Introduction
Synchronous endometrial and ovarian carcinomas occur
in approximately 5% of endometrial carcinomas and 10–20% of ovarian
carcinomas, respectively (1,2). The distinction between independent
primary tumors and metastasis from one site to the other
(endometrium to the ovary or ovary to the endometrium) can be
complicated but it is clinically significant. Historically, the
criteria for this distinction evolved and were based mostly on
morphological features (3,4). However, a great benefit has been
expected from ancillary methods (especially molecular analyses)
which are now becoming, together with methodological development,
more complex and also more available. Surprisingly, the results of
recent molecular studies have shown that most SEOs share clonal
origin irrespectively of their clinico-pathological features
(5–7).
The benefit of molecular analysis regarding differential diagnosis
between independent primary tumors and metastatic disease is, from
this point of view, disputable. Nevertheless, in one recent study
the authors suggested that molecular profiling might be beneficial
in this setting (8). However, this
suggestion was based on molecular profiling of one SEO only. In our
study we focused on a comprehensive clinico-pathological and
molecular study of 22 cases of SEO.
Materials and methods
Patients and materials
Archive files of the: i) Institute of Pathology,
First Faculty of Medicine, Charles University and General
University Hospital in Prague, Czech Republic; ii) The Fingerland
Department of Pathology, Charles University Faculty of Medicine and
University Hospital Hradec Králové, Czech Republic; and iii)
Department of Pathology, University of Debrecen, Hungary, were
searched for synchronous cases of endometrioid endometrial and
ovarian carcinomas. Twenty-two SEOs were found with corresponding
formalin-fixed paraffin-embedded tissue (FFPE) blocks from both
endometrial and ovarian tumors. One patient with SEO also had
synchronous CCCO arising in the second ovary. Another patient had
endometrioid carcinoma of the left ovary and after 49 months she
developed SEO (of the endometrium and right ovary). Histological
type, staging and grading of EEC and EOC were assessed for each
tumor separately according to the standard criteria (9). A review of the hematoxylin and eosin
stained slides was performed in all cases, and the area of tumor
tissue for macrodissection was marked (percentage of tumor cells in
selected area ranges between 30 and 90%).
DNA from FFPE blocks was isolated using standard
procedures implementing QIAamp DNA Tissue kit (Qiagen, Hilden,
Germany) or cobas® DNA Sample Preparation kit (Roche,
Basel, Switzerland), respectively.
Sequencing analysis
The whole project and all auxiliary files are
designed for genome build GRCh37 (hg 19) coordinates. Samples for
sequence capture NGS (massive parallel sequencing) were prepared
using the KAPA HyperPlus kit. Target sequences were enriched using
commercial hybridization probes (Nimblegen, Roche) designed for
human DNA regions of our interest (73 genes or gene parts; 219
kbp). The panel included the following genes: AKT1, AKT3,
ARID1A, ARID2, ATM, BAP1, BARD1, BIRC5 (promoter), BRAF
(exon 11,15), BRCA1, BRCA2, BRIP1, CCND2, CCND3, CDH1, CDK4,
CDKN2A, CYP19A1, ERBB2, ERCC3, ESR1, ESR2, F11R, FOXL2, GNA11,
GNAQ, HNF1B, HRAS, IDH1, JAM2, JAM3, KDR, KIT, KRAS, MAP2K1,
MAP2K2, MAPK1, MAPK3, MDM2, MET, MITF, MLH1, MLH3, MSH2, MSH6, MYC,
NBN, NRAS, PALB2, PARD3, PDGFRA (exon 12,14,18), PIK3CA,
POLE, POT1, PPM1D, PPP6C, PTEN, RAD51C, RAD51D, RB1, SF3B1,
SMARCA4, SMARCB1, SNAI1, SNAI2, SNAI3, TERT (promoter),
TJP1, TP53, TWIST1, TWIST2, ZEB1, ZEB2. The library was
pair-end sequenced by MiSeq instrument (Illumina, Inc., San Diego,
CA, USA).
Selected germline variants and selected variants
with a frequency higher than 10% were confirmed by direct Sanger
sequencing using BigDye v3.1 and ABI3500 analyzer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
The processing of raw sequencing data was performed
to analyze the spectrum of genetic variants, such as single
nucleotide variants and short insertions or deletions using
NextGENe software (SoftGenetics LLC, State College, PA, USA)
according to the standardized biostatistical methods for NGS data.
Primary raw data were trimmed and demultiplexed by MiSeq system
during post-sequencing process. Output in .fastq format was
complexly analyzed by NextGENe software (SoftGenetics LLC). The PCR
duplicate reads were removed by Sequence Operation Tool (default
settings), then .fastq files were converted by using Format
Conversion Tool to .fasta format. During conversion, reads with low
quality were removed (Settings: Median score threshold ≥25; Max #
of uncalled bases ≤2; Called base number of each read ≥40; Trim or
reject read when ≥3 base(s) with score ≤2). After format
conversion, reads were mapped on genome by Project Wizzard Tool
(Settings: Instrument type-Illumina; Application type-SNP/Indel
discovery; Steps-Sequence Alignment; Reference
file-Human_GRCh_v37p10_dbsnp135; Allowable mismatched bases-0;
Allowable ambiguous alignments-10; Seeds: 21 bases, move step-1
base; Allowable alignments-80; Overall matching base percentage
≥95%). Results of NextGENe software analysis (mutation report,
expression report, coverage report, CNV analysis report) were
filtered for the region of interest and mutation report
additionally for the frequency of mutation allele >5%.
