Introduction
Pituitary adenomas (PAs) are monoclonal tumors that
originate from the anterior and posterior lobes of the pituitary
gland and residual cells of the cranial pharyngeal epithelium
(1). PA is a common intracranial
tumor and accounts for ~15–20% of intracranial tumors, and its
incidence ranks third among intracranial tumors and second among
gliomas and meningiomas (2). High
mobility group A2 (HMGA2) is a member of the HMGA family and a
non-histone protein. HMGA2 is named after the high migration
ability of HMGA2 on polyacrylamide gel electrophoresis (3). HMGA2 is a chromatin remodeling factor
that contains AT-hook basic domains, which assign them the ability
to bind the DNA minor groove of AT-rich DNA sequences (4). By changing the chromatin architecture
and assembling transcriptional complexes on chromatin, HMGA2 may
activate or impair the activity of transcriptional enhancers in
various genes indirectly (5). In
addition to serving as a transcriptional co-regulator, HMGA2 may
also directly regulate the transcription activation and expression
of various genes (6). It has been
demonstrated that HMGA2 regulates various biological processes,
including proliferation and cell cycle. Additionally, increased
expression of HMGA2 is associated with the occurrence of various
tumors, including prostate (7),
colorectal (3), lung (4) and breast cancer (8), and serves an important function in the
occurrence, progression and metastasis. Previous studies have
revealed that increased expression of HMGA2 is associated with the
occurrence of PA and prognosis, and the survival time of patients
with PA (9–11). microRNA (miRNA) is an endogenous
non-coding single-stranded RNA of 21–25 nucleotides in length,
which may regulate the expression of target genes by inhibiting the
translation or degradation of mRNAs (12) Previous studies have demonstrated that
the expression of miR-16 is deregulated in PAs, and is associated
with the occurrence of cancer and poor prognosis (13–15).
Bioinformatics analysis indicated there is a complementary region
between seed region of miR-16 and 3′-UTR of HMGA2 gene. In the
present study, it was investigated whether miR-16 may regulate the
expression of HMGA2 and whether it is involved in the pathogenesis
of PAs.
Materials and methods
Reagents
The human pituitary adenoma cell line HP75 was
provided by ScienCell Research Laboratories (USA). The HEK293 cell
line was purchased from the American Type Culture Collection
(Manassas, VA, USA). Dulbecco's modified Eagle's medium (DMEM) was
purchased from Lonza Group, Ltd. (Basel, Switzerland). Streptomycin
was purchased from Corning Incorporated (Corning, NY, USA). Horse
serum, fetal bovine serum (FBS), An Opti-MEM, enhanced
chemiluminesence (ECL) Western Blotting Substrate Kit, TRIzol and
Lipofectamine® 2000 were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The PrimeScript™ RT reagent
kit and SYBR were purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). miRNA nucleotide fragments and primers for
polymerase chain reaction (PCR) were designed and synthesized by
Guangzhou Ribobio (Guangzhou, China). Rabbit anti-HMGA2 antibody
was purchased from GeneTex Inc. (cat no. GTX50799; Irvine, CA,
USA). Mouse anti-histone H3.1 was purchased from Cell Signaling
Technology, Inc. (cat no. 3638; Danvers, MA, USA). Horseradish
peroxidase-conjugated secondary antibodies were purchased from
Abcam (cat no. ab6721; Cambridge, UK). The TdT-UTP nick end
labeling assay (TUNEL) Apoptosis Detection kit,
radioimmunoprecipitation assay buffer and Antifade mounting medium
were purchased from Jiangsu Beyotime Biotechnology Co., Ltd.
(Jiangsu, China). Cell Counting Kit-8 (CCK-8) kit was purchased
from Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China).
The Dual-Luciferase® Reporter Assay System and
pGL3-promoter plasmid were purchased from Promega Corporation
(Madison, WI, USA).
Patients
A total of 52 patients with PAs, including 27 males
and 25 females, were recruited in Mudanjiang Forestry Hospital
between March 2014 and May 2016. The average age of patients was
36.7 years (range, 31–56 years). Normal brain tissues from 16
patients with neurotrauma were collected during surgery and were
used as controls. Among these, 9 patients were males and 7 patients
were females. The average age of patients was 38.1 years (range,
33–57 years). All patients provided written informed consent and
the study was approved by the Ethics Committee of Mudanjiang
Forestry Hospital.
