Introduction
Glioma is the most common primary intracranial
tumors, representing 81% of malignant brain tumors (1). Despite certain conclusive prognostic
factors (e.g. extent of tumor resection, age at diagnosis and
Karnofsky performance status) for glioma having been identified
(2,3),
prognosis remains unsatisfactory, which indicates the involvement
of other factors. Therefore, a more accurate prognostic indicator
for patients with glioma is required.
Of note, emerging evidence has indicated that the
systemic inflammation response is a key determinant of progression
in cancer (4), which presents with
alterations in circulating blood indicators (5). To date, several peripheral blood
inflammation-based scores such as the neutrophil/lymphocyte ratio
(NLR), platelet/lymphocyte ratio (PLR) have been validated as
prognostic markers in various types of tumor (6–10).
Furthermore, as a corrected parameter that reflects the host
nutritional and immune condition, the prognostic nutritional index
(PNI) has been identified to be associated with the progression of
malignant tumors (11,12). Generally, these combined scores are
derived from established measures of full blood count, namely
albumin, neutrophil, platelet and lymphocyte counts, reflecting a
comprehensive individual state in aspects of systemic inflammation,
nutritional and immune status.
However, the function of these blood-derived
biomarkers in the prognosis of glioma is less well-investigated. As
these parameters can be determined routinely in day-to-day clinical
practice, it indicates promise for providing simpler and cheaper
objective biomarkers for glioma prognosis. The aim of the present
study was to identify the predictive value of these prognostic
factors (i.e. NLR, PLR and PNI) in patients with glioma. To the
best of our knowledge, for the first time, a predictive nomogram
for prognosis in patients with glioma based on these biomarkers and
the clinicopathological characteristics has been established.
Materials and methods
Ethics
The present study is a sequential study, with the
patient information used acquired from a study previously published
(13), which was approved by the
Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China; no. 2016-18). Written informed consent
was obtained from all individual participants.
Study population
The medical records of patients with newly diagnosed
glioma who underwent brain tumor resection at The First Affiliated
Hospital of Xi'an Jiaotong University between January 2008 and to
December 2012 were retrospectively reviewed. Patients were included
on the basis of eligibility criteria, which were: i) Diagnosis was
confirmed by post-operative pathological examination; ii) full
pre-operative laboratory data were available (i.e. serum albumin
level, neutrophil, platelet and lymphocyte counts); and iii) no
hematological system disorders, impaired liver function or primary
tumor at other sites.
Data collection
Demographic information of patients, such as age at
diagnosis, sex and clinical characteristics, including
histopathological diagnosis of tumor [based on 2007 World Health
Organization (WHO) brain tumor classification] (14), tumor location, extent of surgical
resection (total gross resection or incomplete resection) were
collected from the medical records. Tumor characteristics was
assessed using pre-operative magnetic resonance imaging images. The
extent of surgical resection was determined by the neurosurgeon
during surgery.
Overall survival (OS) time was defined as the
interval between the time of surgery and mortality. Patients were
censored at the end of follow-up, if mortality had not occurred.
The last follow-up was performed in October 2016.
NLR was calculated as neutrophil count
(109 cells/l) divided by lymphocyte count
(109 cells/l); PLR was calculated as platelet count
(109 cells/l) divided by lymphocyte count
(109 cells/l); and PNI was calculated as albumin level
(g/l) + 5× total lymphocyte count (109 cells/l)
(15).
Statistical analyses
The optimal cut-off values for NLR and PNI were
determined by plotting receiver operating characteristic (ROC)
curves for OS prediction. Continuous variables are presented as the
mean ± standard deviation or median (range) on the basis of whether
they fitted normal distribution or not (determined using a
Kolmogorov-Smirnov test; P<0.05). Categorical data are presented
as percentages. Survival analyses on categorical variables were
performed using the Kaplan-Meier method and significant differences
between groups were identified using the log-rank test. The
associations of prognostic indicators with other categorized
clinicopathological parameters were determined using the
χ2 and unpaired t-test according to the type of
variable. The Cox proportional hazards regression model was applied
to perform univariate and multivariate analyses, and those
variables that were identified to be statistically significant in
the univariate analysis were analyzed further by multivariate
analysis.
The rms package in R software was used to develop
nomograms that predicted the probability of survival at 1, 3 and 5
years, taking into account all the possible prognostic factors.