A variant comparison tool (NextGENe software;
SoftGenetics, LLC) was used for the evaluation of shared/different
mutations between the tumors of respective patients. Nonsynonymous
variants in exons and adjacent intronic regions with frequency ≥10%
in at least one tumor were evaluated and manually controlled using
an IGV viewer (Broad Institute). Mutations detected with coverage
under 100×, which is caused by a limitation of the DNA quality from
FFPE tissue, were manually controlled using an IGV viewer in both
endometrial and ovarian tumors. These mutations are considered as
true variants (not artefacts) if: i) The mutation was detected in
both tumors of respective patient (in case of shared mutation); ii)
reads with mutation excluded duplicates; and iii) variant was
detected in both strands with different orientation of paired-end
reads.
False calls of detected mutation in PIK3CA
evaluated by NextGENe: p.(R524K), p.(Y644H), p.(E707K) were
filtered out. The presence of a PIK3CA pseudogene on
chromosome 22, with >95% sequence homology interferes with the
detection of these variants (10).
The single nucleotide polymorphisms (SNPs; mutation
allele frequency-MAF-above 0.01, i.e., above 1% in population) were
filtered out according to the data from the SNP databases (ExAC,
1,000 g, ESP6500) which are part of the mutation report generated
by the NextGENe® Software. In order to assess the impact
of the detected missense variants, several widely used in silico
prediction programs or databases imported in NexteGENe Software
were employed, comprising: ClinVar database, COSMIC database, and
dbNSFP database (MetaSVM, MetaLR, RS_DBSNP141, SIFT,
Polyphen2_HDIV, Polyphen2_HVAR, LRT, MutationTaster,
MutationAssessor, FATHMM, PROVEAN GERP++, phyloP46, SiPhy).
Clinical significance and ensemble prediction scores were included
in the NextGENe mutation reports.
Germline variants possibly associated with cancer
predisposing syndromes were selected according to the following
rules: i) VAF >40% in both tumors; ii) variants are not
described as SNPs; and iii) clinical significance of the variant
was assessed to be pathogenic and/or of uncertain significance
according to the ClinVar database. These variants were confirmed as
germline mutations by Sanger sequencing of DNA isolated from
non-tumor tissue.
Analysis of microsatellite
instability
Analysis of MSI was performed by fragmentation
analysis on ABI 3500 (Thermo Fisher Scientific, Inc.) with the set
of five quasimonomorphic mononucleotide microsatellite markers
BAT-26, BAT-25, NR-21, NR-22 and NR-24. The phenotype MSI-high
(MSI-H) was defined as the presence of at least two instable loci
and MSI-low as the presence of one instable locus, respectively.
Microsatellite stable (MSS) tumors showed no instability.
Results
Clinico-pathological findings
The characteristics of the patients and tumors are
summarized in Fig. 1. From 22 cases,
ten SEO cases were classified based on conventional morphological
criteria as independent tumors, and twelve cases were considered as
single primary tumors with metastasis to the other location (ovary