Cell culture
The human PA cell line HP75 was cultured in DMEM
supplemented with 10% horse serum, 5% fetal bovine serum and 1%
streptomycin, and cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2. The culture medium was
changed every 3 days. When cells reached 60–80% confluence, cells
were used for subsequent experiments.
Dual-luciferase reporter assay
The HEK293 cell line genome was used as a template
to amplify the full-length 3′-UTR fragment of the HMGA2 gene,
cloned into the luciferase reporter vector pGL-3M and transformed
into DH5α Escherichia coli competent cells. The positive
clones were screened by colony PCR. Following sequencing, the
positive clones were used for subsequent experiments. The HEK293
cell line was co-transfected with pGL3-HMGA2-3′-UTR (400 ng),
miR-16 mimic/miR-control (25 nmol) and pRL-TK (25 ng) using
Opti-MEM and Lipofectamine 2000. Luciferase assays were performed
48 h after transfection, The activity of firefly luciferase was
measured and normalized to the corresponding Renilla luciferase
activity.
Cell transfection
The sequences of siRNAs used in the present study
were as follows: si-HMGA2, 5′-CAGCCUGAAUAACUUGAACTT-3′ (sense) and
5′-GUUCAAGUUAUUCAGGCUGTT-3 (anti-sense); si-negative control (NC),
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(anti-sense). HP75 cells were divided into the following groups:
miR-control (scramble), miR-16 mimic, si-NC transfection group,
si-HMGA2 group and miR-16 mimic+si-HMGA2 groups.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for transfection, according the
manufacturer's protocol. Briefly, HP75 cells were cultured in 100
mm dish at a density of 1.5–2×105/ml, Following
cultivation for 24 h, the cells were transfected with 100 nmol/l
miRNAs using Lipofectamine 2000. Cell were cultured for 72 h before
subsequent experiments.
Bioinformatics analysis
The potential direct common target genes of miR-16
were predicted using miRNA databases, including TargetScan Human
7.2 (16), miRanda (17), and miRDB (18). Intersecting the results of these three
databases demonstrated that the HMGA2 gene was a prediction target
gene.
Reverse transcription quantitative
(RT-q)PCR
Total RNA was isolated from cells using TRIzol
reagent. RNA was reverse-transcribed into cDNA using PrimeScript™
RT Reagent kit, according to the manufacturer's protocol. qPCR was
performed using Bio-Rad CFX96 quantitative PCR system and SYBR,
according to the manufacturer's protocol. The following primers
were used: miR-16, 5′-AACCCGUAGAUCUUGGAUCCUG-3′ (forward) and
5′-CAAGAUCAUCUACGGUUUGGGU-3′ (reverse); U6,
5′-ATTGGAACGATACAGAGAAGATT-3′ (forward) and
5′-GGAACGCTTCACGAATTTG-3′ (reverse); HMGA2,
5′-ACCCAGGGGAAGACCCAAA-3′ (forward) and 5′-CCTCTTGGCCGTTTTTCTCCA-3′
(reverse); β-actin, 5′-GAACCCTAAGGCCAAC-3′ (forward) and
5′-TGTCACGCACGATTTCC-3′ (reverse). PCR was conducted as follows:
Initial denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 30 sec.
All the samples were assessed by relative quantification
(2−ΔΔCq method) (19).
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay buffer and centrifuged at 10,000 × g for 10 min. Proteins (40
µg) were separated by SDS-PAGE (10% gels) and then transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat milk for 60 min, followed by incubation with primary
antibodies against HMGA2 (dilution, 1:500) and histone H3.1
(dilution, 1:1,000, serving as loading control) overnight at 4°C.
Membranes were then washed with 0.1% Tween-20 in PBS (PBST) three
times. Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibody (dilution, 1:5,000) for 60
min at room temperature and then washed with PBST three times.
Immunoreactive bands were visualized by an ECL Western Blotting
Substrate kit. X-ray film was used to analyze the image and
intensity of bands via ImageJ software (version 1.51b; National
Institutes of Health, Bethesda, MA, USA).
CCK-8 assay
Cells were seeded in 96-well cell plates at a
density of 1×104 cells/well for 24 h. Cells were
cultured for additional 24, 48, 72 and 96 h at 37°C in a humidified
atmosphere containing 5% CO2. Then, CCK-8 reagent (10
µl) was added to each well and cells were cultured for 4 h at 37°C
in a humidified atmosphere containing 5% CO2. The
absorbance values were read at 450 nm wavelength using a microplate
reader.