Harrell's concordance index (C-index) was used to evaluate the
accuracy of predictions. The value ranged from 0.5 (agreement by
chance) to 1 (perfect prediction) (16).
All statistical analyses were performed using SPSS
(version 20.0; IBM Corp., Armonk, NY, USA) and R software (version
3.3.1; Institute for Statistics and Mathematics, Vienna, Austria).
All statistical tests used in the present study were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline demographic and clinical
measures
Following a thorough review of the medical records,
a total of 128 patients (71 men and 57 women) were included in the
present study. The median OS time was 19 months (range, 1–81
months). At the last follow-up, 79/128 patients had succumbed to
various causes. The baseline demographic and clinical
characteristics are presented in Table
I.
 | Table I.Baseline characteristics of 128
patients with glioma and their associations with NLR and PNI. |
Table I.
Baseline characteristics of 128
patients with glioma and their associations with NLR and PNI.
| Variable | All patients
(n=128) | NLR <2.8
(n=72) | NLR ≥2.8 (n=56) | P-value | PNI <45
(n=24) | PNI ≥45 (n=104) | P-value |
|---|
| Sex, n (%) |
|
|
| 0.858 |
|
| 0.260 |
|
Female | 57 (44.5) | 33 (45.8) | 24 (42.9) |
| 8 (33.3) | 49 (47.1) |
|
| Male | 71 (55.5) | 39 (54.2) | 32 (57.1) |
| 16 (66.7) | 55 (52.9%) |
|
| Histology, n (%) |
|
|
| <0.001 |
|
| 0.266 |
| LGG | 67 (52.3) | 48 (66.7) | 19 (33.9) |
| 10 (41.7) | 57 (54.8) |
|
| HGG | 61 (47.7) | 24 (33.3) | 37 (66.1) |
| 14 (58.3) | 47 (77.0) |
|
| Lateral, n (%) |
|
|
| 0.759 |
|
| 0.587 |
|
Right | 60 (46.9) | 32 (44.4) | 28 (50.0) |
| 9 (37.5) | 51 (49.0) |
|
| Left | 55 (43.0) | 33 (45.8) | 22 (39.3) |
| 12 (50.0) | 43 (41.3) |
|
|
Bilateral | 13 (10.2) | 7 (9.7) | 6 (10.7) |
| 3 (12.5) | 10 (9.6) |
|
| Tumor location, n
(%) |
|
|
| 0.773 |
|
| 0.268 |
| Frontal
lobe | 63 (49.2) | 38 (52.8) | 25 (44.6) |
| 10 (41.7) | 53 (51.0) |
|
| Temporal
lobe | 34 (26.6) | 18 (25.0) | 16 (28.6) |
| 10 (41.7) | 24 (23.1) |
|
|
Occipital/parietal lobe | 13 (10.2) | 6 (8.3) | 7 (12.5) |
| 1 (4.2) | 12 (11.5) |
|
|
Other | 18 (14.1) | 10 (13.9) | 8 (14.3) |
| 3 (12.5) | 15 (14.4) |
|
| Extent of surgery,
n (%) |
|
|
| 0.857 |
|
| 0.005 |
| Gross
total | 77 (60.2) | 44 (61.1) | 33 (58.9) |
| 8 (33.3) | 69 (66.3) |
|
|
Subtotal | 51 (39.8) | 28 (38.9) | 23 (41.1) |
| 16 (66.7) | 35 (33.7) |
|
| Age, years | 47.84±13.958 | 46.22±13.67 | 49.93±14.42 | 0.137 | 56.79±10.43 | 45.78±13.89 |
<0.001 |
| Albumin (g/l) | 41.1±3.7 | 41.13±3.53 | 41.1±3.9 | 0.966 | 37.3±3.2 | 41.99±3.2 |
<0.001 |
| Platelets
(×109/l) | 176.8±61.9 | 175.29±51.4 | 178.82±73.76 | 0.750 | 148.13±47.97 | 183.46±63.07 | 0.011 |
Among all the patients, 67 cases were diagnosed as
low-grade glioma (WHO G1 and G2) and 61 cases were diagnosed as
high-grade glioma (WHO G3 and G4). The majority of the lesions were
at one side of the cerebral hemisphere, with 55 (43.0%) on the left
and 60 (46.9%) on the right. In addition, 63 (49.2%) lesions were
located in the frontal lobe, 34 (26.6%) were in the temporal lobe,
13 (10.2%) were in the occipital or parietal lobes, and the
remainder of the lesions (18, 14.1%) were at the lateral ventricle
or callosum.