or endometrium). In three cases classified as metastasis from one
location to the other peritoneal involvement was also found, and in
one of these cases there was Fallopian tube involvement. A
lymphadenectomy procedure was performed in 11 patients, and only in
one patient there was a metastasis found in one iliac lymph
node.
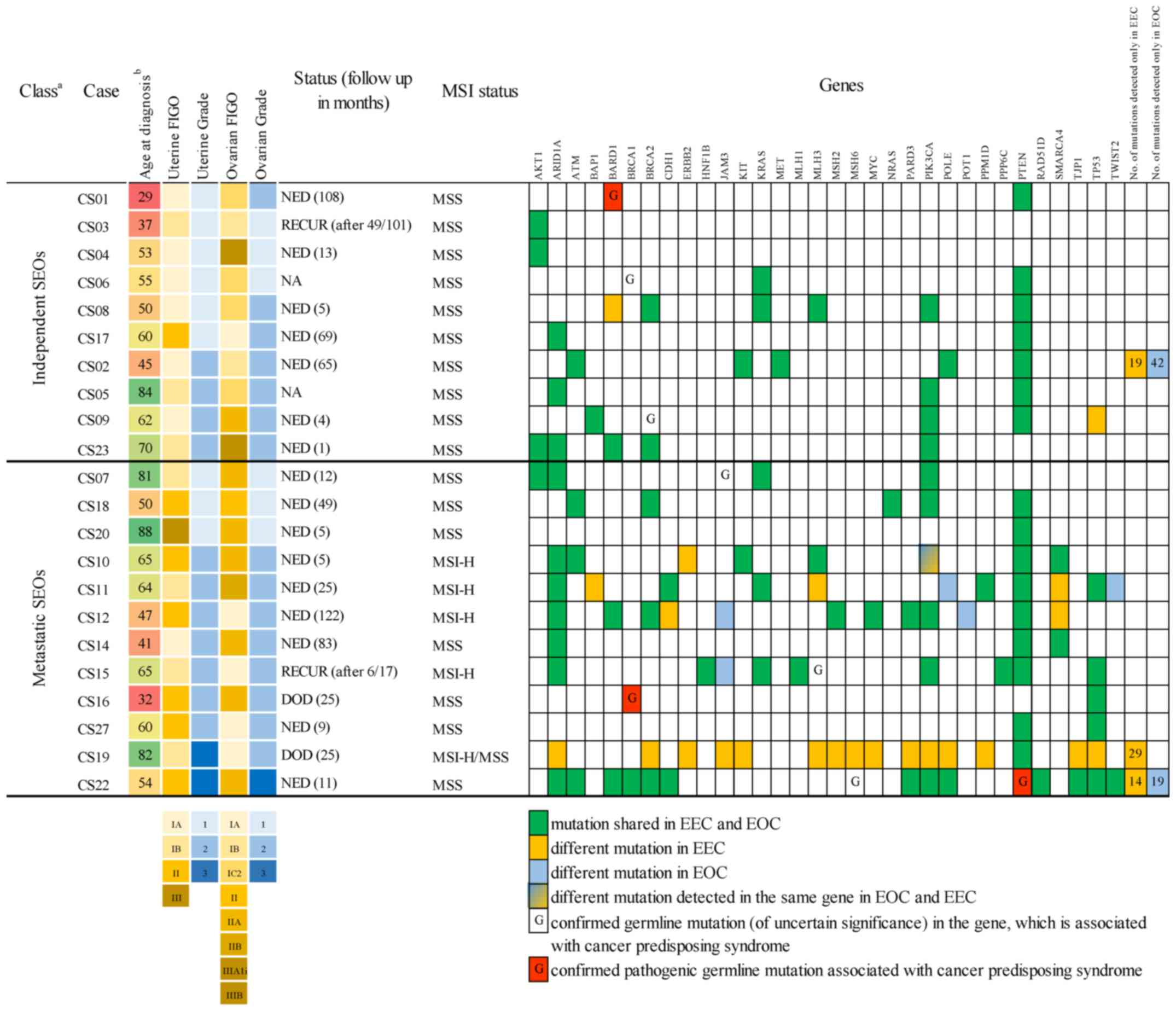 | Figure 1.Spectrum of detected mutations. Tumor
CS11 spreads to the peritoneum, CS18 spreads to the peritoneum,
cervix and fallopian tube and CS22 spreads to the peritoneum and
cervix. Patient CS12 was diagnosed with colorectal carcinoma 15
months after primary EEC/EOC diagnosis, and patient CS15 was
diagnosed with EEC/EOC 41 months after a previous follicular
lymphoma diagnosis. aClass according to the examination
by pathologist using current criteria. Independent SEOs are tumors
classified as independent synchronous endometrial and ovarian
carcinoma. Metastatic SEOs are tumors classified as metastasis from
one location to the other. bColored scale from red to
green indicates the age of patients at diagnosis. CS, case
specific; NED, no evidence of disease; RECUR, the recurrent disease
was found during follow-up; DOD, died of disease; NA, not
available; MSS, microsatellite stable in EEC/EOC; MSI-H,
microsatellite instable in EEC/EOC; MSI-H/MSS, case CS19 MSI-H in
EEC and MSS in EOC; EEC, endometrioid endometrial carcinoma; EOC,
endometrioid ovarian carcinoma; FIGO, Fédération Internationale de
Gynécologie et d'Obstétrique; SEO, synchronous endometrial and
ovarian carcinoma; MSI, microsatellite instability. |
The average age of diagnosis was 58 years (range,
29–88 years). The average follow-up for all patients with available
data (20 from 22 patients) was 37 months (range, 1–122 months).
Only 1 of the 10 (10%) patients (CS03) classified as independent
SEO developed recurrent disease. This patient had a
well-differentiated endometrioid carcinoma of the left ovary
treated by adnexectomy with subsequent pelvic and para-aortic
lymphadenectomy, omentectomy and appendectomy. The SEO was found
after 49 months in the form of a well-differentiated endometrioid
carcinoma in the contralateral (right) ovary and in the
endometrium, and it was treated by a combined radical hysterectomy
and right adnexectomy with subsequent chemotherapy [6 cycles of
paclitaxel (PTX) and carboplatin (CBDCA)]. The patient is without
any signs of disease 52 months after surgery. No patient from this
group died of the disease. On the contrary, 2/12 patients with SEO
classified as metastasis from one location to the other died of the
disease. Another patient from this group developed metastatic
disease 6 months after the diagnosis, the metastatic lesion was
located in the abdominal wall and treated by a surgical excision.
This patient was without any signs of the disease 11 months after
the excision and then was lost to follow-up.
Molecular findings
The detected mutations are summarized in Fig. 1. Comparison of molecular profiles of
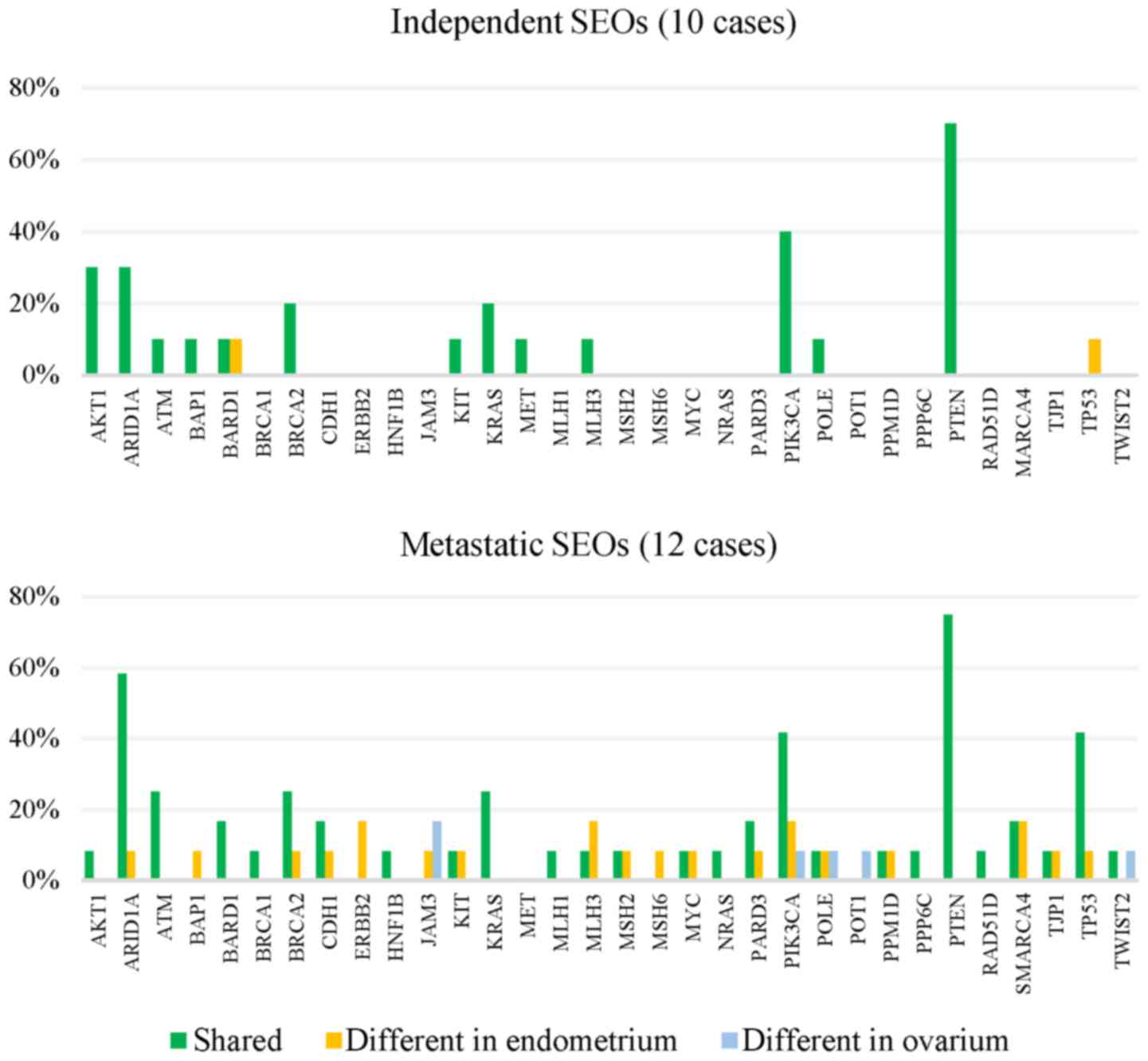
independent and metastatic SEOs are shown in Fig. 2.
All of the SEOs (100%, 22/22) showed to be clonally