TUNEL assay
Cells were washed with PBS and fixed with 4%
paraformaldehyde for 60 min. PBS containing 0.1% Triton X-100 was
added and cells were incubated on ice for 2 min and washed twice
with PBS. Then, 50 µl of TUNEL solution was added and cells
incubated for 60 min at 37°C in dark, washed twice with PBS.
Antifade mounting medium were added onto the cells and the
fluorescein isothiocyanate-labeled TUNEL-positive cells were imaged
under a fluorescent microscope. The cells with green fluorescence
were defined as apoptotic cells. A total of 5 fields of each sample
were observed.
Statistical analysis
Data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean
± standard deviation. Results were analyzed using Student's t-test
when only 2 groups were compared. One-way analysis of variance
followed by Bonferroni post hoc test was used to examine
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
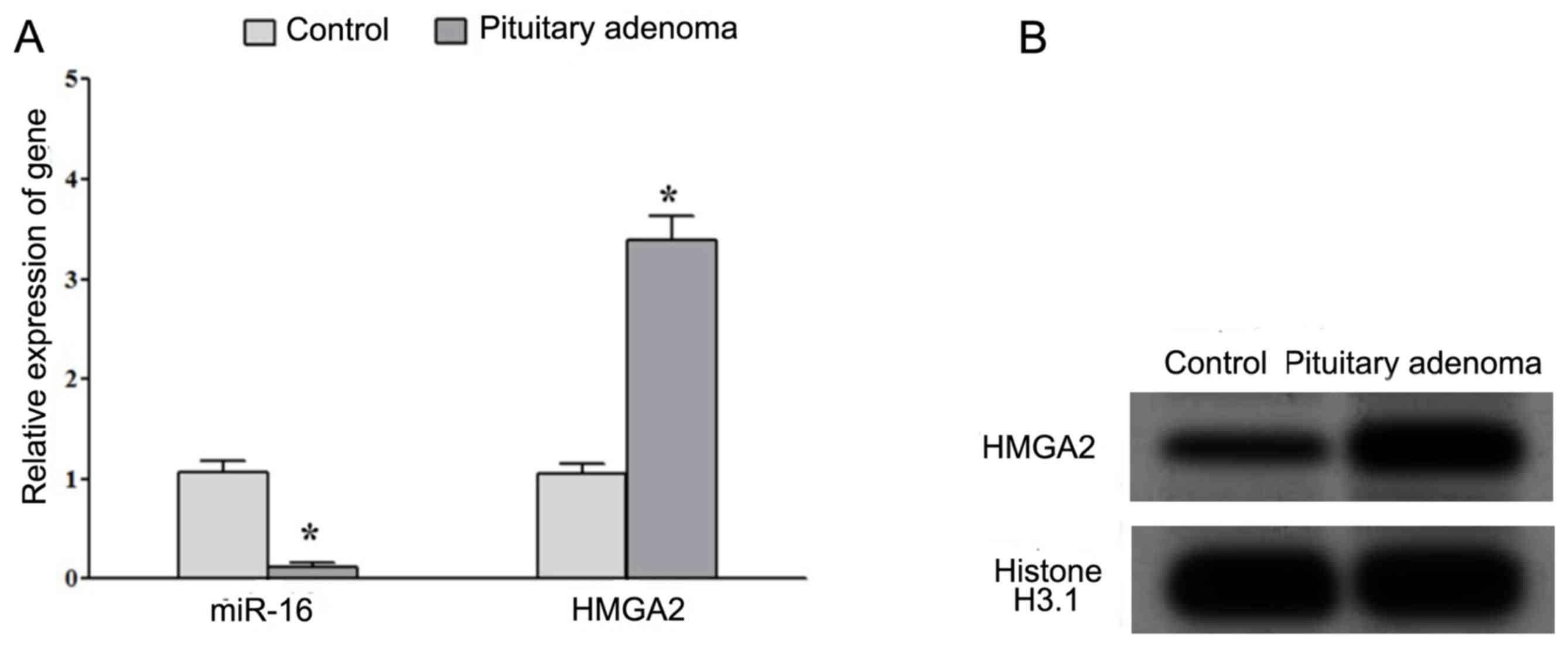
Decreased expression of miR-16
decreased and increased expression of HMGA2 in PA tissues
RT-qPCR analysis demonstrated that the expression of
miR-16 was significantly decreased whereas the expression of HMGA2
mRNA was significantly increased in patients with PAs compared with
that in normal pituitary tissues (Fig.
1A). Western blot analysis indicated that the protein
expression of HMGA2 was increased in patients with PAs compared
with that in normal pituitary tissues (Fig. 1B). These results suggested that the
decreased expression of miR-16 and increased expression of HMGA2
may be associated with the development of PA.