Regarding treatment details, 77 (60.2%) underwent a
microsurgical total gross resection of the tumor, whereas 51
(39.8%) had subtotal resection due to the inaccessible areas or
eloquent brain regions in which the tumor was located. No patient
in our cohort had palliative decompression surgery or tumor
biopsy.
Associations of NLR and PNI with
clinicopathological variables
As presented in Table
I, the associations of NLR and PNI with clinicopathological
variables were evaluated. In this cohort, it was identified that
high NLR was significantly associated with advanced tumor grade
(P<0.001). With regard to PNI, significant associations were
identified with the extent of resection, age, the level of albumin
and the level of platelet. Furthermore, it was identified that the
level of PNI was negatively associated with NLR (r=−0.203;
P<0.05).
Effect of NLR and PNI on OS time
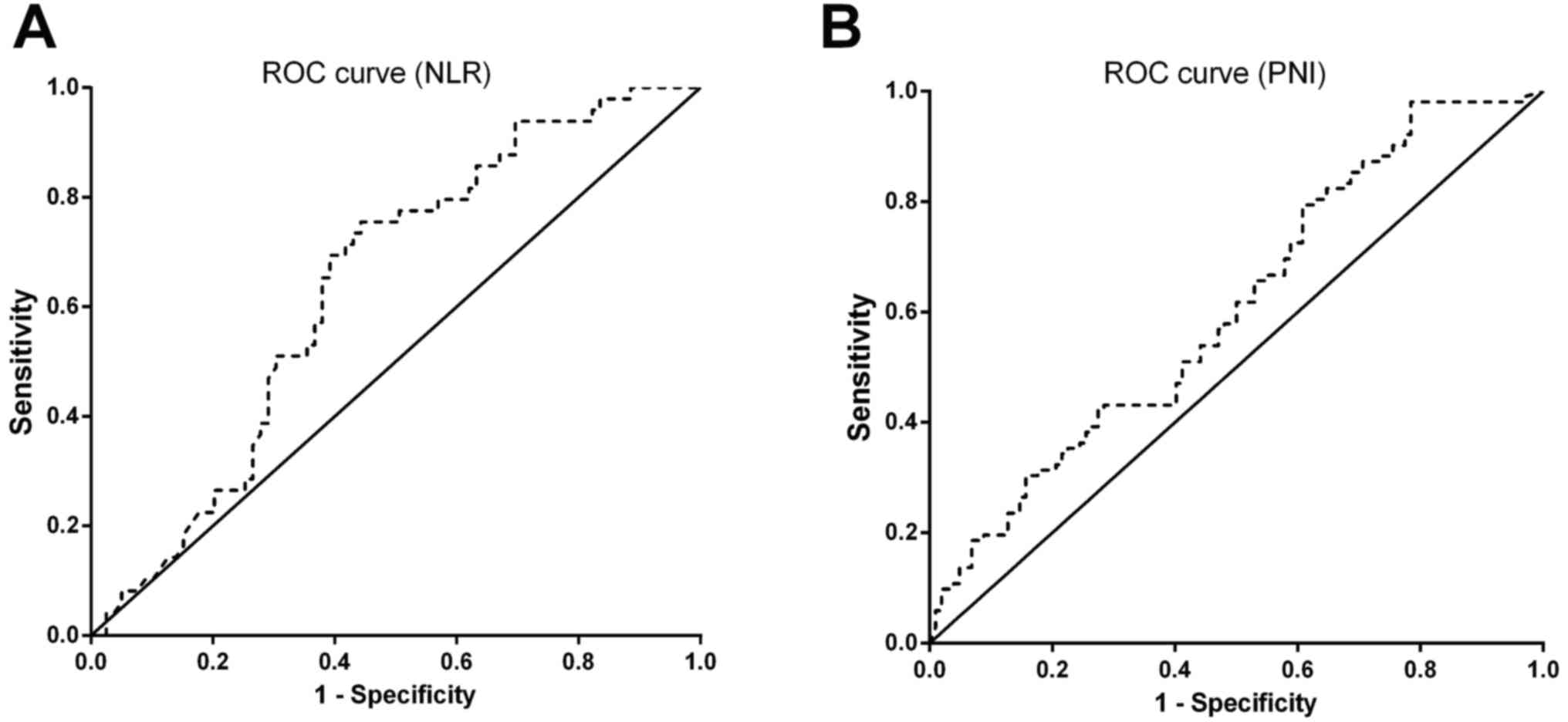
Using the OS rate as the endpoint, ROC curves for
NLR and PNI were plotted (Fig. 1A and
B).