related according to the presence of shared mutations (at least
one). In 77.3% cases (17/22) a shared mutation in PTEN was
detected and out of those, eight SEOs shared additional
ARID1A mutation. Two SEOs (9%, 2/22) carried mutations in
ARID1A, AKT1 and PIK3CA while PTEN was a
wild-type. Another two cases carried only AKT1 mutation,
which is a known pathogenic hot-spot p.(E17K) variant.
Interestingly, one microsatellite instable SEO (CS15, both tumors
of grade 2) shared pathogenic somatic mutation in HNF1B
NM_000458.2: c.1561_1562insC, p.(Q521Pfs*30), both tumors carried
mutations in MLH3, MLH1 and also further mutations in
PTEN NM_000314.4: c.955_958del, p.(T319X), ARID1A
NM_006015.4: c.5981delT, p.(V1994Gfs*21) and c.3667C>T,
p.(R1223C), KRAS NM_004985.3: c.35G>A, p.(G12D) and
TP53 NM_001126112.2: c.960_961del, p.(K321Tfs*15).
Two SEOs (CS02 and CS22) shared POLE mutation
causing hypermutator phenotype (24 in EEC and 42 in EOC, 27 in EEC
and 32 in EOC nonsynonymous mutations, respectively). As the
evidence suggests, POLE hypermutator phenotype defines a unique
subclass of endometrioid tumors. Moreover, in one patient
(54-year-old patient-CS22) a germline PTEN mutation
NM_000314.4: c.389G>A, rs121909229, p.(R130Q) was detected.
Germline pathogenic PTEN mutations are usually associated
with Cowden syndrome (PTEN hamartoma tumor syndrome). The
association of the detected germline PTEN mutation with
carcinogenesis in this particular patient is however questionable,
due to the relatively high patient's age at the time of diagnosis
and POLE hypermutator phenotype. In patient CS19,
POLE mutation (29 nonsynonymous mutations detected in
endometrial tumor), and microsatellite instability were detected in
the endometrial tumor but not in the paired ovarian tumor, while
both tumors shared the PTEN mutation.
Fragment analysis showed microsatellite instable
phenotype in 22.7% (5/22) of cases, including the above-mentioned
case which was microsatellite instable only in the endometrial
tumor.
Several germline mutations (germline status was
confirmed by Sanger sequencing of DNA isolated from non-tumor
tissue) in the genes which have been previously associated with
cancer predisposing syndromes in the literature were detected. Two
patients carried the pathogenic germline variant in BARD1 or
BRCA1. The tumors of a 29-year-old patient (CS01) shared
pathogenic nonsense germline mutation in BARD1 NM_000465.2:
c.1690C>T, rs587780021, p.(Q564X) together with shared somatic
PTEN mutation. Another (32-year old patient-CS16) shared
pathogenic frameshift germline mutation in BRCA1
NM_007294.3: c.5266_5267insC, rs80357906, p.(Q1756Pfs*73) together
with somatic TP53 mutation. Other germline mutations of
uncertain clinical significance were detected in the genes
BRCA1, BRCA2, MLH3 or MSH6.
In the case of CS06, a patient diagnosed with clear
cell carcinoma of the ovary (CCCO) together with SEO, a different
mutation profile was found in the respective tumors. The SEO shared
common mutation in PTEN and KRAS, which was absent in
CCCO, while the MSH6 mutation NM_000179.2: c.2873_2874del,
p.(Q958Pfs*7) was detected only in CCCO.