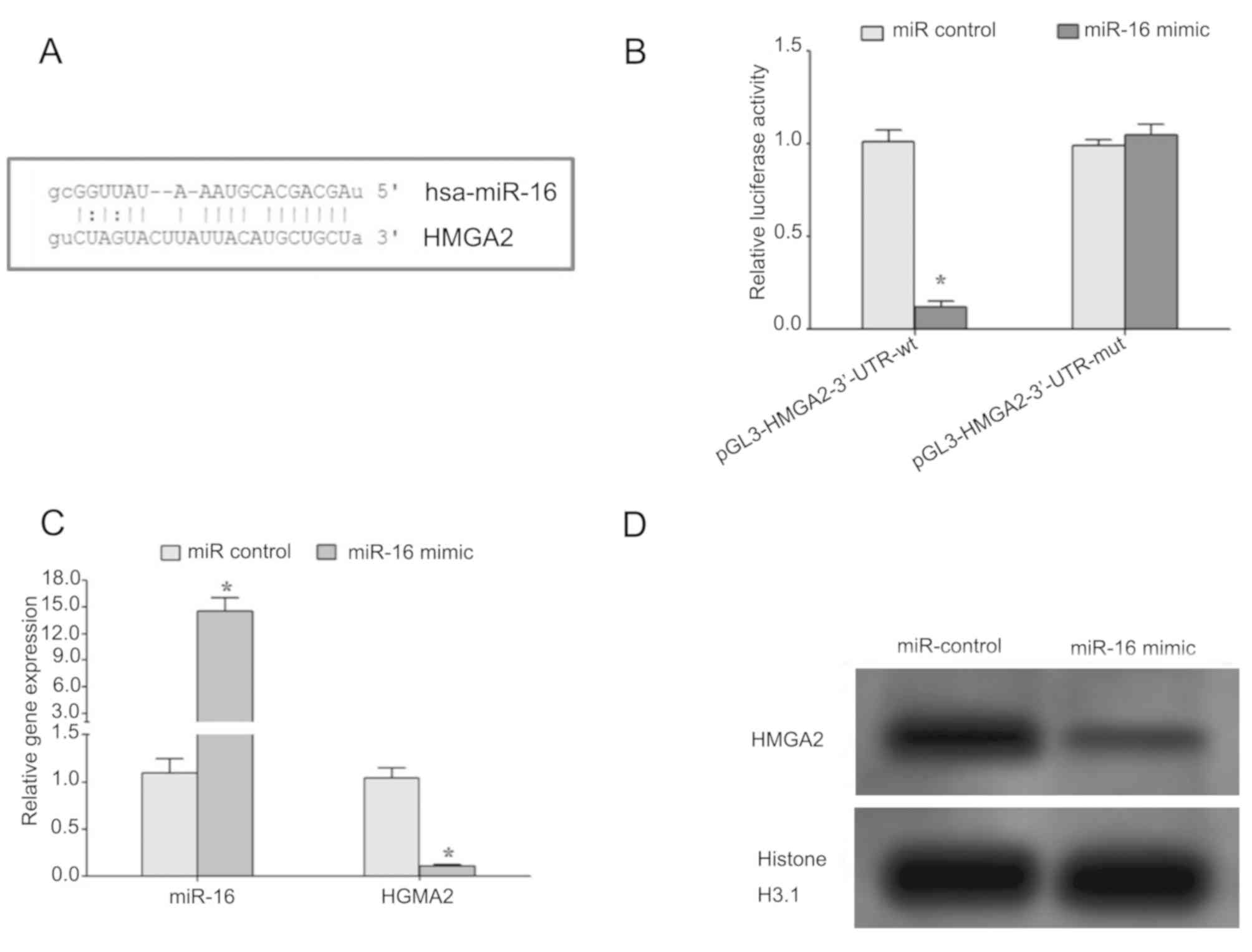
Regulation of HMGA2 by miR-16
Bioinformatics analysis revealed that there is a
complementary region between seed region of miR-16 and 3′-UTR of
HMGA2 gene (Fig. 2A). Dual-luciferase
reporter assay demonstrated that miR-16 mimic significantly
decreased the relative luciferase activity in the wild-type but not
mutant constructs (Fig. 2B),
suggesting that miR-16 may target the 3′-UTR region of HMGA2 mRNA
and regulate the expression of HMGA2. Additionally, the
introduction of miR-16 mimic in HP75 cells led to decrease in gene
and protein expression levels of HMGA2, thus confirming that miR-16
may regulate the expression of HMGA2 (Fig. 2C and D).
