Introduction
The screening results of a large number of growth
hormone (GH)-secreting pituitary tumors revealed that gsp oncogenes
were observed in 4–59% of patients with acromegaly (1–9). A
number of studies indicated that the gsp oncogene may result in
increased serum GH levels and smaller tumors in patients with
gsp-positive tumors, compared with those with gsp-negative tumors
(9–11).
Maternally expressed gene 3 (MEG3) is a maternally
imprinted gene encoding a long non-coding RNA that suppresses tumor
cell proliferation (12,13). MEG3 is highly expressed in
GH-secreting pituitary tumors, but not in clinically
non-functioning pituitary tumors (14). A previous study indicated that a
cyclic adenosine monophosphate (cAMP) response element (CRE)
located at the MEG3 proximal promoter region was critically
important for promoter activity (15,16).
Furthermore, gsp oncogene could increase intracellular cAMP levels
and promote the phosphorylation of cyclic adenosine
monophosphate-responsive element binding (p-CREB) protein, which
consequently may result in the constitutive GH hypersecretion
(17,18). To investigate the mechanism of gsp
oncogene underlying different biochemical and clinical features of
GH-secreting pituitary tumors, we hypothesized in the present study
that MEG3 may serve a major role in gsp-positive tumors, as it
could increase GH levels and reduce tumor volume, compared with
gsp-negative tumors. Clinical and biochemical data, as well as
pathological features of patients with acromegaly were carefully
analyzed with respect to gsp oncogenes.
Materials and methods
Patients and clinical
characteristics
A retrospective analysis of data from 25 patients
with acromegaly, 13 male and 12 female (range, 24–61 years of age,
mean: 45.16±10.16 years), was conducted. Patients underwent
endoscopic endonasal transsphenoidal surgery at the Department of
Neurosurgery of Nanjing Jinling Hospital (Nanjing, China) between
November 2015 and November 2016. Approval for the study was
obtained from the Ethical Committee of Nanjing Jinling Hospital.
Written informed consent was obtained from all patients.
All patients had manifested signs of active
acromegaly, and the diagnosis of acromegaly was on the basis
presence of classic clinical features and the lack of GH
suppression to 1 µg/l during an oral glucose tolerance test (OGTT)
and immunohisto-chemical staining of the tumors for GH. [Tumor
size=(length × width2)/2] Fresh samples of 10 clinically
non-functioning pituitary tumors were pathologic confirmed at the
Department of Pathology of Nanjing Jinling Hospital were also
obtained. No patients had previously undergone radiation therapy.
Approval for the 10 fresh samples was obtained from the Ethical
Committee of Nanjing Jinling Hospital, and informed consent was
obtained from all patients.
The preoperative clinical and biochemical data of
all patients with acromegaly were retrospectively collected by
reviewing medical charts. Magnetic resonance (MR) technologists
measured tumor volumes with standard AW VolumeShare 5 (AW4.6),
GEHealthcare (GE Healthcare Life Sciences, Little Chalfont, UK)
imaging software to manually trace the contrast-enhancing tumor
boundary on each image. Knosp classification was based on the
degree of lateral extension to the cavernous sinus (CS) space
through MR Imaging (19), and Knosp
grade 3 and 4 were defined as CS invasion (20).
Polymerase chain reaction (PCR) for
detecting gsp mutations
DNA was extracted and isolated from the frozen 25
GH-secreting tumor tissues with a DNA minikit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol. PCR
amplification of exons 8 and 9, including codons 201 and 227,
respectively, which are sites for G-protein α subunit (Gsα)
mutations, was performed on human genomic DNA with oligonucleotide
primers, as previously described (12). Each of the 50 µl PCR reaction mixes
contained 2 µl DNA solution isolated from glass slides, 5 units of
Taq DNA-Polymerase (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 20 pmol of each primer. Following denaturing
for 15 min at 94°C, amplification was performed for 40 cycles at
94°C for 15 sec, at 64°C for 10 sec, annealing and elongation steps
were combined, and at 72°C for 1 min, the PCR amplification
products were purified by a PCR Purification kit (Qiagen GmbH), and
were further used for direct sequencing with an ABI3730XL analyzer
(Applied Biosystems; Thermo Fisher Scientific, Inc.).
Immunohistochemistry
The pathological specimens of 25 patients with
acromegaly were stained by immunohistochemistry. Monoclonal
antibodies that were directed against p-CREB (dilution, 1:250;
Abcam, Cambridge, MA, USA), Ki-67 and p53 (dilution, 1:200; Abcam)
were used. Briefly, tumor tissue grown on coverslips were fixed
with 4% paraformaldehyde for 15 min at room temperature, and
incubated sections with 1% Triton X-100 were diluted in PBS for 30
min at room temperature. The 5 µm sections were subsequently
directly incubated with 10% normal goat serum (Beyotime institute
of Biotechnology, Shanghai, China) blocking solution for 30 min at
room temperature. Furthermore, these sections were incubated with
the primary antibody and secondary antibodies. For the primary
antibodies, the dlides were incubated with p-CREB (ab32096, 1:250,
Abcam, Cambridge, UK), Ki-67 (ab15580, 1:200, Abcam) and p53 (ab26,
1:200, Abcam) overnight at 4°C. The slides were then incubated with
secondary antibody (goat anti-rabbit horseradish
peroxidase-conjugated IgG; #A0208; 1:50; Beyotime Institute of
Biotechnology, (Shanghai, China) and goat anti-mouse rabbit
horseradish peroxidase-conjugated IgG; #A0216; 1:50 (Beyotime
Institute of Biotechnology) for 15 min at 37°C.
The number of the p-CREB protein positively stained
tissues were subsequently counted in each section in 10 random
microscope fields (magnification, ×400).
Expression levels were defined as follows: High
expression when GH-secreting tumors revealed abundant p-CREB
staining in ≥50% of the cell nucleus; and low expression when
tumors exhibited p-CREB staining in <50% of the nucleus.
The Ki-67 labeling index (Li) was defined as
follows: The percentage of labeled cells/the total number of cells
analyzed in each field with ≥1,000 cells (13). Ki-67 index that had a 3% cutoff value
was highlighted for distinguishing the level of proliferation
activity (14). Qualitative analysis
of p53 expression was conducted in GH-secreting pituitary tumors.