Discussion
Synchronous endometrioid endometrial and ovarian
carcinomas have been a matter of dispute especially because of the
difficulties in differential diagnostics between two independent
primary tumors and metastasis from one site to the other. The
morphological criteria involved in their diagnostics comprise
mainly the grade and stage of the diseases (3,4,8). In endometrial tumors the criteria
include: Size of the tumor and depth of invasion, direct extension
to the adnexa, lymphovascular space invasion, presence of atypical
hyperplasia in the surrounding endometrium, and grading. In ovarian
tumors the criteria include: The presence of endometriosis, size
and laterality of the tumor, surface implants, hilar location,
lymphovascular space invasion, and multinodularity. In general,
several studies have confirmed that low stage (organ confined) and
low-grade SEOs behave clinically as independent primary tumors.
Moreover, it has been shown that women with stage I endometrioid
endometrial cancer with synchronous stage I endometrioid ovarian
cancer have a survival outcome similar to those patients with stage
I endometrioid endometrial cancer without synchronous ovarian
cancer (1,11–21). This
is in concordance with the results of our study, as only 1/10
patients classified as independent SEO developed recurrent disease
and none of these patients died of the disease.
Few studies have focused on which ancillary methods
may be potentially helpful in the decision process, including
immunohistochemical and molecular studies. The use of
immunohistochemistry seems to be very limited and only few studies
focused on assessing the expression of selected markers. One such
study assessed the expression of cytokeratins, vimentin, CEA, Ca125
and Ca19.9 (22). The results between
endometrial and ovarian tumors showed overlapping features and the
authors concluded that in this setting, immunohistochemistry is not
beneficial. In another study, the authors used different antibodies
including ER, PR, HER2, p53, and Ki-67. They found out that some
antibodies (ER, PR, bcl2) showed different immunostaining patterns
between two primaries and can be used as a surrogate marker in the
distinction of these tumors (23).
Other study focused on vimentin expression in endometrial and
ovarian endometrioid carcinoma (24).
In this study the authors found that vimentin was negative in 97%
of primary ovarian carcinomas and positive in 82% of primary
endometrial carcinomas. In SEOs the expression of vimentin was
discordant in 53% of cases. They concluded that the expression of
vimentin between endometrial and ovarian endometrioid carcinoma is
different. However, the practical meaning of immunohistochemistry
in this setting is disputable in light of the current knowledge
that almost all EOC are clonal lesions and also, with respect to
immunophenotypic tumor heterogeneity, can also be influenced by
tumor microenvironment.
Molecular studies used variable approaches,
including the assessing of microsatellite instability (MSI), the
pattern of X chromosome inactivation, loss of heterozygosity (LOH),
and mutation of single or small group of genes, especially
PTEN and CTNNB1 (22,25–35). Most
of the studies failed to identify the clonal origin in most of the
cases analyzed. The results of these studies are disputable and
limited, particularly with regards to the used methodology.
Analysis of a limited number of markers did not allow a
comprehensive evaluation and comparison of possible clonal origin
of all tumors. In view of current knowledge, it is clear that the
intratumoral and intermetastatic heterogeneity is relatively common
and can be influenced by several factors such as tumor type,
presence of driver mutations, mutation load, and also by immune
host reaction with clonal selection. Only three recent studies
focused on the comparison between endometrial and ovarian tumors by
NGS approach (5–7). One of these studies analyzed 18 SEO
cases (all but two were endometrioid in type; the two exceptions
were ovarian clear cell carcinoma and endometrial endometrioid
carcinoma), 11 of them were classified based on conventional
criteria as independent primary tumors. The authors used targeted
sequencing of 35 genes commonly altered in endometrial and ovarian
tumors. Their results showed that 17/18 cases were of clonal
origin, including 10/11 cases based on conventional criteria
classified as independent tumors (6).
The second study analyzed 23 SEOs, with 15 of being classified as
independent primary tumors. Five of these cases were analyzed by
whole-exome sequencing, the remaining 18 cases were analyzed by NGS
targeting of 341 (n=4) or 410 (n=14) genes, respectively. Their
results showed that all sporadic SEOs were clonally related. The
only exception was a SEO in patient with Lynch syndrome with
germline MSH6 mutation in which the somatic mutations were
different. The last study analyzed 14 cases by NGS targeting of 409
genes and by genome-wide copy number analysis by MIP microarrays
(7). Thirteen of their cases showed
evidence of clonality. In our present study we found that all 22
synchronous EECs and EOCs shared nonsynonymous mutations in at
least one gene: PTEN, AKT1, PIK3CA, KRAS, TP53 and
ARID1A. These findings suggest that the relatively small
panel of genes included in the NGS analysis can be a very useful
approach to confirming clonal relation of SEOs.
The results of all these 3 molecular studies were
very similar and are in concordance with the results of our study,
the most important findings being: i) almost all SEOs are of clonal
origin and even low stage and low-grade tumors seem to represent
dissemination from one site to the other (however, without a
possibility to conclusively assess the directionality); ii) all
sporadic SEOs shared nonsynonymous mutations in at least one cancer
driver gene of EEC and/or EOC. The authors suggest that the low
grade and low stage SEOs, despite being clonal in origin, probably
represent phenomenon of restricted dissemination colonizing only
certain types of microenvironments and represent clinically
indolent spread and not a sign of a ‘fully’ metastatic disease. A
possible explanation is that SEOs classified as independent tumors,
using conventional criteria, represent mostly primary indolent
endometrial tumors with spread through the Fallopian tube and
seeding of the implant into the ovary. This explanation is
supported by the finding that SEO tumors showed a lower frequency
of ovarian endometriosis than sporadic ovarian endometrioid
carcinomas (36).
Based on the results of our study and other three
recent studies focusing on this topic the benefit of molecular
analysis used for differential diagnosis between independent
primary tumors and metastatic disease is disputable (5–7). The
finding of clonal origin of almost all SEOs is very important and
contrary to the belief that molecular testing could be used as a
tool for differential diagnosis. Still, this issue has not been
solved and in one recent study the authors suggest that molecular
profiling may be beneficial in this setting (8). However, this suggestion was based on
whole exome sequencing of only one case of SEO. In their case the
authors detected 253 shared mutations (including ARID1A, PIK3CA,
MSH6 and intronic mutation in PTEN), but more mutations
were found only in EEC or EOC. Despite the findings of shared
mutations, they proposed that these tumors were clonally unrelated
and classified them as synchronous independent tumors.