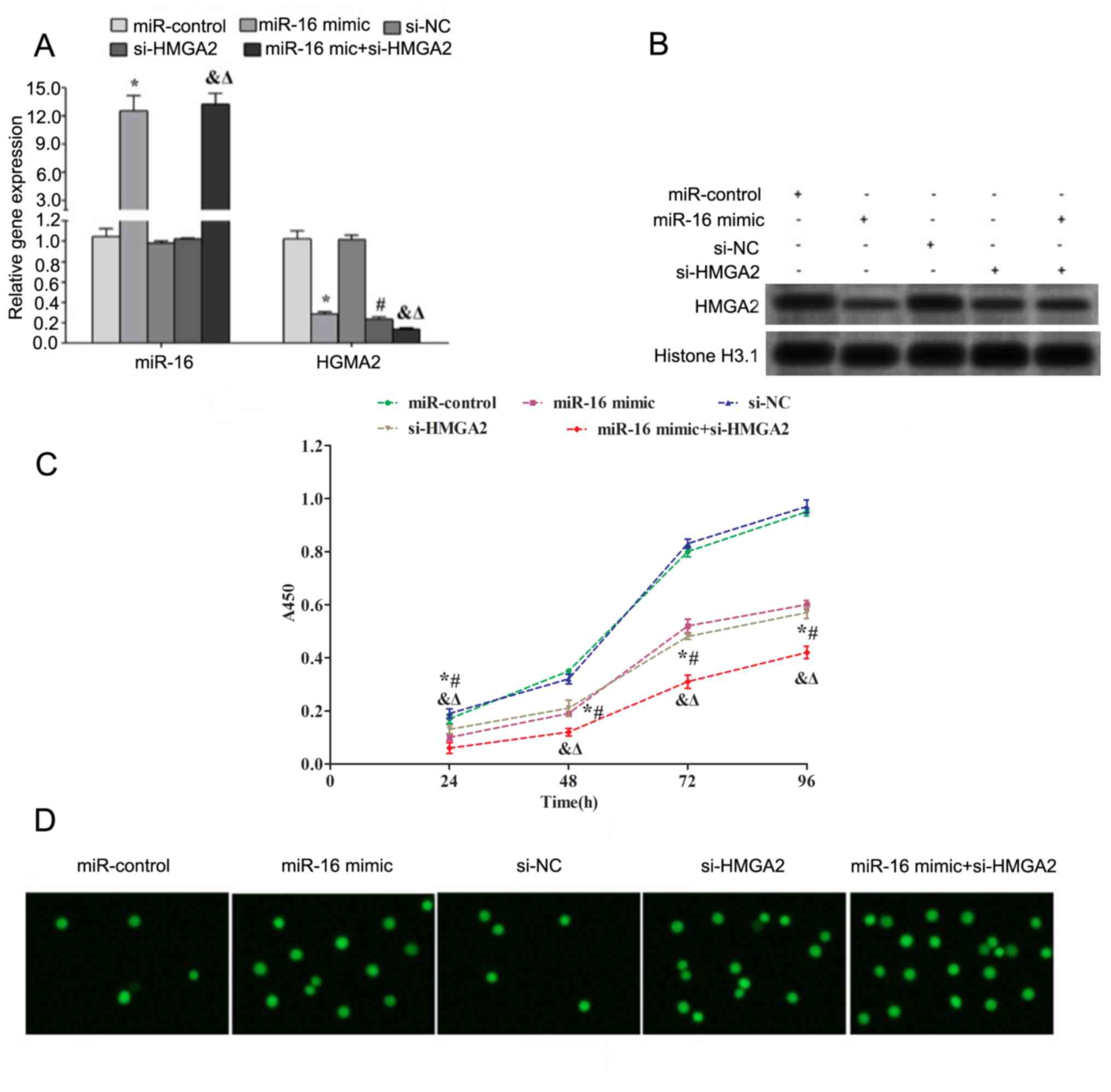
Overexpression of miR-16 promotes
apoptosis and inhibits proliferation of HP75 cells
Transfection with miR-16 mimic and/or si-HMGA2
downregulated the gene and protein expression levels of HMGA2 in
HP75 cells (Fig. 3A and B). Cell
proliferation was assessed using a CCK-8 assay. The results
demonstrated that transfection with miR-16 and/or si-HMGA2
significantly decreased the proliferation of HP75 cells after 24,
48, 72 and 96 h (Fig. 3C).