The expression of p53 negative was primarily detected no staining
in the nuclear of tumor cells or nucleus staining is observed in
<10% of tumor cells in 10 randomly-selected microscope fields of
view. A positive nuclear staining visual score of ≤10% for tumor
cells was considered p53 positive. The number of positive tumor
cells were determined by Image-Pro Plus 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Reverse transcription-quantitative PCR
(RT-qPCR) for examining the expression levels of MEG3
Total RNA was extracted from tumors (n=25) with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The isolated RNA
was reversely transcribed into cDNA with a PrimeScript™ RT reagent
Kit (Perfect Real Time) (RR037A, Takara Biomedical Technology,
Beijing, China) reverse transcription kit. The expression was
quantified by RT-qPCR, using SYBR® Advantage qPCR Premix
(Takara Biotechnology, Dalian, China), according to the
manufacturer's protocol, on an ABI 7500 fast Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The PCR reaction was conducted at 95°C for 30 sec, followed
by 40 cycles of 95°C for 5 sec, and 64°C for 10 sec, which was
performed in combination with annealing and elongation. Each sample
was analyzed in triplicate, and the relative expression was
calculated with the 2−ΔΔCq method relative to
β-actin.
The expression levels of MEG3 in non-functioning
tumors (n=10) were used as negative controls. The MEG3 primers used
for RT-qPCR were forward, 5′-CCTGCTGCCCATCTACACCTC-3′ and reverse,
5′-CCTCTTCATCCTTTGCCATCCTGG-3′. As a control, transcript of β-actin
was also detected. The β-actin primers were forward,
5′-CACCCAGCACAATGAAGATCAAGAT-3′ and reverse,
5′-CCAGTTTTTAAATCCTGAGTCAAGC-3′. MEG3 level in GH-secreting tumors
was given by formula 2−ΔΔCq, where ΔCq=Cq (MEG3
tumor-β-actin tumor), and ΔΔCq=ΔCq (MEG3 GH tumor-β-actin
tumor)-ΔCq (MEG3 non-functioning tumor-β-actin tumor). The MEG3
with 2−∆∆Cq=479.75 was set as a cut-off value, according
to the You den's index to separate low MEG3 expression from high
MEG3 expression, according to Youden's index (16). With this value as the reference, 25
patients were categorized into groups of low and high-MEG3
expression, which was determined by the cut-off value (stated in
results).
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used to perform the statistical analysis. A comparison between the
two groups was performed by the Student's unpaired t-test with
results presented as mean ± standard deviation and Fisher's exact
test. Correlation was conducted by Spearman's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Gsα mutations and clinical data of
GH-secreting tumors
All patients were categorized into gsp-positive
(n=7) and gsp-negative (n=18) groups, according to the detection of
Gsα mutations. The prevalence of gsp oncogene among GH-secreting
pituitary tumors reached 28%. A total of 6 mutations were in codon
227 and 1 mutation was in codon 201. No significant differences in
age (P=0.140) and sex distribution (P=0.576) were indicated between
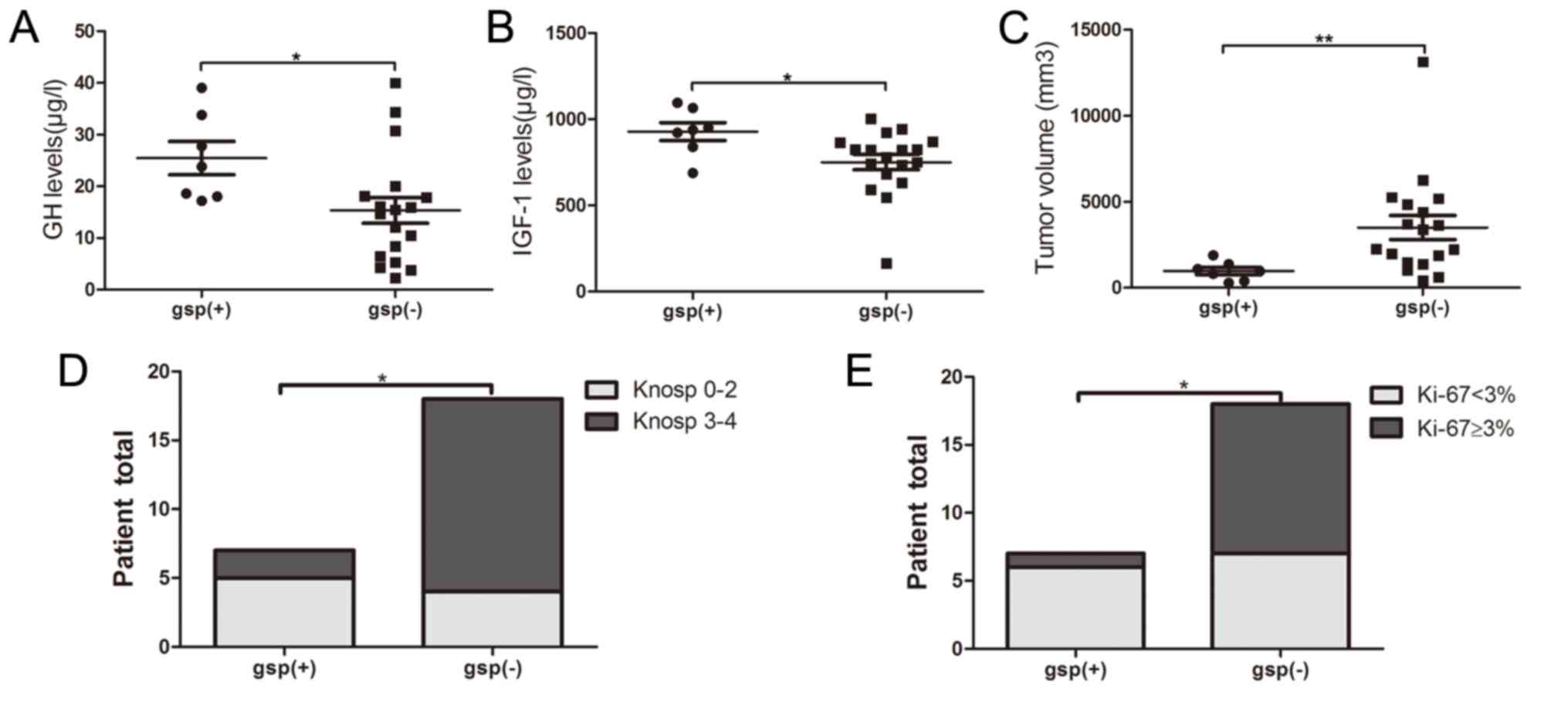
the gsp-positive and gsp-negative groups. The gsp-positive group
indicated significantly increased levels of baseline GH (25.5±8.5
vs. 15.4±10.7 µg/l; P=0.035) and IGF-1 (928.3±137.3 vs. 751.2±189.6
µg/l; P=0.035), and a reduced tumor size (1,928.0±1,109.1 vs.
