| Vascular invasion
(yes vs. no) | 3.446
(1.029–11.456) | 0.045 | 2.384
(0.655–8.678) | 0.188 |
| Distant metastasis
(yes vs. no) | 3.354
(0.788–14.273) | 0.101 |
|
|
| TNM stage (III+IV
vs. I+II) | 2.632
(1.200–5.773) | 0.016 | 0.465
(0.151–1.431) | 0.182 |
| MMP-9 score (≥6
points vs. <6 points) | 16.665
(4.975–55.826) |
<0.001 | 12.210
(3.404–43.798) |
<0.001 |
|
| B, SPRD |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristic | OR (95%
CI) | P-value | OR (95%
CI) | P-value |
|
| Age (≥45 years old
vs. <45 years old) | 3.696
(0.768–17.796) | 0.103 | 0.204
(0.003–16.021) | 0.475 |
| Sex (male vs.
female) |
1.661(0.345–7.995) | 0.527 |
|
|
| BMI (≥25 kg/m2 vs.
<25 kg/m2) | 3.533
(0.734–17.010) | 0.115 |
|
|
| Tumor size (>2
cm vs. ≤2 cm) | 10.131
(2.103–48.810) | 0.004 | 1.949
(0.160–23.801) | 0.601 |
| Capsule invasion
(yes vs. no) | 8.522
(1.1066–68.143) | 0.043 | 2.477
(0.171–35.817) | 0.506 |
| Multifocality (yes
vs. no) | 3.401
(0.850–13.600) | 0.084 |
|
|
| CLNM (yes vs.
no) | 6.338
(1.316–30.521) | 0.021 | 0.434
(0.048–3.939) | 0.458 |
| LLNM (yes vs.
no) | 17.896
(3.709–86.335) |
<0.001 | 3.484
(0.461–26.302) | 0.226 |
| Extrathyroidal
invasion (yes vs. no) | 5.814
(1.453–23.265) | 0.013 | 0.738
(0.092–5.944) | 0.775 |
| Vascular invasion
(yes vs. no) | 16.064
(3.985–64.746) |
<0.001 | 17.258
(2.434–122.345) | 0.004 |
| Distant metastasis
(yes vs. no) | 18.993
(3.866–93.306) |
<0.001 | 3.023
(0.476–14.376) | 0.052 |
| TNM stage (III+IV
vs. I+II) | 8.492
(1.763–40.902) | 0.008 | 3.542
(0.428–29.316) | 0.241 |
| MMP-9 score (≥8
points vs. <8 points) | 10.471
(2.173–50.450) | 0.003 | 15.329
(1.368–171.717) | 0.027 |
By univariate Cox regression analysis, a tumor size
>2 cm, capsule invasion, CLNM, LLNM, extrathyroidal invasion,
vascular invasion, distant metastasis, advanced TNM stage and an
MMP-9 score of ≥8 points were risk factors for SPRD (all
P<0.05). Furthermore, multivariate analysis was performed to
evaluate these univariate predictors, and the results revealed that
vascular invasion (OR, 17.258; 95% CI, 2.434–122.345; P=0.004) and
an MMP-9 score of ≥8 points (OR, 15.329; 95% CI, 1.368–171.717;
P=0.027) were independent risk factors for SPRD (Table III).