Additionally, apoptosis of HP75 cells was assessed using TUNEL
assay. The results demonstrated that transfection with miR-16
and/or si-HMGA2 increased the number of apoptotic cells (Fig. 3D).
Discussion
PA may lead to a series of endocrine symptoms,
including acromegaly, decreased sexual function, headache, vision
loss, visual field defect, hypothalamic syndrome, cerebrospinal
fluid rhinorrhea and other symptoms of adjacent local tissue
compression. Invasive PAs (IPAs) account for ~30% of PAs and are
large tumors with infiltration of adjacent tissue. Patients with
IPAs exhibit an increased postoperative recurrence rate of 50% and
poor prognosis (20). Therefore,
future studies investigating the underlying molecular mechanisms
are required for the diagnosis and treatment of PA.
HMGA2 is a non-histone chromatin protein that is
involved in the regulation of chromatin. HMGA2 is involved in
biological processes, including cell proliferation,
differentiation, cell cycle and migration. Under physiologic
conditions, HMGA2 is not expressed or minimally expressed in normal
tissues. Abnormal expression of HMGA2 is detected in various types
of cancer, including prostate (7),
colorectal (3), lung (4) and breast cancer (8). Increased expression of HMGA2 is
associated with the occurrence, progression and poor prognosis of
cancer. Shi et al (7) revealed
that HMGA2 may be involved in the occurrence of prostate cancer by
regulating cell proliferation, apoptosis, epithelial mesenchymal
transition and invasion. Wu et al (21) demonstrated that upregulation of HMGA2
may be used as a biomarker of breast cancer progression and poor
prognosis. Borrmann et al (22) reported that HMGA2 negatively regulated
the activity of the Nucleotide excision repair cross complementing
gene 1 (ERCC1) gene promoter. Additionally, HMGA2 inhibited the
nucleotide excision repair pathway. Thus, HMGA2 may be involved in
the occurrence of tumor by regulating DNA damage and repair.
Previous studies (9–11) have demonstrated that elevated
expression of HMGA2 may be associated with the occurrence of PA,
the survival and prognosis of patients with PA. Previous studies
(13–15) revealed that the expression of miR-16
was deregulated in PA tissues. Bioinformatics analysis indicated
there is a complementary region between seed region of miR-16 and
3′-UTR of HMGA2 gene. In the present study, it was investigated
whether miR-16 may regulate the expression of HMGA2 and whether it
is involved in the pathogenesis of PAs.
The results of present study demonstrated that the
expression level of miR-16 was decreased, whereas the expression
level of HMGA2 was increased in PA tissues compared with normal
pituitary tissues. These results suggest that the decreased
expression of miR-16 may serve a function in upregulating the
expression of HMGA2 and promoting the occurrence of PA. D'Angelo
et al (10) demonstrated that
the expression level of HMGA2 in tissues from patients with growth
hormone-secreting PAs was significantly increased compared with
that in normal pituitary glands. Oruckaptan et al (20) revealed that HMGA2-transgenic mice have
an increased incidence of PA compared with normal wild-type mice.