6,765.3±5,897.9 mm3; P=0.003), compared with the
gsp-negative group (Fig. 1A-C). The
percentage of invasiveness (29 vs. 78%; P=0.024) and Ki-67 Li
<3% (86 vs. 39%; P=0.039) was significantly reduced in
gsp-positive tumors, compared with gsp-negative tumors (Table I; Fig. 1D
and E).
 | Table I.Clinical features of patients with
gsp-positive and negative tumors. |
Table I.
Clinical features of patients with
gsp-positive and negative tumors.
| Variables | Gsp positive | Gsp negative | P-value | Total |
|---|
| Patients (n) | 7 (28%) | 18 (72%) |
| 25 |
| Age (years) | 50.0±10.2 | 43.3±9.8 | 0.140 | 45.2±10.2 |
| Sex |
|
Male | 3 | 10 | 0.576 | 13 |
|
Female | 4 | 8 |
| 12 |
| GH (µg/l) | 25.5±8.5 | 15.4±10.7 | 0.035a | 18.2±11.0 |
| IGF-1 (µg/l) | 928.3±137.3 | 751.2±189.6 | 0.035a | 800.8±191.8 |
| Volume
(mm3) | 1,237.3±482.3 |
5,871.8±3,980.5 | 0.003a |
2,785.4±2,766.2 |
| Knosp grade
(%) |
|
| 0.024a |
|
|
0–2 | 71% (5/7) | 22% (4/18) |
| 36% (9/25) |
|
3–4 | 29% (2/7) | 78% (14/18) |
| 64% (16/25) |
| Ki-67 (%) |
|
| 0.039a |
|
| Ki-67
<3% | 86% (6/7) | 39% (7/18) |
| 52% (13/25) |
| Ki-67
≥3% | 14% (1/7) | 61% (11/18) |
| 48% (12/25) |
| p53 (%) |
|
| 0.576 |
|
| + | 57% (4/7) | 44% (8/18) |
| 48% (12/25) |
| − | 43% (3/7) | 56% (10/18) |
| 52% (13/25) |
p-CREB and clinical data of
GH-secreting tumors
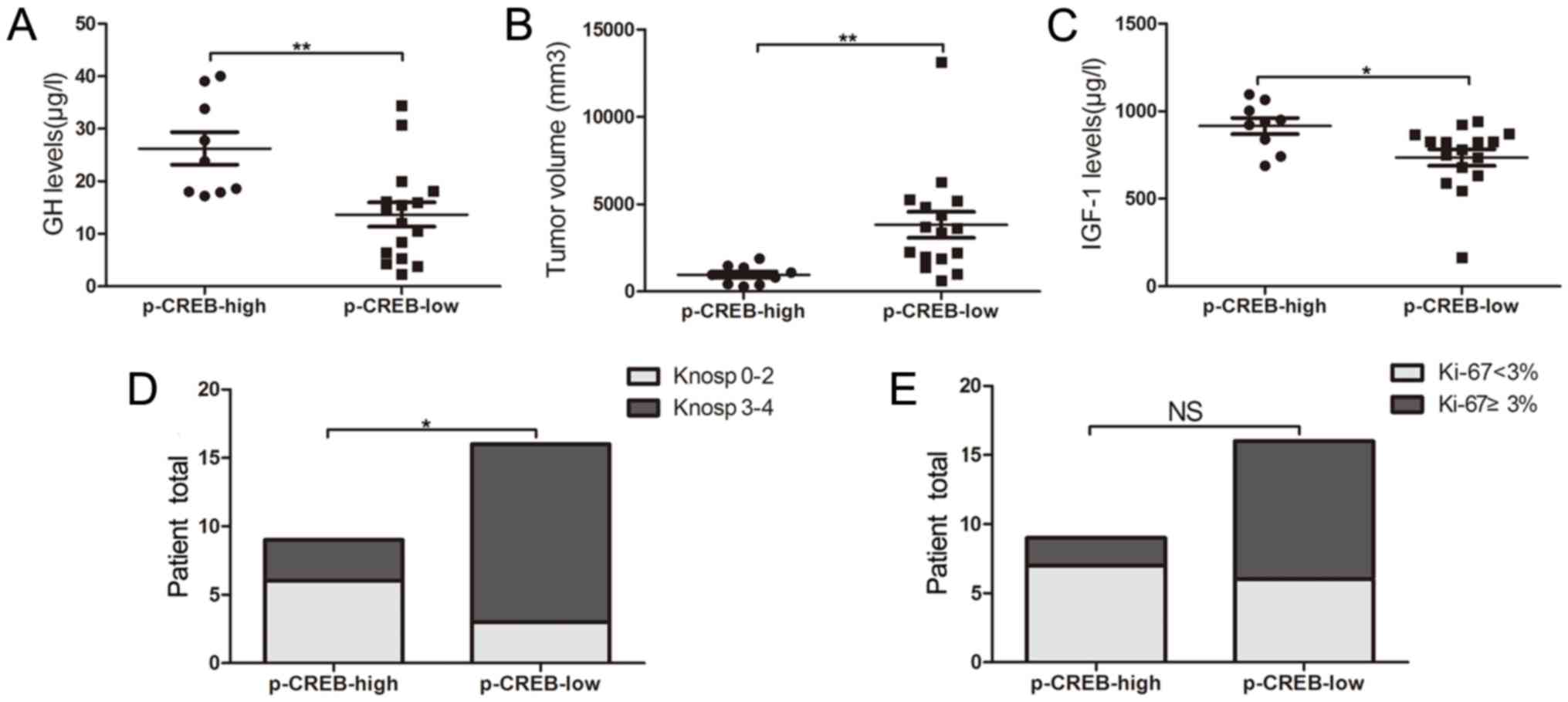
Patients with acromegaly were categorized into the
group of p-CREB-low expression (n=16) and that of p-CREB-high
expression (n=9), based on immunohistochemical staining results.