For NLR [area under the curve (AUC), 0.639; 95%
confidence interval (CI), 0.542–0.733; P=0.009], a cut-off of 2.8
led to the maximal Youden value in ROC curve analysis. Patients
were subsequently categorized into two groups according to this
cut-off value, with the high NLR group at ≥2.8 and the low NLR
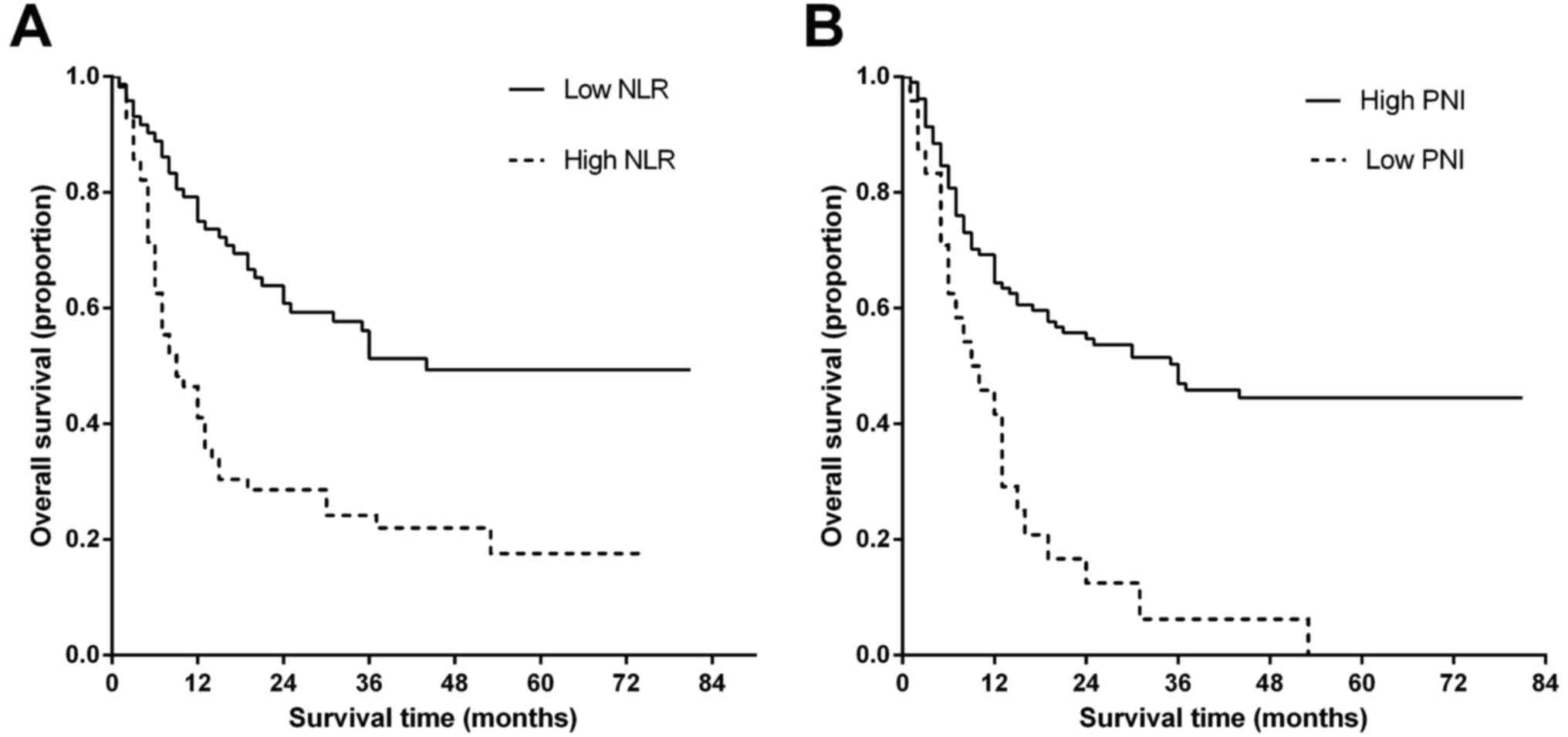
group at <2.8. Kaplan-Meier survival analysis and log-rank tests
indicated a significant difference in the median OS time between
the high NLR group and low NLR group (22.78±3.61 vs. 48.31±4.01
months; P<0.001), which indicated a marked association between
high NLR and decreased OS time (Fig.