Fedele et al (11)
demonstrated that overexpression of HMGA2 may lead to the
development of prolactin or growth hormone adenomas in 85% of
transgenic mice within 6 months. In the present study, it was
demonstrated that the expression level of HMGA2 in tumor tissues
from patients with PA was significantly increased compared with
that in normal pituitary tissues, thus confirming the results from
a previous study (10). Amaral et
al (13) demonstrated the
expression level of miR-16 in tumor tissues from patients with PA
was significantly decreased compared with normal tissues. Bottoni
et al (14) revealed that the
expression level of miR-16 was significantly decreased in PA
tissues compared with normal pituitary tissues. Renjie and Haiqian
(15) demonstrated that the
expression level of miR-16 was significantly decreased in PA cell
lines compared with that in control. Additionally, the expression
level of miR-16 was significantly increased in patients with IPA
compared with that in patients with non-invasive PAs (15), suggesting that decreased expression of
miR-16 may be involved in the pathogenesis of PA. The present study
demonstrated that the expression level of miR-16 in PA tissues was
significantly decreased compared with that in normal pituitary
tissues, thus confirming the results from previous studies
(13,14). The dual-luciferase reporter assay
demonstrated that transfection of miR-16 mimic decreased the
relative luciferase activity, and the protein and gene expression
level of HMGA2 in HP75 cells, suggesting that miR-16 may target
HMGA2. Additionally, upregulation of miR-16 or transfection of
si-HMGA2 significantly decreased the expression level of HMGA2 in
HP75 cells. Additionally, transfection with miR-16 and/or si-HMGA2
significantly decreased the proliferation but increased apoptosis
of HP75 cells. Renjie and Haiqian (15) reported that miR-16 may regulate the
proliferation, migration and invasion of pituitary tumor cells by
inhibiting the expression of sex determining region Y-box 5, and
overexpression of miR-16 inhibited the proliferation of PA cell
lines, GH3 and MMQ and attenuated their invasive ability. In the
present study, overexpression of miR-16 significantly attenuated
the malignant biological behavior of HP75 cells, thus confirming
the findings from Renjie and Haiqian (15). Fedele et al (23) demonstrated that histone deacetylase
(HDAC) 2 interacted with retinoblastoma protein (pRB) and
substituted HDAC1 in the phosphorylated pRB/E2F transcription
factor 1 complex to enhance E2F1 acetylation and transcription
activity, and promote cells to enter S phase. Thus, HMGA2 may be
involved in the occurrence of PA. De Martino et al (24) confirmed that HMGA2 binds to the
promoter region of the G2/mitotic-specific cyclin-B2 (CCNB2) gene
to upregulate the expression of CCNB2 and promote cell mitosis and
proliferation, thus serving a function in the occurrence of PAs.
Additionally, the association between HMGA2 and apoptosis has been
demonstrated by previous studies (25,26). In
the present study, si-HMGA2 interference and miR-16 overexpression
inhibited cell proliferation and promoted apoptosis by inhibiting
the expression of HMGA2, which in turn may regulate cell cycle,
mitosis proliferation and apoptosis. The molecular mechanism
underlying the function of HMGA2 in PAs needs to be further
identified.
In summary, the results of the present study
demonstrated that the expression of miR-16 was decreased and the
expression of HMGA2 was increased in PA tissues. miR-16 may inhibit
the proliferation of HP75 cells and promote apoptosis by inhibiting
the expression of HMGA2.
Acknowledgements
The authors would like to thank Mudanjiang Medical
University (Mudanjiang, China) for providing assistance in the
development of related experiments, Dean Li Xiaoxia and Dean Liu
Fenghai (School of Public Health, Mudanjiang Medical University,
Mudanjiang, China) for giving guidance on the design of relevant
experiments.
Funding
The present study was supported by the Scientific
Research Project of Heilongjiang Provincial Health Department
(grant no. 2012-290), the Science and Technology Research Project
of Mudanjiang Medical University (grant nos. ZS201523 and ZS201526)
and the Key Laboratory of Mudanjiang Center for Disease Control and
Prevention.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
NA, YN and YW made substantial contributions to
conception and design. YL and HZ were responsible for the analysis
and interpretation of data. CF and JX made substantial
contributions to acquisition and analysis of data. NA, CF and JX
were involved in drafting the manuscript and revising it critically
for important intellectual content. NA agreed to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All patients provided written informed consent and
the study was approved by the Ethics Committee of Mudanjiang
Forestry Hospital.
Patient consent for publication
All patients provided consent for the publication of
this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leone V, Langella C, D'Angelo D, Mussnich
P, Wierinckx A, Terracciano L, Raverot G, Lachuer J, Rotondi S,
Jaffrain-Rea ML, et al: Mir-23b and miR-130b expression is
downregulated in pituitary adenomas. Mol Cell Endocrinol. 390:1–7.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esposito F, De Martino M, D'Angelo D,
Mussnich P, Raverot G, Jaffrain-Rea ML, Fraggetta F, Trouillas J
and Fusco A: HMGA1-pseudogene expression is induced in human
pituitary tumors. Cell Cycle. 14:1471–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siahmansouri H, Somi MH, Babaloo Z,
Baradaran B, Jadidi-Niaragh F, Atyabi F, Mohammadi H, Ahmadi M and
Yousefi M: Effects of HMGA2 siRNA and doxorubicin dual delivery by
chitosan nanoparticles on cytotoxicity and gene expression of HT-29
colorectal cancer cell line. J Pharm Pharmacol. 68:1119–1130. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhuo HC, Song YF, Ye J, Lai GX and Liu DL:
MicroRNA-154 functions as a tumor suppressor and directly targets
HMGA2 in human non-small cell lung cancer. Genet Mol Res.