Serum GH (26.2±9.3 vs. 13.7±9.2 µg/l; P=0.003) and IGF-1 expression
levels (915.9±137.9 vs. 736.0±190.4 µg/l; P=0.021) were
significantly increased, and tumor volume (1235.8±472.2 vs.
6015.0±3955.0 mm3, P=0.002) (Fig. 2A-C) was significantly reduced in the
high-expression group, compared with the low-expression one. A
significant difference was also observed in the percentage of
invasiveness between high- and low-expression groups (33 vs. 81%;
P=0.019) (Fig. 2D). No significant
difference was indicated in the proportion of KI-67 Li <3%
between tumors with high p-CREB expression and tumors with low
p-CREB expression (78 vs. 38%, P=0.058; Fig. 2E).
MEG3 and clinical data of GH-secreting
tumors
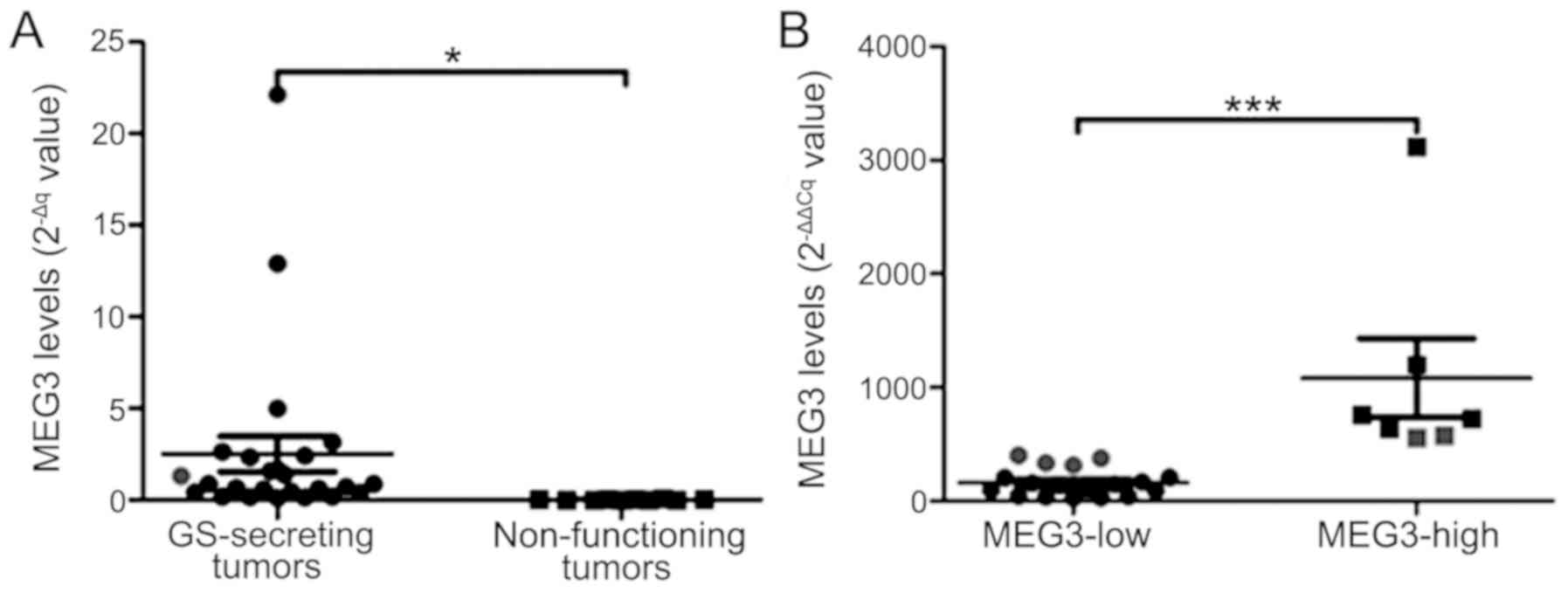
The 2−∆Cq values of MEG3 were
significantly increased in 25 GH-secreting tumors, compared with
the 10 non-functioning pituitary tumors (2.5±4.8 vs. 0.02±0.02;
P=0.02; Fig. 3A). The mean MEG3
level (2−∆∆Cq value) in GH-secreting tumors was
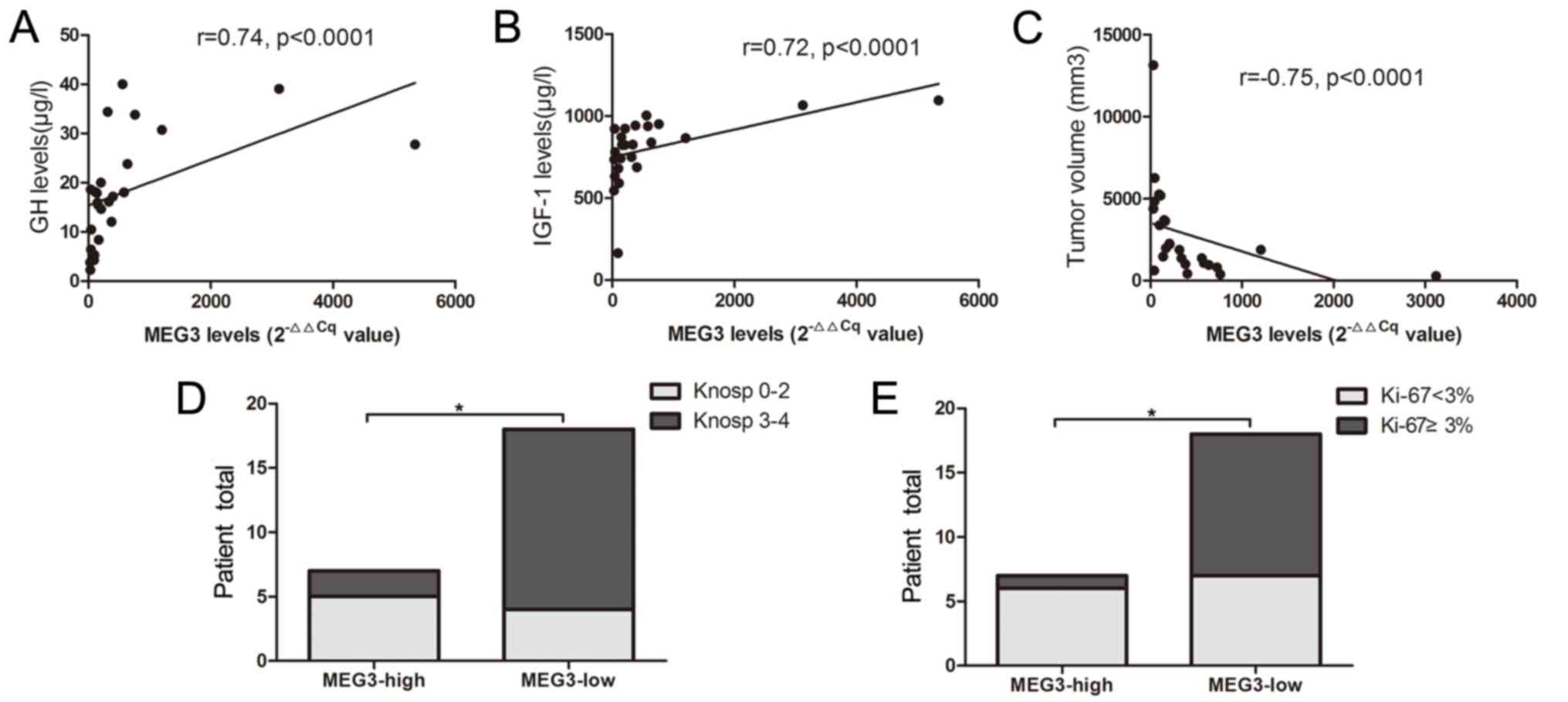
420.9±633.4. Correlation analysis indicated that MEG3 is positively