Regardless of the significance in assessing the
clonality of an SEO, our results showed some interesting findings
with respect to the spectrum of molecular changes occurring in the
analyzed tumors. Pathogenic germline mutations in BARD1 or
BRCA1, which are usually associated with cancer predisposing
syndromes, were detected in two patients with SEOs. Germline
BARD1 mutation p.(Q564X) has been previously described in
families with members affected by breast, colon and uterine cancer
(37). Germline BRCA1 mutation
p.(Q1756Pfs*73) is a known founder mutation in the Ashkenazi Jewish
population and in several European countries including the Czech
Republic (38,39). This finding is important for proper
genetic counseling besides Lynch syndrome, the common predisposing
syndrome associated with uterine tumors.
Pathogenic somatic HNF1B mutation
NM_000458.2: c.1561_1562insC, p.(Q521Pfs*30) shared in the SEO of
one patient (CS15) represents the insertion of one nucleotide
(cysteine affecting codon 521 located in exon 8 polyC (C)7
sequence) resulting in frameshift and protein truncation. This
mutation in the polyC segment might possibly be the result of the
microsatellite instability of these tumors (mutated MLH3 and
MLH1). The tumors carried further pathogenic or likely
pathogenic somatic mutations in PTEN, ARID1A, KRAS, and
TP53. Nevertheless, the HNF1B p.(Q521Pfs*30) mutation
has been previously described in two stomach adenocarcinomas
(40) and in four renal clear cell
carcinomas according to the TCGA atlas (study Kidney Renal Clear
Cell Carcinoma) (41). All those
tumors carried also at least one of the mutations in ARID1A,
TP53 (frameshift variant), PTEN and/or KRAS,
which suggests a similar mutation profile in different types of
tumors. The incidence and significance of HNF1B mutations in
EEC is unknown. However, in our previous study we detected another
pathogenic truncation mutation of this gene c.454C>T, p.(Q152X)
in 1/30 EEC (42).
In conclusion, based on our results and the results
of three previously published comprehensive molecular studies of
SEOs, these tumors are clonally related in almost all cases
irrespectively of their clinico-pathological features. Even low
grade and low stage tumors classified as independent primaries,
according to the conventional morphological criteria, have a clonal
origin. From the practical point of view, only the conventional
morphological criteria should be used for the classification of
these tumors, while molecular profiling does not seem, in this
context, helpful. However, analysis of more cases is needed to draw
a definite conclusion. Despite this fact, the molecular studies of
SEOs can help us to better understand the pathogenesis of SEOs and
can be beneficial in clinical practice for example in cases of
metachronous tumors affecting female genital organs and/or
peritoneum, or in cases of metastatic tumors (43,44).
Moreover, molecular profiling of SEOs can be also significant with
respect to prognostic and also predictive meaning.
Acknowledgements
The authors would like to thank Mr. Zachary H.K.
Kendall (Institute for History of Medicine and Foreign Langauges,
First Faculty of Medicine, Charles University in Prague, Prague,
Czech Republic) for English language editing.
Funding
The present study was supported by Ministry of
Health, Czech Republic (Conceptual Development of Research
Organization 64165, General University Hospital in Prague, and by
project AZV 17-28404A), by Charles University (Project Progress
Q28/LF1 and Q40/11, UNCE 204065 and SVV 260367), by European
Regional Development Fund (Project BBMRI-CZ No. EF16_013/0,001674)
and by OPPK (Research Laboratory of Tumor Diseases,
CZ.2.16/3.1.00/24509).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PD, NH and IT contributed to the conception and
design of the study. DC, JL, TG, GM, KN, PD, MB and MZ acquired the
data. PD, NH, KN, JH, IT and MB performed the experiments, and
acquired and interpreted the data. NH, JH, RM and IT processed the
bioinformatics data. PD, NH and IT drafted the manuscript. PD and
IT proofread the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
In compliance with the Helsinki Declaration, the
study has been approved by The Ethics Committee of General
University Hospital in Prague (Czech Republic).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have co competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CBDCA
|
carboplatin
|
|
CCCO
|
clear cell carcinoma of ovary
|
|
CNV
|
copy number variation
|
|
CS
|
case specification
|
|
EEC
|
endometrioid endometrial carcinoma
|
|
EOC
|
endometrioid ovarian carcinoma
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
LOH
|
loss of heterozygosity
|
|
MSI
|
microsatellite instability
|
|
MSS
|
microsatellite stable
|
|
MSI-H
|
microsatellite instability-high
|
|
NGS
|
next generation sequencing
|
|
PCR
|
polymerase chain reaction
|
|
PTX
|
paclitaxel
|
|
SEO
|
synchronous endometrial and ovarian
carcinomas
|
|
SNP
|
single nucleotide polymorphisms
|
|
VAF
|
variant allele fraction
|
References
|
1
|
Zaino R, Whitney C, Brady MF, DeGeest K,
Burger RA and Buller RE: Simultaneously detected endometrial and
ovarian carcinomas-a prospective clinicopathologic study of 74
cases: A gynecologic oncology group study. Gynecol Oncol.