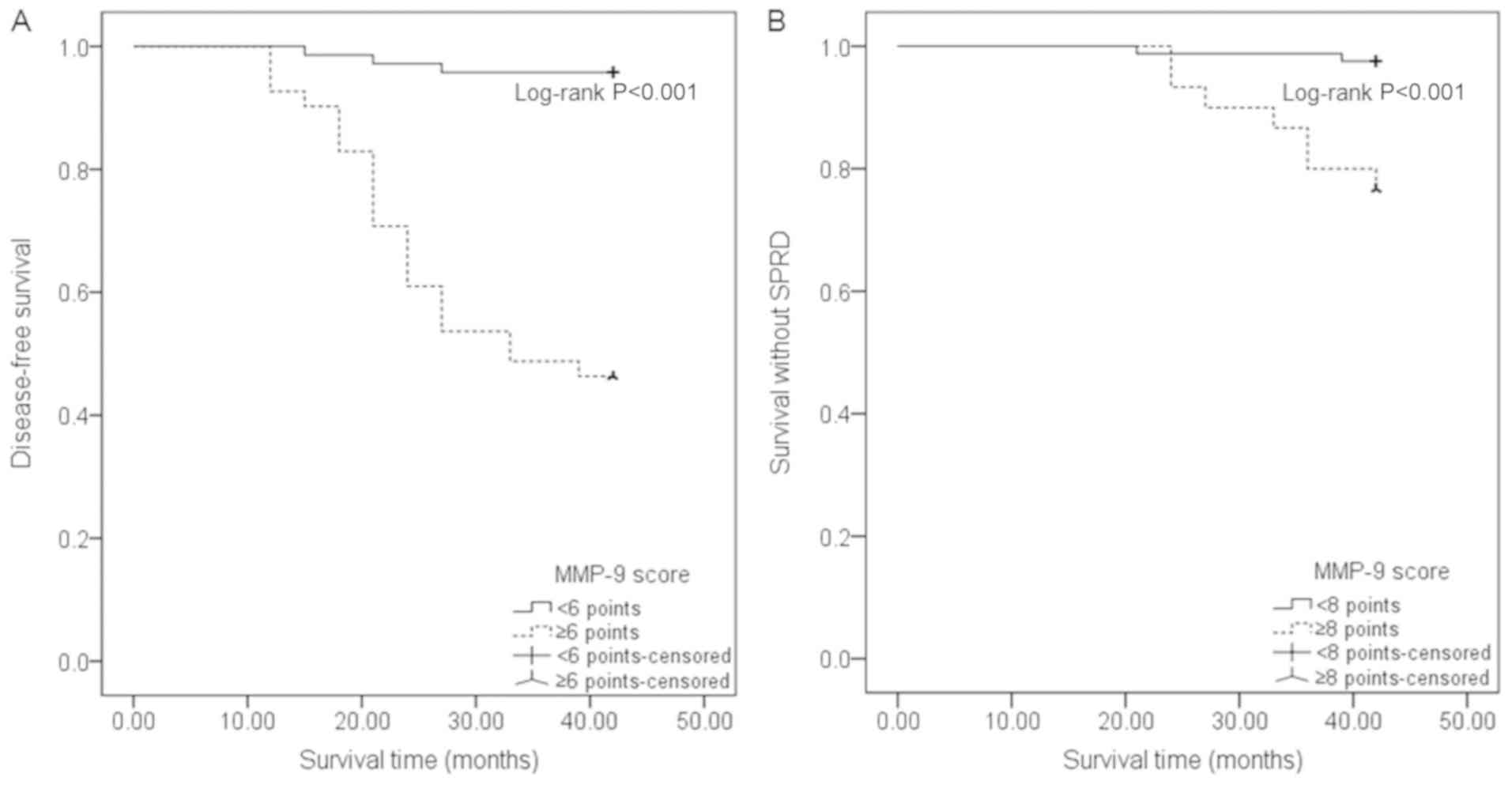
Clinical outcomes stratified by MMP-9
scores
DFS time was shorter in PTC patients with an MMP-9
score of ≥6 points (31.46±11.43 months), when compared to patients
with an MMP-9 score of <6 points (41.11±4.37 months)
(P<0.001). Similarly, PTC patients with an MMP-9 score of ≥8
points had shorter survival time without SPRD (39.6±5.42 months),
when compared with patients with an MMP-9 score of <8 points
(41.70±2.34 months) (P=0.001). The Kaplan-Meier curves of survival
demonstrated that patients with an MMP-9 score of ≥6 points had
lower cumulative DFS rates, when compared to patients with a score
of <6 points (P<0.001). Furthermore, patients with an MMP-9
score of ≥8 points had a lower cumulative rate of survival without
SPRD, when compared to patients with a score of <8 points
(P<0.001, Fig. 5).