correlated with GH and IGF-1 levels, and negatively correlated with
tumor size (r=0.74, P<0.0001; r=0.72, P<0.0001; r=−0.75,
P<0.0001, respectively; Fig.
4A-C). The MEG3 with 2−∆∆Cq=479.75 was set as a
cut-off value, according to the Youden's index. The total number of
patients (n=25) with GH-secreting tumors were categorized into low-
and high-expression groups. A significant difference was indicated
in MEG3 expression between the two groups (P=0.0001; Fig. 3B). The percentage of invasiveness (29
vs. 78%; P=0.024) and Ki-67 Li <3% (86 vs. 39%, P=0.039) were
significantly reduced in the group with high MEG3 expression,
compared with the group with low MEG3 expression (Fig. 4D and E).
Association among gsp oncogene, p-CREB
and MEG3
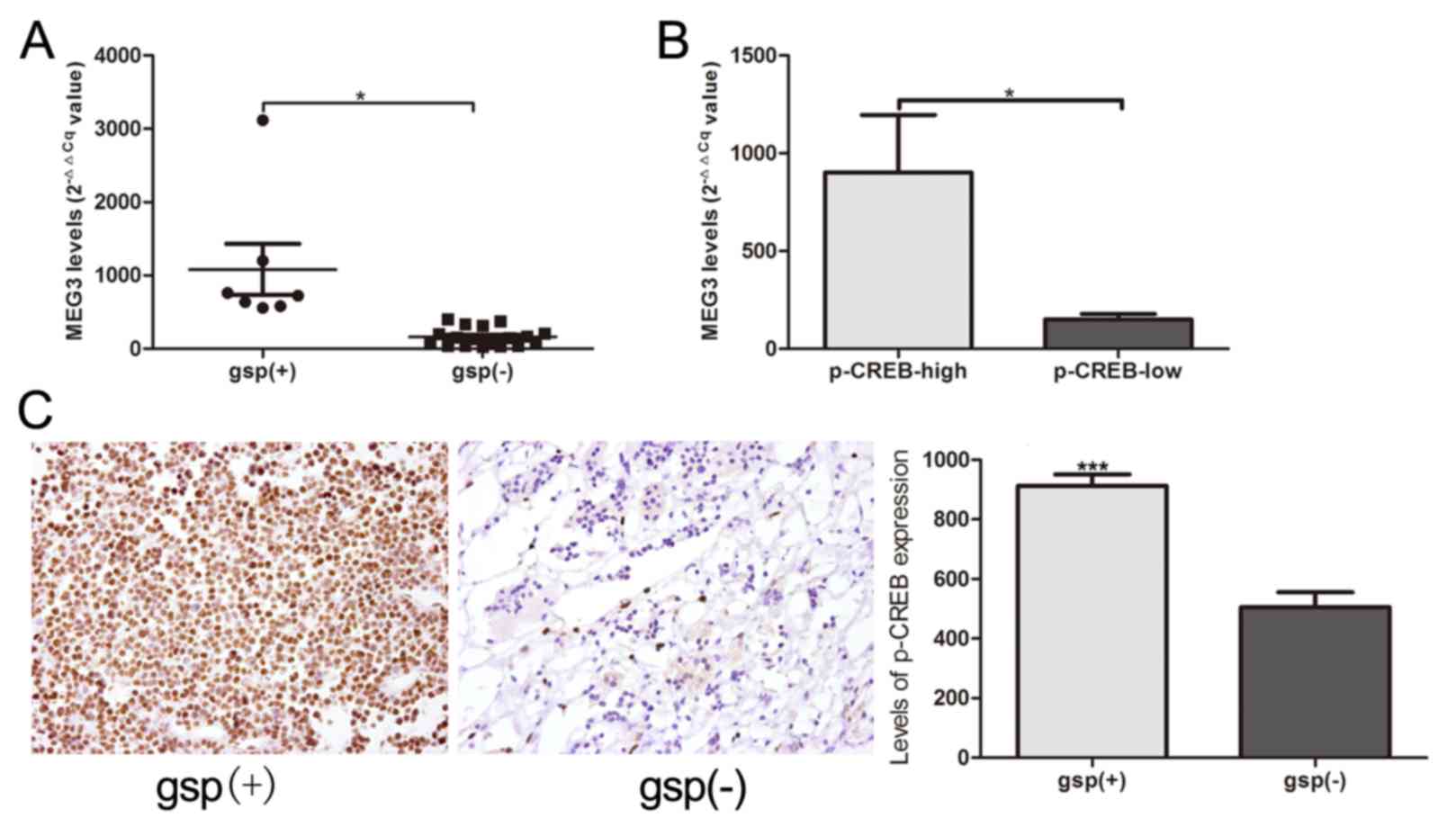
MEG3 expression was significantly increased in the 7
gsp-positive tumors, compared with the 18 gsp-negative tumors
(1,083.2±922.9 vs. 163.3±121.9; P=0.039; Fig. 5A). Patients with high p-CREB
expression indicated significantly increased expression levels of
MEG3, compared with low p-CREB expression (902.2±878.7 vs.