2A).
Accordingly, the prognostic effect of PNI was
evaluated. Using ROC curves analysis, the AUC for PNI was 0.618
(95% CI, 0.520–0.716; P=0.025). The optimal cut-off value was
further determined as 45.0. The median OS time was 43.55±3.44
months in the high PNI group and 13.33±2.73 months in the low PNI
group (P<0.001), which indicated that low PNI was significantly
associated with decreased OS time (Fig.
2B).
With regard to the remaining parameters, the cut-off
values were determined by respective median or relative diagnosis
criteria, as presented in Table
II.
 | Table II.Univariate and multivariate analyses
for overall survival time of patients with glioma. |
Table II.
Univariate and multivariate analyses
for overall survival time of patients with glioma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 1.000 |
|
|
|
|
Male | 0.910
(0.583–1.419) | 0.676 |
|
|
| Age, years |
|
|
|
|
|
<50 | 1.000 |
|
|
|
|
≥50 | 3.166
(1.973–5.081) |
<0.001 | 2.328
(1.386–3.908) | 0.001 |
| Tumor location |
|
|
|
|
| Frontal
lobe | 1.303
(0.629–2.698) | 0.476 |
|
|
|
Temporal lobe | 1.942
(0.904–4.171) | 0.089 |
|
|
|
Occipital/parietal lobe | 1.245
(0.463–3.347) | 0.664 |
|
|
|
Other | 1.000 |
|
|
|
| Lateral |
|
|
|
|
|
Right | 1.109
(0.519–2.372) | 0.790 |
|
|
|
Left | 0.853
(0.392–1.858) | 0.689 |
|
|
|
Bilateral | 1.000 |
|
|
|
| Histology |
|
|
|
|
|
LGG | 1.000 |
|
|
|
|
HGG | 3.675
(2.284–5.912) |
<0.001 | 3.088
(1.893–5.037) |
<0.001 |
| Extent of
resection |
|
|
|
|
| Gross
total | 0.557
(0.357–0.869) | 0.010 | 0.606
(0.380–0.965) | 0.035 |
|
Subtotal | 1.000 |
|
|
|
| Albumin, g/l |
|
|
|
|
|
≥35 | 1.000 |
|
|
|
|
<35 | 0.313
(0.124–0.787) | 0.014 | 0.675
(0.243–1.880) | 0.452 |
| Platelets,
×109/l |
|
|
|
|
|
<350 | 1.000 |
|
|
|
|
≥350 | 3.279
(0.449–23.956) | 0.242 |
|
|
| NLR |
|
|
|
|
|
<2.8 | 1.000 |
|
|
|
|
≥2.8 | 2.525
(1.611–3.957) |
<0.001 | 2.037
(1.264–3.281) | 0.003 |
| PLR |
|
|
|
|
|
≥100 | 1.000 |
|
|
|
|
<100 | 1.348
(0.850–2.138) | 0.205 |
|
|
| PNI |
|
|
|
|
|
<45 | 1.000 |
|
|
|
|
≥45 | 0.346
(0.210–0.569) |
<0.001 | 0.716
(0.400–1.280) | 0.260 |
Additionally, Cox univariate analysis was used to
further investigate the predictive value of NLR, PLR, PNI and other
clinicopathological parameters. The results indicated that age,
histology, extent of resection, level of albumin, NLR and PNI were
significantly associated with OS. Furthermore, multivariate
analysis indicated that age ≥50 years [hazard ratio (HR), 2.328;
95% CI, 1.386–3.908; P<0.001], high-grade glioma (HR, 3.088; 95%
CI, 1.893–5.037; P<0.001), gross total resection (HR, 0.606; 95%
CI, 0.380–0.965; P=0.035) and NLR ≥2.8 (HR, 2.037; 95% CI,
1.264–3.281; P=0.003) were independent prognostic factors for poor
prognosis (Table II).