Discussion
To the best of our knowledge, only a small number of
studies regarding MMP-9 in thyroid tumorigenesis have been
conducted, in comparison with tumors from other organs, including
breast, ovarian, liver, lung and colon cancer, in which MMP-9
expression has already been extensively studied (27–31). In
the present study, the IHC results revealed that MMP-9 expression
was significantly higher in PTC tissues than in BTN specimens. In
addition, the MMP-9 staining score was greater in the presence of
CLNM, LLNM or in the case of advanced TNM stage. Furthermore, the
MMP-9 score yielded good sensitivity and specificity for PTC
diagnosis and prognostic prediction. A MMP-9 score of ≥6 and ≥8
indicated shortened DFS and survival without SPRD,
respectively.
MMPs have essential roles in various biological
functions, including cell proliferation, differentiation, ECM
degradation and remodeling, angiogenesis and cell migration.
Furthermore, the expression and activity of MMP-9 are elevated in
numerous types of human cancer (32–34). In
addition, the IHC positive staining of MMP-9 has been reported in
57.0–92.4% of patients with PTC (3,24). In the
present study, MMP-9 was expressed at low or almost undetectable
levels in BTN specimens (median: 1.0; IQR: 0.0–1.0). Conversely,
overexpression of MMP-9 (median: 4.0; IQR: 2.0–8.0) was detected in
PTC tissues, and was associated with tumor malignancy. The majority
of MMPs are secreted in an inactive form, and can be
proteolytically activated by extracellular proteinase, which is
critically implicated in carcinogenesis (35). The present findings revealed that the
MMP-9 IHC score may be useful for the differential diagnosis of PTC
from BTN, with a sensitivity and specificity of 81.3 and 85.7%,
respectively, and at a cutoff value of 2.0 points. Consistently,
Meng et al demonstrated that the immunohistochemical
staining of MMP-9 yields a sensitivity and specificity of 92.4 and
80%, respectively, for PTC diagnosis from BTN (24), thus suggesting a role for MMP-9 in PTC
diagnosis. In addition, vasculature is important for tumor size and
growth; if the tumor vasculature is not developed, tumors are
restricted in size within a tissue-diffusion distance of 0.2–2.0 mm
(36). MMP-9 has been demonstrated to
participate in angiogenesis, as a result, MMP-9 overexpression is
likely to be associated with a larger tumor size and is the
dependent risk factor for disease status and SPRD (23,37). Above
all, MMP-9 is associated with the prognosis of PTC. MMP-9 has been
demonstrated to participate in the switch from vascular quiescence
to angiogenesis, which is a crucial process required for persistent
tumor growth (38). The MMP-dependent
release of growth factors, including VEGF and FGF, stimulates the
progressive growth of tumor cells (39). This may explain the present finding
that stronger MMP-9 immunostaining was likely to be associated with
a larger tumor size (>2 cm). Furthermore, tumor size (>2 cm)
was dependent predictor for disease status and SPRD, indicating
that MMP-9 was correlated with the prognosis of PTC.
Invasion and metastasis substantially contribute to
cancer-associated mortality. The disruption of basement membranes
is a crucial step in the process of cancer invasion to surrounding
tissues and of metastasis to distant organs. The role of MMP-9 is
to degrade type IV collagen, which is the main structural component
of the basement membrane, and is of particular importance in tumor
cell migration (40). MMP-9-mediated
tumor angiogenesis creates a microenvironment favorable to tumor
cell invasion by promoting gas exchange and supplying nutrients
(41). Furthermore, MMP-9 expression
has been reported to be associated with microvessel density in
colorectal carcinoma (42). In
addition, MMP-9 is an essential mediator of epithelial-mesenchymal
transition, which is a key step in tumor progression and metastasis
(43). The association between MMP-9
overexpression and the invasiveness of cancer has been reported in
numerous studies (32,44–47). In
patients with PTC, positive MMP-9 staining is correlated with
lymphatic spreading and the degree of tumor infiltration (3). Consistent with these findings, the IHC
score of MMP-9 was greater in the presence of CLNM, LLNM or
advanced TNM stage, indicating that MMP-9 may be used as a
predictive biomarker of aggressive PTC behaviors.