150.1±113.4, P=0.034; Fig. 5B). A
comparison between gsp-negative tumors and gsp-positive tumors
additionally indicated a significantly increased level of p-CREB
expression, according to immunohistochemical staining analysis
(882.3±88.1 vs. 609.0±102.7; P<0.0001; Fig. 5C).
Association between MEG3 and p53
No significant difference was indicated in p53
mutation between the low- and high-MEG3 expression groups,
according to the immunohistochemical staining results (52 vs. 48%;
P=0.576; Table I).
Discussion
A number of studies have been conducted to
investigate the association between gsp oncogenes and clinical
characteristics of patients with GH-secreting pituitary tumor
(8,11,12,17–19). The
majority of the observations suggested that gsp-positive tumors
indicated an increased secretory activity and reduced size,
compared with gsp-negative tumors (9–11).
Another study demonstrated that there were no significant
differences of basal serum GH levels, IGF-I levels and tumor size
in patients with or without a gsp oncogene (8). In the present study, the data indicated
that a reduced tumor size, together with increased serum GH and
IGF-1 levels, was indicative of the presence of a gsp-positive
tumor. This observation is consistent with the previous studies
that examined the association between gsp and the biochemical
characteristics in patients with acromegaly (9–11).
According to Knosp classification, the incidence of invasiveness
was markedly reduced in the gsp-positive tumors, at 29%, compared
with the gsp-negative tumors, at 78% (P=0.024). This conclusion is
consistent with previous research (4). Although no difference was indicated in
terms of Ki-67 Li between the two groups in a previous study
(8), the results of the present
study indicated that the proliferation index was significantly
reduced in gsp-positive tumors, compared with gsp-negative ones
(P=0.039). The aforementioned observations demonstrated that gsp
correlates with GH hypersecretion and reduces tumor size, as well
as suppresses the rate of invasiveness and proliferation of
GH-secreting tumors.
Biochemical analyses explained that this was
attributable to a defect in the Gsα, which controlled adenylyl
cyclase activity and increased intracellular cAMP levels (20,21). The
underlying mechanism of GH hypersecretion in GH-secreting tumors
may indicate that GH-releasing hormone uses cAMP as a second
messenger to modulate GH secretion in somatotroph cells (22). For the effect on proliferation, cAMP
may induce cell arrest or even inhibit mitogenic action of growth
factors in a number of cell lines and conversely it may stimulate
proliferation in other cell lines (23,24). The
mechanism that underlines high probability of gsp-positive tumors
to bear small tumor volume remains unknown. The majority of the
effects of cAMP were mediated by activating cAMP-dependent protein
kinase A (PKA). CREB was phosphorylated by PKA and transactivates
transcription of genes in response to hormonal stimulation of the
cAMP pathway (25). Additionally,
dimers of CREB bound to the enhancer-binding site, referred to as
CRE, was indicated in the control regions of numerous genes,
including c-jun, pit-1 and c-myc (26). Among the aforementioned genes, MEG3,
as an imprinted gene, encoded a novel noncoding RNA that suppressed
tumor cell proliferation (27), and
was activated by the binding of CREB to CRE site as a downstream
target gene of cAMP (28). A
‘cross-talk’ may occur between the cAMP signal pathway and MEG3
activation. Accordingly, it was speculated that the gsp oncogene
may mediate MEG3 expression in suppressing proliferation and
invasiveness, and promoting hormone hypersecretion of GH-secreting
pituitary tumors through the gsp/p-CREB/MEG3 signal pathway.
Previous study observations supported that the
expression of MEG3 cDNA suppresses proliferation in a number of
human tumor cell lines such as cervical carcinoma HeLa, breast
adenocarcinoma MCF-7, and neuroglioma H4 (29). The study of Zhang et al
(29) indicated that MEG3
represented a novel tumor suppressor gene, which may be involved in
the pathogenesis of pituitary adenomas. In the present study, a
strong expression of MEG3 RNA was observed in all 25 GH-secreting
tumors, but almost no MEG3 RNA expression was detected in the 10
clinically nonfunctioning tumors, which is consistent with the
previous research results (29). The
most prominent observation of the present study is that MEG3 mRNA
level is positively correlated with GH and IGF-1 levels, and
negatively correlated with tumor volume. The aforementioned data
indicate that MEG3 may serve an important role in a specific
pathway controlling the GH secretion and cell proliferation.
Additionally, the incidence of invasiveness was indicated to be
notably reduced in tumors with high MEG3 expression, at 29%,
compared with tumors with low MEG3 expression, at 78% (P=0.024).
The Ki-67 index was significantly increased in the group with low
MEG3 expression, compared with in the group with high MEG3
expression (P=0.039). The aforementioned results further confirm
that a strong regulation effect of MEG3 overexpression on cell
proliferation in GH-secreting pituitary tumors exists. Overall,
this may indicate that MEG3 is physiologically involved in the
control of GH production and proliferation.
A previous research has observed that p-CREB
activates pituitary-specific transcription factor-1, which promotes
the transcription of GH gene (30).