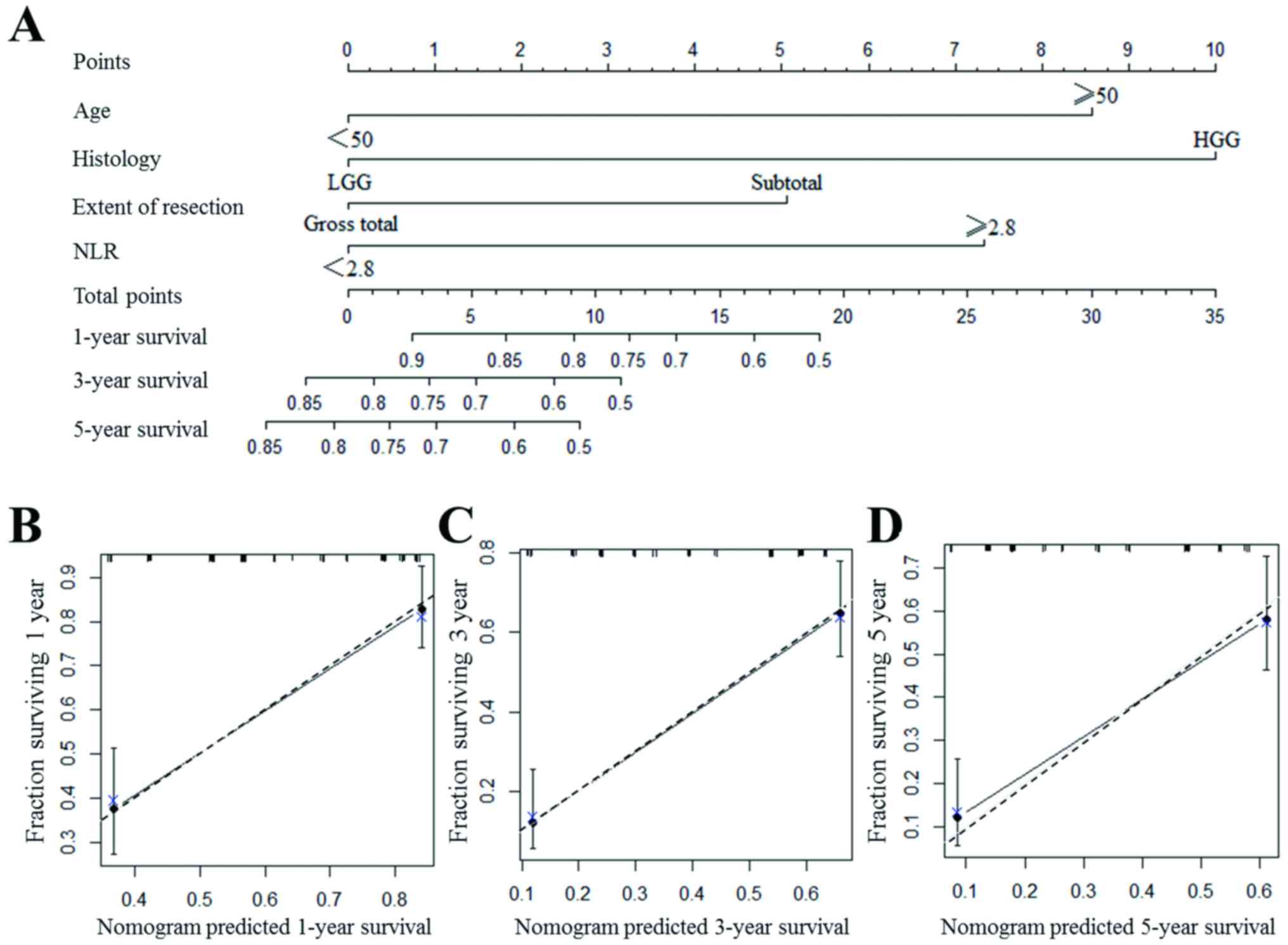
Predictive nomogram for prognosis in
patients with glioma
A nomogram to predict the survival of patients with
glioma following surgical resection is presented in Fig. 3A. With all the independent prognostic
indicators included, the nomogram is able to provide an approximate
prediction of the survival rate at 1, 3 and 5 years for patients
with glioma. In the nomogram, all the indicators were scored as a
number. For instance, if the patient had a high-grade glioma, the
patient was attributed with 10 points. The total points of the
patient were corresponding to a certain survival rate at 1, 3 and 5
years, and a greater number of points indicates a worse
prognosis.