83:355–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matias-Guiu X and Stewart CJR:
Endometriosis-associated ovarian neoplasia. Pathology. 50:190–204.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ulbright TM and Roth LM: Metastatic and
independent cancers of the endometrium and ovary: A
clinicopathologic study of 34 cases. Hum Pathol. 16:28–34. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scully RE, Young RH and Philip B: Clement
MD: Tumors of the ovary, maldeveloped gonads, Fallopian tube and
broad ligament: Atlas of tumor pahology (AFIP, Atlas of tumor
pathology, No. 23). American registry of pathology. (Washington,
DC). 1999.
|
|
5
|
Schultheis AM, Ng CK, De Filippo MR,
Piscuoglio S, Macedo GS, Gatius S, Perez Mies B, Soslow RA, Lim RS,
Viale A, et al: Massively parallel sequencing-based clonality
analysis of synchronous endometrioid endometrial and ovarian
carcinomas. J Natl Cancer Inst. 108:djv4272016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anglesio MS, Wang YK, Maassen M, Horlings
HM, Bashashati A, Senz J, Mackenzie R, Grewal DS, Li-Chang H,
Karnezis AN, et al: Synchronous endometrial and ovarian carcinomas:
Evidence of clonality. J Natl Cancer Inst. 108:djv4282016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao A, Wu RC, Jung SM, Lee YS, Chen SJ,
Lu YL, Tsai CL, Lin CY, Tang YH, Chen MY, et al: Implication of
genomic characterization in synchronous endometrial and ovarian
cancers of endometrioid histology. Gynecol Oncol. 143:60–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Zhang L, Huang Q, Liu C, Qi L, Li
L, Qu T, Wang Y, Liu S, Meng B, et al: Combination of scoring
criteria and whole exome sequencing analysis of synchronous
endometrial and ovarian carcinomas. Int J Gynecol Cancer.
28:704–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurman RJ, Carcangiu LM, Herrington SC and
Young HR: International Agency for Research on Cancer and World
Health Organization: WHO classification of tumours of female
reproductive organs. International Agency for Research on Cancer
(Lyon). 2014.
|
|
10
|
Baker CL, Vaughn CP and Samowitz WS: A
PIK3CA pyrosequencing-based assay that excludes pseudogene
interference. J Mol Diagn. 14:56–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuo K, Machida H, Frimer M, Marcus JZ,
Pejovic T, Roman LD and Wright JD: Prognosis of women with stage I
endometrioid endometrial cancer and synchronous stage I
endometrioid ovarian cancer. Gynecol Oncol. 147:558–564. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Natee J, Kietpeerakool C, Srisomboon J,
Khunamornpong S, Suprasert P, Phongnarisorn C, Cheewakriangkrai C,
Charoenkwan K, Siriaree S and Pantusart A: Clinicopathologic
analysis of women with synchronous primary carcinomas of the
endometrium and ovary: 10- year experience from Chiang Mai
University Hospital. Asian Pac J Cancer Prev. 7:234–238.
2006.PubMed/NCBI
|
|
13
|
Narin MA, Karalok A, Basaran D, Ureyen I,
Turkmen O, Turan T and Tulunay G: Does synchronous endometrioid
endometrial cancer have any prognostic effect on Stage I
endometrioid ovarian cancer? Eur J Obstet Gynecol Reprod Biol.
200:113–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim YK, Padma R, Foo L, Chia YN, Yam P,
Chia J, Khoo-Tan H, Yap SP and Yeo R: Survival outcome of women
with synchronous cancers of endometrium and ovary: A 10 year
retrospective cohort study. J Gynecol Oncol. 22:239–243. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dębska-Szmich S, Czernek U, Frąckowiak M,
Frackowiak M, Zięba A, Czyzykowski R, Kulejewska D and Potemski P:
Synchronous primary ovarian and endometrial cancers: A series of
cases and a review of literature. Prz Menopauzalny. 13:64–69.
2014.PubMed/NCBI
|
|
16
|
Eifel P, Hendrickson M, Ross J, Ballon S,
Martinez A and Kempson R: Simultaneous presentation of carcinoma
involving the ovary and the uterine corpus. Cancer. 50:163–170.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karki SC and Chapagain U: Synchronous
primary tumors of the endometrium and ovary. J Pathol Nepal.
2:189–192. 2012. View Article : Google Scholar
|
|
18
|
Liu Y, Li J, Jin H, Lu Y and Lu X:
Clinicopathological characteristics of patients with synchronous
primary endometrial and ovarian cancers: A review of 43 cases.
Oncol Lett. 5:267–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soliman PT, Slomovitz BM, Broaddus RR, Sun
CC, Oh JC, Eifel PJ, Gershenson DM and Lu KH: Synchronous primary
cancers of the endometrium and ovary: A single institution review
of 84 cases. Gynecol Oncol. 94:456–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song T, Seong SJ, Bae DS, Suh DH, Kim DY,
Lee KH, Lim MC and Lee TS: Synchronous primary cancers of the
endometrium and ovary in young women: A Korean Gynecologic Oncology
Group Study. Gynecol Oncol. 131:624–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sozen H, Vatansever D, Iyibozkurt AC,
Topuz S, Ozsurmeli M, Salihoglu Y, Guzelbey B and Berkman S:
Clinicopathologic and survival analyses of synchronous primary
endometrial and epithelial ovarian cancers. J Obstet Gynaecol Res.
41:1813–1819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prat J, Matias-Guiu X and Barreto J:
Simultaneous carcinoma involving the endometrium and the ovary. A
clinicopathologic, immunohistochemical, and DNA flow cytometric
study of 18 cases. Cancer. 68:2455–2459. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halperin R, Zehavi S, Hadas E, Habler L,
Bukovsky I and Schneider D: Simultaneous carcinoma of the
endometrium and ovary vs endometrial carcinoma with ovarian
metastases: A clinical and immunohistochemical determination. Int J
Gynecol Cancer. 13:32–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desouki MM, Kallas SJ, Khabele D, Crispens
MA, Hameed O and Fadare O: Differential vimentin expression in
ovarian and uterine corpus endometrioid adenocarcinomas: Diagnostic
utility in distinguishing double primaries from metastatic tumors.