The observations of the present study revealed that p-CREB and MEG3
expression levels were significantly increased in gsp-positive
tumors, compared with gsp-negative tumors (P<0.0001 and P=0.039,
respectively). Additionally, MEG3 expression was frequently
increased in the group with high p-CREB expression, compared with
the group with low p-CREB expression (P=0.034). The aforementioned
results indicated that the clinical characteristics of tumors with
high p-CREB expression were similar to that of gsp-positive tumors
and the high MEG3 expression group.
Additionally, the p53-dependent and p53-independent
pathways have been reported to mediate tumor suppression induced by
MEG3 (31). To investigate the role
of p53 in suppressing MEG3 in GH-secreting pituitary tumors, p53
expression was analyzed in groups with low and high MEG3 expression
levels. The data indicated no significant differences in p53
expression between the two groups. Therefore, in GH-secreting
pituitary tumors, MEG3 may serve a role in suppressing tumors
through the p53-independent signaling pathways.
Further research is required to investigate whether
gsp oncogene upregulates p-CREB expression levels to subsequently
promote MEG3 expression. This information would further result in
substantial differences in biochemical and clinical characteristics
of GH-secreting tumors. There are, however, a number of limitations
namely the gsp/p-CREB/MEG3 signal pathway has not been verified in
GH3 cell. The role of MEG3 in regulating the GH3 cell proliferation
and invasiveness has not been verified. MEG3 expression has
reportedly caused apoptosis in numerous tumor cell lines, including
tongue squamous cell carcinoma lines CAL-27 and SCC-15 (32), non-small cell lung cancer lines
SPC-A1 and A549 (33), and glioma
line U251 (34). Previous data
indicated that MEG3 suppresses tumor growth by causing cell cycle
G1 arrest (35). Therefore, the
underlying mechanism of tumor suppression through MEG3 in
GH-secreting pituitary tumors remains to be investigated.
The correlation between MEG3 and gsp oncogene in
gsp-positive and gsp-negative GH-secreting pituitary tumors, to the
best of our knowledge, has not been previously reported.
Collectively, the present study indicated that gsp oncogene
promoted the overexpression of p-CREB, thereby enhancing MEG3
expression and eventually promoting hormone hypersecretion, as well
as suppressing proliferation and invasiveness of GH-secreting
pituitary tumors.
Acknowledgements
Not applicable.
Funding
The present study received grants from the Applied
Basic Research Programs of Science and Technology Commission
Foundation of Jiangsu Province (grant no., BE2015684; Nanjing,
China), and National Natural Science Foundation of China (grant
no., 30801178; Beijing, China).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CT, CZ and CM designed the experiments. CT and CZ
analyzed and interpreted the patient data. CM supervised the
project. CT, CZ, JY, ZC, GW and JZ performed PCR, RT-qPCR and
immunohistochemistry. CT, CZ and CM wrote the manuscript.
Ethics approval and consent to
participate
Approval for the study was obtained from the Ethical
Committee of Nanjing Jinling Hospital. Informed consent was
obtained from all patients.
Patient consent for publication
Informed consent for publication was obtained from
all patients.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
Gsα
|
G-protein α subunit
|
|
MEG3
|
maternally expressed gene 3
|
|
CREB
|
cyclic adenosine
monophosphate-responsive element binding
|
|
GH
|
growth hormone
|
|
IGF-1
|
insulin-like growth factor 1
|
|
p-CREB
|
phosphorylated CREB
|
References
|
1
|
Freda PU, Chung WK, Matsuoka N, Walsh JE,
Kanibir MN, Kleinman G, Wang Y, Bruce JN and Post KD: Analysis of
GNAS mutations in 60 growth hormone secreting pituitary tumors:
Correlation with clinical and pathological characteristics and
surgical outcome based on highly sensitive GH and IGF-I criteria
for remission. Pituitary. 10:275–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasufuku-Takano J, Takano K, Morita K,
Takakura K, Teramoto A and Fujita T: Does the prevalence of gsp
mutations in GH-secreting pituitary adenomas differ geographically
or racially? Prevalence of gsp mutations in Japanese patients
revisited. Clin Endocrinol (Oxf). 64:91–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park C, Yang I, Woo J, Kim S, Kim J, Kim
Y, Sohn S, Kim E, Lee M, Park H, Jung J and Park S: Somatostatin
(SRIF) receptor subtype 2 and 5 gene expression in growth
hormone-secreting pituitary adenomas: The relationship with
endogenous srif activity and response to octreotide. Endocr J.
51:227–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buchfelder M, Fahlbusch R, Merz T,
Symowski H and Adams EF: Clinical correlates in acromegalic
patients with pituitary tumors expressing GSP oncogenes. Pituitary.
1:181–185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballare E, Mantovani S, Lania A, Di Blasio
AM, Vallar L and Spada A: Activating mutations of the Gs alpha gene
are associated with low levels of Gs alpha protein in growth
hormone-secreting tumors. J Clin Endocrinol Metab. 83:4386–4390.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang I, Park S, Ryu M, Woo J, Kim S, Kim
J, Kim Y and Choi Y: Characteristics of gsp-positive growth
hormone-secreting pituitary tumors in Korean acromegalic patients.
Eur J Endocrinol. 134:720–726. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosoi E, Yokogoshi Y, Hosoi E, Horie H,
Sano T, Yamada S and Saito S: Analysis of the Gs alpha gene in
growth hormone-secreting pituitary adenomas by the polymerase chain
reaction-direct sequencing method using paraffin-embedded tissues.