The association between MMP-9 expression and
malignant neoplasms has garnered much interest, and MMP-9 may be
considered a predictor of patient prognosis. It has been reported
that elevated MMP-9 expression, as an independent risk factor, is
associated with advanced tumor stage and shortened survival in
various types of cancer (47,48). In the present study, MMP-9 expression
in PTC samples was revealed to represent an independent risk factor
for predicting the presence of disease and SPRD following
thyroidectomy. Subsequently, high MMP-9 expression was associated
with shortened DFS and survival without SPRD in patients with PTC.
These results are consistent with a previous study, which reported
that MMP-9 is an independent prognostic factor for predicting the
worse outcome in patients with PTC following radiofrequency
ablation (49). In addition, Lin
et al revealed that median event-free survival was shorter
in patients with T-cell acute lymphoblastic leukemia with the CT+TT
genotype of MMP-9-1562C>T, when compared to patients with the CC
genotype, suggesting that MMP-9 genetic polymorphism may affect
tumor progression and prognosis (50). Therefore, the genetic characteristics
of MMP-9 and its association with PTC prognosis require further
investigation.
The present study differs from previous studies in
many ways. To the best of our knowledge, it is the first to
carefully assess the diagnostic and prognostic values of MMP-9
immunostaining in patients with PTC and after thyroidectomy. PTC is
a more common malignancy in women than in men (51); however, male patients with PTC are
common in China (52). The sex and
age were well matched between the BTN group and PTC group, in order
to minimize any influences caused by age and sex. In addition, the
relationships between MMP-9 and SPRD, and survival without SPRD,
were examined, which has rarely been reported in previous studies
investigating the predictive role of MMP-9 in PTC prognosis.
Finally, previous studies have focused on other proteins, and MMP-9
has only been examined with regards to a few aspects of PTC
prognosis (1,3,22,23). The present study systematically
analyzed the diagnostic and prognostic roles of immunohistochemical
MMP-9 in patients with PTC, and identified it as an independent
risk factor for disease status and survival. Consequently, the
present study may provide detailed evidence for deciding the
usefulness of MMP-9 in PTC prediction.
The present study presented numerous limitations.
Firstly, this study was a single-center, retrospective cohort
analysis with a relatively small sample size. In addition, a longer
follow-up period is required to further analyze the relationship
between alterations in MMP-9 and the prognosis of patients.
Secondly, IHC staining is only a semi-quantitative method for
testing MMP-9 protein levels. Quantitative measurements of MMP-9
expression levels and protein activation should be conducted to
validate these findings. Thirdly, selection bias at the time of
study enrollment may have occurred. Notably, some patients with
small PTC lesions or BTNs who refused dissection were not recruited
in the study. Finally, although there were five subtypes of PTC in
the present study, the number of patients with each of them was
very small; therefore, all PTC subtypes were added and analyzed as
a whole.
In conclusion, the present study demonstrated that
immunohistochemical MMP-9 expression was markedly increased in PTC.
In addition, elevated MMP-9 expression was associated with central
lymph node metastasis, lateral lymph node metastasis, advanced TNM
stage and shortened patient survival. Therefore, the assessment of
MMP-9 expression in thyroid carcinoma samples may represent a novel
tool for PTC diagnosis and prognostic prediction.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special
Project for Health in Jilin Province (grant nos. 2018SCZWSZX-039
and 2018SCZWSZX-050), Projects of the Education Department of Jilin
Province (grant nos. 20150125, 2016453 and 2016482), and the
Project of the Health Department of Jilin Province (grant no.