The nomogram exhibited a good accuracy for
predicting the survival rate of patients with glioma, with a
c-index of 0.750. The value became 0.727 if NLR was not included in
the nomogram. The internal calibration plots of the nomogram
predicting 1-, 3- and 5-year survival revealed good agreement with
the ideal model (Fig. 3B-D).
Discussion
Currently, maximal safe resection and adjunctive
chemo/radiotherapy are considered to be the most effective
treatment modalities for glioma. Since patients who have undergone
the same treatment may have significant variation in their
prognosis, a more accurate prognostic model is required to guide
post-operative counseling and post-operative adjuvant
treatment.
Notably, evidence has indicated the relevance
between deteriorated nutritional and immunological condition with
progress of cancer (17). As
biomarkers of systemic inflammation, NLR and PLR have been
identified as independent prognostic factors in a wide range of
malignancies (6–8). In the present study, it was identified
that NLR ≥2.8 was significantly associated with decreased OS rates
as an independent risk factor, which is consistent with the results
of previous studies (18,19). There are several potential mechanisms
for how systemic inflammation is involved in tumor progression and
recurrence. One is that the inflammation response deteriorated the
cellular immune response to malignancy by depleting lymphocytes
(20). Secondly, the relatively
increased neutrophil level was deemed to contain and promote the
release of inflammatory mediators and vascular endothelial growth
factor, leading to the promotion of angiogenesis, DNA damage and
genetic instability which have been widely accepted to be
associated with tumor proliferation and metastasis (21–23).
PNI, a corrected indicator for the nutritional and
immunological condition of patients, was proposed a few decades
ago. In the early days, PNI was used to assess the risk of
post-operative complications, particularly the surgery for
gastrointestinal malignancy (24).
With the predictive value of PNI being validated in other tumors
(25), it began to be accepted that
this factor was not determined by the digestive or liver function
alone. A previous study revealed that the inflammatory environment
alteration induced by cancer cells may be the reason for the
deterioration of the nutritional condition of patients (26). In the present study, a significant
difference in OS time between the patients with high PNI and low
PNI was identified. However, the multivariate analysis indicated
that the PNI was not an independent prognostic factor. The reason
for this may be that NLR weakened the test power of PNI, as NLR is
a relatively more direct indicator for systemic inflammation
compared with PNI.
As a novel prediction method for prognosis,
nomograms have been widely used in oncology (27). In the present study, age at diagnosis,
tumor character, extent of resection and the inflammatory condition
of the patients were taken into account when the predictive
nomogram was established, providing an improved prediction of the
individual prognosis following surgery in a more intuitive way and
provided guidance for clinicians to decide whether adjuvant
treatment is required. Additionally, to the best of our knowledge,
the present study is the first to use a nomogram to evaluate the
prognostic value of NLR for patients with glioma.
The present study has a number of limitations.
First, the sample size is relatively small and all the subjects
included were from a single institution. Secondly, owing to the
retrospective nature of the study, information bias and selection
bias are essentially inevitable. Therefore, prospective studies
involving a large sample size and thorough follow-up are required
to investigate further the association between inflammatory
biomarkers and prognosis in patients with glioma.
In conclusion, the present study indicated that high
NLR was an independent risk factor for OS time in patients with
glioma, which indicated its value for improving the current
prognostic model.
Acknowledgements
Not applicable.
Funding
The present study was funded by Natural Science
Foundation of Shaanxi Province (grant no., 2015JM8462) and National
Natural Science Foundation of China (grant no., 81602207).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY analyzed data and drafted the manuscript. PM
participated in data interpretation and manuscript revision. XC
participated in data acquisition and analysis. XN participated in
data analysis and interpretation. GX and XB participated in study
concept and data acquisition. WX participated in study concept and
design. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study is a sequential study, with the
patient information used acquired from a study previously published
(13), which was approved by the
Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China; no. 2016-18). Written informed consent
was obtained from all individual participants.
Patient consent for publication
Consent was obtained from all patients for the
publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A ‘state of the
science’ review. Neuro Oncol. 16:896–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gorlia T, van den Bent MJ, Hegi ME,
Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K,
Brandes AA, Allgeier A, et al: Nomograms for predicting survival of
patients with newly diagnosed glioblastoma: Prognostic factor
analysis of EORTC and NCIC trial 26981-22981/CE. 3. Lancet Oncol.