Acta Endocrinol (Copenh). 129:301–306. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams EF, Brockmeier S, Friedmann E, Roth
M, Buchfelder M and Fahlbusch R: Clinical and biochemical
characteristics of acromegalic patients harboring gsp-positive and
gsp-negative pituitary tumors. Neurosurgery. 33:198–203. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spada A, Arosio M, Bochicchio D, Bazzoni
N, Vallar L, Bassetti M and Faglia G: Clinical, biochemical, and
morphological correlates in patients bearing growth
hormone-secreting pituitary tumors with or without constitutively
active adenylyl cyclase. J Clin Endocrinol Metab. 71:1421–1426.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spada A, Arosio M, Bassetti M, Vallar L,
Clementi E and Bazzoni N: Mutations in the alpha subunit of the
stimulatory regulatory protein of adenylyl cyclase (Gs) in human
GH-secreting pituitary adenomas. Biochemical, clinical, and
morphological aspects. Pathol Res Pract. 187:567–570. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Landis CA, Harsh G, Lyons J, Davis RL,
McCormick F and Bourne HR: Clinical characteristics of acromegalic
patients whose pituitary tumors contain mutant Gs protein. J Clin
Endocrinol Metab. 71:1416–1420. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto Y, Kinoshita M, Oshino S, Arita H,
Kitamura T, Otsuki M, Shimomura I, Yoshimine T and Saitoh Y: Gsp
mutation in acromegaly and its influence on TRH-induced paradoxical
GH response. Clin Endocrinol (Oxf). 80:714–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fusco A, Zatelli MC, Bianchi A, Cimino V,
Tilaro L, Veltri F, Angelini F, Lauriola L, Vellone V, Doglietto F,
et al: Prognostic significance of the Ki-67 labeling index in
growth hormone-secreting pituitary adenomas. J Clin Endocrinol
Metab. 93:2746–2750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thapar K, Scheithauer BW, Kovacs K,
Pernicone PJ and Laws ER Jr: p53 expression in pituitary adenomas
and carcinomas: Correlation with invasiveness and tumor growth
fractions. Neurosurgery. 38:765–771. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruopp MD, Perkins NJ, Whitcomb BW and
Schisterman EF: Youden Index and optimal cut-point estimated from
observations affected by a lower limit of detection. Biom J.
50:419–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Kim MS, Park YJ, Kim SW, Park DJ,
Park KS, Kim SY, Cho BY, Lee HK, Jung HW, et al: Prevalence of Gs
alpha mutations in Korean patients with pituitary adenomas. J
Endocrinol. 168:221–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Metzler M, Luedecke DK, Saeger W, Grueters
A, Haberl H, Kiess W, Repp R, Rascher W and Doetsch J: Low
prevalence of Gs alpha mutations in śomatotroph adenomas of
children and adolescents. Cancer Genet Cytogenet. 166:146–151.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Efstathiadou ZA, Bargiota A, Chrisoulidou
A, Kanakis G, Papanastasiou L, Theodoropoulou A, Tigas SK,
Vassiliadi DA, Alevizaki M and Tsagarakis S: Impact of gsp
mutations in somatotroph pituitary adenomas on growth hormone
response to somatostatin analogs: A meta-analysis. Pituitary.
18:861–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clementi E, Malgaretti N, Meldolesi J and
Taramelli R: A new constitutively activating mutation of the Gs
protein alpha subunit-gsp oncogene is found in human pituitary
tumours. Oncogene. 5:1059–1061. 1990.PubMed/NCBI
|
|
21
|
Landis CA, Masters SB, Spada A, Pace AM,
Bourne HR and Vallar L: GTPase inhibiting mutations activate the
alpha chain of Gs and stimulate adenylyl cyclase in human pituitary
tumours. Nature. 340:692–696. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Billestrup N, Swanson LW and Vale W:
Growth hormone-releasing factor stimulates proliferation of
somatotrophs in vitro. Proc Natl Acad Sci USA. 83:6854–6857. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mantovani G, Bondioni S, Ferrero S, Gamba
B, Ferrante E, Peverelli E, Corbetta S, Locatelli M, Rampini P,
Beck-Peccoz P, et al: Effect of cyclic adenosine
3′,5′-monophosphate/protein kinase a pathway on markers of cell
proliferation in nonfunctioning pituitary adenomas. J Clin
Endocrinol Metab. 90:6721–6724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pertuit M, Barlier A, Enjalbert A and
Gérard C: Signalling pathway alterations in pituitary adenomas:
Involvement of Gsalpha, cAMP and mitogen-activated protein kinases.
J Neuroendocrinol. 21:869–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto KK, Gonzalez GA, Menzel P, Rivier
J and Montminy MR: Characterization of a bipartite activator domain
in transcription factor CREB. Cell. 60:611–617. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyer TE, Waeber G, Lin J, Beckmann W and
Habener JF: The promoter of the gene encoding 3′,5′-cyclic
adenosine monophosphate (cAMP) response element binding protein
contains cAMP response elements: Evidence for positive
autoregulation of gene transcription. Endocrinology. 132:770–780.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Rice K, Wang Y, Chen W, Zhong Y,
Nakayama Y, Zhou Y and Klibanski A: Maternally expressed gene 3
(MEG3) noncoding ribonucleic acid: Isoform structure, expression,
and functions. Endocrinology. 151:939–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Zhang X, Zhou Y, Ansell PJ and
Klibanski A: Cyclic AMP stimulates MEG3 gene expression in cells
through a cAMP-response element (CRE) in the MEG3 proximal promoter
region. Int J Biochem Cell Biol. 38:1808–1820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Zhou Y, Mehta KR, Danila DC,
Scolavino S, Johnson SR and Klibanski A: A pituitary-derived MEG3
isoform functions as a growth suppressor in tumor cells. J Clin
Endocrinol Metab. 88:5119–5126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frutos MG, Cacicedo L, Méndez CF, Vicent
D, González M and Sánchez-Franco F: Pituitary alterations involved
in the decline of growth hormone gene expression in the pituitary
of aging rats. J Gerontol A Biol Sci Med Sci. 62:585–597. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou Y, Zhong Y, Wang Y, Zhang X, Batista
DL, Gejman R, Ansell PJ, Zhao J, Weng C and Klibanski A: Activation
of p53 by MEG3 non-coding RNA. J Biol Chem. 282:24731–24742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia LF, Wei SB, Gan YH, Guo Y, Gong K,
Mitchelson K, Cheng J and Yu GY: Expression, regulation and roles
of miR-26a and MEG3 in tongue squamous cell carcinoma. Int J
Cancer. 135:2282–2293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Ren Z and Sun P: Overexpression of
the long non-coding RNA MEG3 impairs in vitro glioma cell
proliferation. J Cell Biochem. 113:1868–1874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chunharojrith P, Nakayama Y, Jiang X, Kery
RE, Ma J, De La Hoz Ulloa CS, Zhang X, Zhou Y and Klibanski A:
Tumor suppression by MEG3 lncRNA in a human pituitary tumor derived
cell line. Mol Cell Endocrinol. 416:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|