20150125).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, JX and CS analyzed and interpreted the patient
data on papillary thyroid diseases and BTNs. DX and NZ performed
the histological examination of the thyroid tissues. HH was the
major contributor in designing the experiment and writing the
manuscript. DZ, HY, WL and GC prepared the figures and conducted
the statistical analyses. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study methodology was approved by the Ethics
Committee of the First Hospital of Jilin University. Patients
provided informed consent.
Patient consent for publication
All participants provided written informed consent
prior to participation.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Luo YK, Zhang MB, Li J, Li CT,
Tang J and Li JL: Values of ultrasound features and MMP-9 of
papillary thyroid carcinoma in predicting cervical lymph node
metastases. Sci Rep. 7:66702017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Šelemetjev S, Ðoric I, Paunovic I, Tatic S
and Cvejic D: Coexpressed high levels of VEGF-C and active MMP-9
are associated with lymphatic spreading and local invasiveness of
papillary thyroid carcinoma. Am J Clin Pathol. 146:594–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang LL, Wang Z, Cao CJ, Ke ZF, Wang F,
Wang R, Luo CQ, Lu X and Wang LT: AEG-1 associates with metastasis
in papillary thyroid cancer through upregulation of MMP2/9. Int J
Oncol. 51:812–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Wu W, Gao M and Fei Z:
MicroRNA-451 as a prognostic marker for diagnosis and lymph node
metastasis of papillary thyroid carcinoma. Cancer Biomark.
19:437–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vuong HG, Duong UN, Altibi AM, Ngo HT,
Pham TQ, Tran HM, Gandolfi G and Hassell L: A meta-analysis of
prognostic roles of molecular markers in papillary thyroid
carcinoma. Endocr Connect. 6:R8–R17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim Y, Kim MH, Jeon S, Kim J, Kim C, Bae
JS and Jung CK: Prognostic implication of histological features
associated with EHD2 expression in papillary thyroid carcinoma.
PLoS One. 12:e01747372017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castro MG, Campos LE, Rodriguez YI and
Alvarez SE: In vitro methods to study the modulation of migration
and invasion by sphingosine-1-phosphate. Methods Mol Biol.
1697:117–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edatt L, Maurya AK, Raji G, Kunhiraman H
and Kumar SVB: MicroRNA106a regulates matrix metalloprotease 9 in a
sirtuin-1 dependent mechanism. J Cell Physiol. 233:238–248. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Q, Guo X and Choksi R: Activation of
focal adhesion kinase and Src mediates acquired sorafenib
resistance in A549 human lung adenocarcinoma xenografts. J
Pharmacol Exp Ther. 363:428–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou G, Peng F, Zhong Y, Chen Y, Tang M
and Li D: Rhein suppresses matrix metalloproteinase production by
regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian
carcinoma cells. Int J Oncol. 50:933–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L and Xue GB: Catalpol suppresses
osteosarcoma cell proliferation through blocking
epithelial-mesenchymal transition (EMT) and inducing apoptosis.
Biochem Biophys Res Commun. 495:27–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai XX, Li G, Lin B and Yang H:
Interference of Notch 1 inhibits the proliferation and invasion of
breast cancer cells: Involvement of the β-catenin signaling
pathway. Mol Med Rep. 17:2472–2478. 2018.PubMed/NCBI
|
|
14
|
Bai XY, Li S, Wang M, Li X, Yang Y, Xu Z,
Li B, Li Y, Xia K, Chen H and Wu H: Krüppel-like factor 9
down-regulates matrix metalloproteinase 9 transcription and
suppresses human breast cancer invasion. Cancer Lett. 412:224–235.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu N, Si M, Yang N, Jing Y, Fu Y, Zhao X,
Lin Z and Yang G: Overexpression of RAS-association domain family 6
(RASSF6) inhibits proliferation and tumorigenesis in hepatocellular
carcinoma cells. Oncol Res. 25:1001–1008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Chen Y, Li S, Lei Y, Xu D, Jiang N,
Zhang Y, Cao J and Ke Z: NanoVelcro-captured CTC number concomitant
with enhanced serum levels of MMP7 and MMP9 enables accurate
prediction of metastasis and poor prognosis in patients with lung
adenocarcinoma. Int J Nanomedicine. 12:6399–6412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nat Cell Biol. 2:737–744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ucuzian AA, Gassman AA, East AT and
Greisler HP: Molecular mediators of angiogenesis. J Burn Care Res.