Int J Gynecol Pathol. 33:274–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii H, Matsumoto T, Yoshida M, Furugen
Y, Takagaki T, Iwabuchi K, Nakata Y, Takagi Y, Moriya T, Ohtsuji N,
et al: Genetics of synchronous uterine and ovarian endometrioid
carcinoma: Combined analyses of loss of heterozygosity, PTEN
mutation and microsatellite instability. Hum Pathol. 33:421–428.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreno-Bueno G, Gamallo C, Pérez-Gallego
L, de Mora JC, Suárez A and Palacios J: Beta-catenin expression
pattern, beta-catenin gene mutations, and microsatellite
instability in endometrioid ovarian carcinomas and synchronous
endometrial carcinomas. Diagn Mol Pathol. 10:116–122. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi Y, Nakamura K, Nomura H, Banno
K, Irie H, Adachi M, Iida M, Umene K, Nogami Y, Masuda K, et al:
Clinicopathologic analysis with immunohistochemistry for DNA
mismatch repair protein expression in synchronous primary
endometrial and ovarian cancers. Int J Gynecol Cancer. 25:440–446.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emmert-Buck MR, Chuaqui R, Zhuang Z,
Nogales F, Liotta LA and Merino MJ: Molecular analysis of
synchronous uterine and ovarian endometrioid tumors. Int J Gynecol
Pathol. 16:143–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujita M, Enomoto T, Wada H, Inoue M,
Okudaira Y and Shroyer KR: Application of clonal analysis.
Differential diagnosis for synchronous primary ovarian and
endometrial cancers and metastatic cancer. Am J Clin Pathol.
105:350–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Irving JA, Catasús L, Gallardo A,
Bussaglia E, Romero M, Matias-Guiu X and Prat J: Synchronous
endometrioid carcinomas of the uterine corpus and ovary:
Alterations in the beta-catenin (CTNNB1) pathway are associated
with independent primary tumors and favorable prognosis. Hum
Pathol. 36:605–619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin WM, Forgacs E, Warshal DP, Yeh IT,
Martin JS, Ashfaq R and Muller CY: Loss of heterozygosity and
mutational analysis of the PTEN/MMAC1 gene in synchronous
endometrial and ovarian carcinomas. Clin Cancer Res. 4:2577–2583.
1998.PubMed/NCBI
|
|
32
|
Matias-Guiu X, Bussaglia E, Catasus L,
Lagarda H, Gras E, Machin P, Prat J; Correspondence re: W.M. Lin, ;
et al: loss of heterozygosity and mutational analysis of the
PTEN/MMAC1 gene in synchronous endometrial and ovarian carcinomas.
Clin. Cancer Res. 4:2577–2583. 1998.Clin Cancer Res 6: 1598-1600,
2000. PubMed/NCBI
|
|
33
|
Nishimura N, Hachisuga T, Nabeshima K and
Kawarabayashi T: Synchronous endometrial and ovarian carcinomas:
Analysis of genetic relationship of the tumors. Int J Oncol.
27:1519–1526. 2005.PubMed/NCBI
|
|
34
|
Ricci R, Komminoth P, Bannwart F, Torhorst
J, Wight E, Heitz PU and Caduff RF: PTEN as a molecular marker to
distinguish metastatic from primary synchronous endometrioid
carcinomas of the ovary and uterus. Diagn Mol Pathol. 12:71–78.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shenson DL, Gallion HH, Powell DE and
Pieretti M: Loss of heterozygosity and genomic instability in
synchronous endometrioid tumors of the ovary and endometrium.
Cancer. 76:650–657. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kelemen LE, Rambau PF, Koziak JM, Steed H
and Köbel M: Synchronous endometrial and ovarian carcinomas:
Predictors of risk and associations with survival and tumor
expression profiles. Cancer Causes Control. 28:447–457. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ratajska M, Antoszewska E, Piskorz A,
Brozek I, Borg Å, Kusmierek H, Biernat W and Limon J: Cancer
predisposing BARD1 mutations in breast-ovarian cancer families.
Breast Cancer Res Treat. 131:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamel N, Feng BJ, Foretova L,
Stoppa-Lyonnet D, Narod SA, Imyanitov E, Sinilnikova O, Tihomirova
L, Lubinski J, Gronwald J, et al: On the origin and diffusion of
BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum
Genet. 19:300–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pohlreich P, Zikan M, Stribrna J, Kleibl
Z, Janatova M, Kotlas J, Zidovska J, Novotny J, Petruzelka L, Szabo
C and Matous B: High proportion of recurrent germline mutations in
the BRCA1 gene in breast and ovarian cancer patients from the
Prague area. Breast Cancer Res. 7:R728–R736. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cerami E, Gao JJ, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Němejcová K, Tichá I, Kleiblová P, Bártů
M, Cibula D, Jirsová K and Dundr P: Expression, epigenetic and
genetic changes of HNF1B in endometrial lesions. Pathol Oncol Res.
22:523–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu RC, Veras E, Lin J, Gerry E,
Bahadirli-Talbott A, Baras A, Ayhan A, Shih IM and Wang TL:
Elucidating the pathogenesis of synchronous and metachronous tumors
in a woman with endometrioid carcinomas using a whole-exome
sequencing approach. Cold Spring Harb Mol Case Stud. 3(pii):
a0016932017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valtcheva N, Lang FM, Noske A, Samartzis
EP, Schmidt AM, Bellini E, Fink D, Moch H, Rechsteiner M, Dedes KJ
and Wild PJ: Tracking the origin of simultaneous endometrial and
ovarian cancer by next-generation sequencing-a case report. BMC
Cancer. 17:662017. View Article : Google Scholar : PubMed/NCBI
|