15:2016.doi: 10.4238/gmr.15028173. View Article : Google Scholar
|
|
5
|
Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL
and Pan CB: Overexpression of HMGA2 promotes tongue cancer
metastasis through EMT pathway. J Transl Med. 14:262016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao X, Dai M, Li Q, Wang Z, Lu Y and Song
Z: HMGA2 regulates lung cancer proliferation and metastasis. Thorac
Cancer. 8:501–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Z, Wu D, Tang R, Li X, Chen R, Xue S,
Zhang C and Sun X: Silencing of HMGA2 promotes apoptosis and
inhibits migration and invasion of prostate cancer cells. J Biosci.
41:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou Q, Wu H, Fu F, Yi W, Pei L and Zhou M:
RKIP suppresses the proliferation and metastasis of breast cancer
cell lines through up-regulation of miR-185 targeting HMGA2. Arch
Biochem Biophys. 610:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Angelo D, Esposito F and Fusco A:
Epigenetic mechanisms leading to overexpression of HMGA proteins in
human pituitary adenomas. Front Med (Lausanne). 2:392015.PubMed/NCBI
|
|
10
|
D'Angelo D, Palmieri D, Mussnich P, Roche
M, Wierinckx A, Raverot G, Fedele M, Croce CM, Trouillas J and
Fusco A: Altered microRNA expression profile in human pituitary GH
adenomas: Down-regulation of miRNA targeting HMGA1, HMGA2, and
E2F1. J Clin Endocrinol Metab. 97:E1128–E1138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fedele M, Battista S, Kenyon L,
Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R,
Pierantoni GM, Outwater E, et al: Overexpression of the HMGA2 gene
in transgenic mice leads to the onset of pituitary adenomas.
Oncogene. 21:3190–3198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma
H, Yu D, Maitikabili A, Xiao H, Zhang C, et al: MicroRNA-543
suppresses colorectal cancer growth and metastasis by targeting
KRAS, MTA1 and HMGA2. Oncotarget. 7:21825–21839. 2016.PubMed/NCBI
|
|
13
|
Amaral FC, Torres N, Saggioro F, Neder L,
Machado HR, Silva WA Jr, Moreira AC and Castro M: MicroRNAs
differentially expressed in ACTH-secreting pituitary tumors. J Clin
Endocrinol Metab. 94:320–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bottoni A, Piccin D, Tagliati F, Luchin A,
Zatelli MC and Degli Uberti EC: miR-15a and miR-16-1
down-regulation in pituitary adenomas. J Cell Physiol. 204:280–285.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Renjie W and Haiqian L: MiR-132, miR-15a
and miR-16 synergistically inhibit pituitary tumor cell
proliferation, invasion and migration by targeting Sox5. Cancer
Lett. 356:568–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human microRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X: miRDB: A microRNA target
prediction and functional annotation database with a wiki
interface. RNA. 14:1012–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oruckaptan HH, Senmevsim O, Ozcan OE and
Ozgen T: Pituitary adenomas: Results of 684 surgically treated
patients and review of the literature. Surg Neurol. 53:211–219.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen
L, Ren X, Yao L, Sheng H, Li L, et al: Elevated HMGA2 expression is
associated with cancer aggressiveness and predicts poor outcome in
breast cancer. Cancer Lett. 376:284–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borrmann L, Schwanbeck R, Heyduk T,
Seebeck B, Rogalla P, Bullerdiek J and Wisniewski JR: High mobility
group A2 protein and its derivatives bind a specific region of the
promoter of DNA repair gene ERCC1 and modulate its activity.
Nucleic Acids Res. 31:6841–6851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fedele M, Visone R, De Martino I, Troncone
G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C,
Melillo RM, et al: HMGA2 induces pituitary tumorigenesis by
enhancing E2F1 activity. Cancer Cell. 9:459–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Martino I, Visone R, Wierinckx A,
Palmieri D, Ferraro A, Cappabianca P, Chiappetta G, Forzati F,
Lombardi G, Colao A, et al: HMGA proteins up-regulate CCNB2 gene in
mouse and human pituitary adenomas. Cancer Res. 69:1844–1850. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Xu G, Liu H and Li T:
MicroRNA-490-3p regulates cell proliferation and apoptosis by
targeting HMGA2 in osteosarcoma. FEBS Lett. 589:3148–3153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai J, Shen G, Liu S and Meng Q:
Downregulation of HMGA2 inhibits cellular proliferation and
invasion, improves cellular apoptosis in prostate cancer. Tumour
Biol. 37:699–707. 2016. View Article : Google Scholar : PubMed/NCBI
|