31:158–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: An overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XZ, Cui SZ, Zeng LS, Cheng TT, Li XX,
Chi J, Wang R, Zheng XF and Wang HY: Overexpression of Rab1B and
MMP9 predicts poor survival and good response to chemotherapy in
patients with colorectal cancer. Aging (Albany NY). 9:914–931.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li
SZ and Fei Z: High expression of MMP9 in glioma affects cell
proliferation and is associated with patient survival rates. Oncol
Lett. 13:1325–1330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo D, Chen H, Li X, Lu P, Long M, Peng X,
Lin S, Tan L, Zhu Y, Ouyang N and Li H: Activation of the
ROCK1/MMP-9 pathway is associated with the invasion and poor
prognosis in papillary thyroid carcinoma. Int J Oncol.
51:1209–1218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang N, Jiang R, Yang JY, Tang C, Yang L,
Xu M, Jiang QF and Liu ZM: Expression of TGF-β1, SNAI1 and MMP-9 is
associated with lymph node metastasis in papillary thyroid
carcinoma. J Mol Histol. 45:391–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng XY, Zhang Q, Li Q, Lin S and Li J:
Immunohistochemical levels of cyclo-oxygenase-2, matrix
metalloproteinase-9 and vascular endothelial growth factor in
papillary thyroid carcinoma and their clinicopathological
correlations. J Int Med Res. 42:619–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JJ, Wang TY, Liu CL, Chien MN, Chen
MJ, Hsu YC, Leung CH and Cheng SP: Dipeptidyl peptidase IV as a
prognostic marker and therapeutic target in papillary thyroid
carcinoma. J Clin Endocrinol Metab. 102:2930–2940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang L, Zhang N, Xia Y, Wang D, Wang G and
Meng X: Serum APN/CD13 as a novel diagnostic and prognostic
biomarker of pancreatic cancer. Oncotarget. 7:77854–77864. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun XF, Shao YB, Liu MG, Chen Q, Liu ZJ,
Xu B, Luo SX and Liu H: High-concentration glucose enhances
invasion in invasive ductal breast carcinoma by promoting
Glut1/MMP2/MMP9 axis expression. Oncol Lett. 13:2989–2995. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou G, Peng F, Zhong Y, Chen Y, Tang M
and Li D: Rhein suppresses matrix metalloproteinase production by
regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian
carcinoma cells. Int J Oncol. 50:933–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu N, Si M, Yang N, Jing Y, Fu Y, Zhao X,
Lin Z and Yang G: Overexpression of RAS-association domain family 6
(RASSF6) inhibits proliferation and tumorigenesis in hepatocellular
carcinoma cells. Oncol Res. 25:1001–1008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu B, Yang J, Zhang P, Shen L, Li X and
Li J: Safety and effectiveness of localized lung resection combined
with neoadjuvant chemotherapy in the treatment of stage I–II
non-small cell lung cancer. Oncol Lett. 13:2344–2348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang R, Zhao J, Xu J, Jiao DX, Wang J,
Gong ZQ and Jia JH: Andrographolide suppresses proliferation of
human colon cancer SW620 cells through the TLR4/NF-κB/MMP-9
signaling pathway. Oncol Lett. 14:4305–4310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ricci S, Guadagno E, Bruzzese D, Del Basso
De Caro M, Peca C, Sgulò FG, Maiuri F and Di Carlo A: Evaluation of
matrix metalloproteinase type IV-collagenases in serum of patients
with tumors of the central nervous system. J Neurooncol.
131:223–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reiner AT, Tan S, Agreiter C, Auer K,
Bachmayr-Heyda A, Aust S, Pecha N, Mandorfer M, Pils D, Brisson AR,
et al: EV-associated MMP9 in high-grade serous ovarian cancer is
preferentially localized to annexin V-binding EVs. Dis Markers.