9:29–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang TH, Kuo SH, Wang CW, Chen WY, Hsu
CY, Lai SF, Tseng HM, You SL, Chen CM and Tseng WY: Adverse
prognosis and distinct progression patterns after concurrent
chemoradiotherapy for glioblastoma with synchronous subventricular
zone and corpus callosum invasion. Radiother Oncol. 118:16–23.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Engl J
Med. 340:448–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Templeton AJ, Ace O, McNamara MG,
Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A,
Tannock IF and Amir E: Prognostic role of platelet to lymphocyte
ratio in solid tumors: A systematic review and meta-analysis.
Cancer Epidemiol Prevention Biomarkers Prev. 23:1204–1212. 2014.
View Article : Google Scholar
|
|
7
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil–lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang HB, Xing M, Ma LN, Feng LX and Yu Z:
Prognostic significance of
neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers:
A meta-analysis. Oncotarget. 7:76769–76778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kijima T, Arigami T, Uchikado Y, Uenosono
Y, Kita Y, Owaki T, Mori S, Kurahara H, Kijima Y, Okumura H, et al:
Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic
marker of advanced esophageal squamous cell carcinoma. Cancer Sci.
108:193–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki R, Takagi T, Hikichi T, Konno N,
Sugimoto M, Watanabe KO, Nakamura J, Waragai Y, Kikuchi H, Takasumi
M, et al: Derived neutrophil/lymphocyte ratio predicts gemcitabine
therapy outcome in unresectable pancreatic cancer. Oncol Lett.
11:3441–3445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao
J, Sun J, Xu Y and Wang Z: Prognostic significance of preoperative
prognostic nutritional index in colorectal cancer: Results from a
retrospective cohort study and a meta-analysis. Oncotarget.
7:58543–58552. 2016.PubMed/NCBI
|
|
12
|
Yang Z, Zhang B, Hou L, Xie Y and Cao X:
Pre-operative prognostic nutritional index predicts the outcomes
for triple-negative breast cancer. Tumor Biol. 35:12165–12171.
2014. View Article : Google Scholar
|
|
13
|
Wang J, Yang T, Xu G, Liu H, Ren C, Xie W
and Wang M: Cyclin-dependent kinase 2 promotes tumor proliferation
and induces radio resistance in glioblastoma. Transl Oncol.
9:548–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
16
|
Harrell FE Jr, Lee KL and Mark DB:
Multivariable prognostic models: Issues in developing models,
evaluating assumptions and adequacy, and measuring and reducing
errors. Stat Med. 15:361–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bambury RM, Teo MY, Power DG, Yusuf A,
Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, et
al: The association of pre-treatment neutrophil to lymphocyte ratio
with overall survival in patients with glioblastoma multiforme. J
Neurooncol. 114:149–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auezova R, Ryskeldiev N, Doskaliyev A,
Kuanyshev Y, Zhetpisbaev B, Aldiyarova N, Ivanova N, Akshulakov S
and Auezova L: Association of preoperative levels of selected blood
inflammatory markers with prognosis in gliomas. OncoTargets Ther.
9:6111–6117. 2016. View Article : Google Scholar
|
|
20
|
Denkert C, Loibl S, Noske A, Roller M,
Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R,
Hanusch C, et al: Tumor-associated lymphocytes as an independent
predictor of response to neoadjuvant chemotherapy in breast cancer.
J Clin Oncol. 28:105–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lord BI, Bronchud MH, Owens S, Chang J,
Howell A, Souza L and Dexter TM: The kinetics of human
granulopoiesis following treatment with granulocyte
colony-stimulating factor in vivo. Proc Natl Acad Sci USA.
86:9499–9503. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buzby GP, Mullen JL, Matthews DC, Hobbs CL
and Rosato EF: Prognostic nutritional index in gastrointestinal
surgery. Am J Surg. 139:160–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma W, Zhang P, Qi J, Gu L, Zang M, Yao H,
Shi X, Wang C and Jiang Y: Prognostic value of platelet to
lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. Sci
Rep. 6:353782016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo HC, Tsao LY, Hsu WY, Chen HN, Yu WK and
Chi CY: Relation of cord serum levels of growth hormone,
insulin-like growth factors, insulin-like growth factor binding
proteins, leptin, and interleukin-6 with birth weight, birth
length, and head circumference in term and preterm neonates.
Nutrition. 18:604–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|