2017:96531942017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skerenova M, Mikulova V, Capoun O, Zima T
and Tesarova P: Circulating tumor cells and serum levels of MMP-2,
MMP-9 and VEGF as markers of the metastatic process in patients
with high risk of metastatic progression. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 161:272–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang J, Shing Y, Wiederschain D, Yan L,
Butterfield C, Jackson G, Harper J, Tamvakopoulos G and Moses MA:
Matrix metalloproteinase-2 is required for the switch to the
angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA.
97:3884–3889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He J, Shen N and Huang X: Thyroid
carcinoma cells produce PLGF to enhance metastasis. Tumour Biol.
36:8601–8607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|
|
39
|
Chang C and Werb Z: The many faces of
metalloproteases: Cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zheng H and Liu JF: Studies on the
relationship between P13K/AKT signal pathway-mediated MMP-9 gene
and lung cancer. Eur Rev Med Pharmacol Sci. 21:753–759.
2017.PubMed/NCBI
|
|
41
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46. 94–112.
2015.
|
|
42
|
Wu XL, Xue J, Wang LK, Yang DD, Qu M, Guo
F, Sun GY, Han L and Yang RM: Expressions of inhibitors of DNA
binding-1 and matrix metalloproteinase-9 in colorectal
adenocarcinoma tissues and their correlations with microvessel
density. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 38:696–701.
2016.PubMed/NCBI
|
|
43
|
Bai X, Li YY, Zhang HY, Wang F, He HL, Yao
JC, Liu L and Li SS: Role of matrix metalloproteinase-9 in
transforming growth factor-β1-induced epithelial-mesenchymal
transition in esophageal squamous cell carcinoma. Onco Targets
Ther. 10:2837–2847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu XM and Sun WF: Association between
matrix metalloproteinases polymorphisms and ovarian cancer risk: A
meta-analysis and systematic review. PLoS One. 12:e01854562017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsu CC, Huang SF, Wang JS, Chu WK, Nien
JE, Chen WS and Chow SE: Interplay of N-cadherin and matrix
metalloproteinase 9 enhances human nasopharyngeal carcinoma cell
invasion. BMC Cancer. 16:8002016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rašić I, Rašić A, Akšamija G, Radović S
and Šehović N: The association between the serum levels of matrix
metalloproteinase 9 and colorectal cancer. Med Glas (Zenica).
14:229–235. 2017.PubMed/NCBI
|
|
47
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
48
|
Lee CY, Shim HS, Lee S, Lee JG, Kim DJ and
Chung KY: Prognostic effect of matrix metalloproteinase-9 in
patients with resected non small cell lung cancer. J Cardiothorac
Surg. 10:442015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He J, Liu G, Shao K, Shen X and Chen L:
Serum contents of matrix metalloproteinase-2 and 9 are correlated
with the prognosis of papillary thyroid carcinoma after
ultrasound-guided radiofrequency ablation. Biomed Res.
28:6711–6716. 2017.
|
|
50
|
Lin CM, Zeng YL, Xiao M, Mei XQ, Shen LY,
Guo MX, Lin ZY, Liu QF and Yang T: The relationship between MMP-2
−1306C>T and MMP-9-1562C>T polymorphisms and the risk and
prognosis of T-cell acute lymphoblastic leukemia in a chinese
population: A case-control study. Cell Physiol Biochem.
42:1458–1468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aschebrook-Kilfoy B, Ward MH, Sabra MM and
Devesa SS: Thyroid cancer incidence patterns in the United States
by histologic type, 1992–2006. Thyroid. 21:125–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan HX, Pang P, Wang FL, Tian W, Luo YK,
Huang W, Yang GQ, Jin N, Zang L, Du J, et al: Dynamic profile of
differentiated thyroid cancer in male and female patients with
thyroidectomy during 2000–2013 in China: A retrospective study. Sci
Rep. 7:158322017. View Article : Google Scholar : PubMed/NCBI
|